Open Journal of Medicinal Chemistry
Vol.06 No.01(2016), Article ID:64766,18 pages
10.4236/ojmc.2016.61002
Synthesis of Novel Acid Dyes with Coumarin Moiety and Their Utilization for Dyeing Wool and Silk Fabrics
Mahmoud S. Bashandy1,2*, Fatma A. Mohamed3,4, Mohamed M. El-Molla3,5, Mahmoud B. Sheier1, Ahmed H. Bedair1
1Chemistry Department, Faculty of Science (Boys), Al-Azhar University, Cairo, Egypt
2Chemistry Department, University College in Al-Jamoum, Umm Al-Qura University, Makkah, Saudi Arabia
3Textile Research Division, National Research Centre, Giza, Egypt
4Balguenvzh University College, Department of Female Student, Umm Al-Qura University, Makkah, Saudi Arabia
5Chemistry Department, College of Sciences & Art, Al Jouf University, El-Quraiat, Saudi Arabia

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 31 December 2015; accepted 17 March 2016; published 21 March 2016
ABSTRACT
This article describes the synthesis of some novel coumarin compounds to use as acid dyes by using compounds 1 - 4 as starting materials, which were prepared by interaction of 2-hydroxyben- zaldehyde with ethyl 3-oxobutanoate, diethylmalonate, 4-nitrobenzenediazonium chloride and 4- sulfobenzene-diazonium chloride, respectively. Compound 1 reacted with bromine and 2-cyano- acetohydrazide to give phenacyl bromide derivative 5 and 2-cyanoacetohydrazone derivative 6, respectively. Coupling of compound 6 with equimolar amount of 2-sulfo-4-((4-sulfophenyl) di- azenyl)benzenediazonium chloride gave coumarin acid dye 8. Phenacyl bromide derivative 5 reacted with potassium cyanide in refluxing ethanol to produce compound 7, which on coupling with equimolar amount of 8-hydroxy-6-sulfonaphthalene-2-diazonium chloride and 8-hydroxy-3,6-di- sulfonaphthalene-1-diazonium chloride gave coumarin acid dyes 9 and 10, respectively. Interaction of compound 2 with 2-amino-5-((4-sulfophenyl)diazenyl)benzenesulfonic acid, benzene-1,4- diamine and 3,3’-dimethoxy-[1,1’-biphenyl]-4,4’-diamine in refluxing ethanol afforded compounds 11, 12 and 14, respectively. Diazonium sulphate of compounds 12 and 14 coupling with 4-amino- 5-hydroxynaphthalene-2,7-disulfonic acid gave compounds 13 and 15, respectively. Cyclocondensation of compound 3 with ethyl 3-oxobutanoate, diethyl malonate and malononitrile afforded derivatives of 3-acetyl-2H-chromen-2-one 16, ethyl 2-oxo-2H-chromene-3-carboxylate 17 and 2- imino-2H-chromene-3-carbonitrile 18, respectively. Reaction of sodium benzenesulfonate deriva- tive 4 with ethyl 3-oxobutanoate and hydrazine hydrate gave compounds 19 and 20, respectively. The structures of the newly synthesized compounds were confirmed by elemental analysis, UV/ VIS, IR, 1H NMR and Ms spectral data. The suitability of the prepared dyestuffs for dyeing of wool and silk fabrics has been investigated. The dyed fabric shows good light fastness, very good rubbing, perspiration, washing and excellent sublimation fastness. These dyes have been color shade from blue to violet with very good depth and levelness on fabrics. The dye bath exhaustion and fixation on fabric has been found to be very good.
Keywords:
Synthesis, Coumarin, Acid Dye, Dyeing, Wool Fabric

1. Introduction
The considerable innovation has been witnessed in past three decades in the field of azo dye chemistry based on heterocyclic systems and studies in the synthesis of such derivatives have been reported [1] - [5] . Most of the recent research has focused on structural variations of existing types, for example, variations in substituent, especially on the side chains of the coupling components. The use of heterocyclic coupling component and diazo components in the synthesis of azo dyes is well established, and the resultant dyes exhibit better tinctorial strength and brighter dyeing than those derived from aniline-based components. Most heterocyclic dyes of technical interest for application to textiles are derived from diazo components consisting of five-membered rings containing one sulphur heteroatom and to which a diazotisable amino group is directly attached. The ring may also possess one or more nitrogen heteroatoms and be fused to another aromatic ring. These diazo components are capable of providing red to blue color dyes that meet the rigorous technical and economical requirements demanded of them by both manufacturer and user. Intensive efforts have been made in the investigation of monoazo dyes in which a heterocyclic system replaces one of the usual carboxylic systems. Many different heterocyclic diazo components have been studied, especially derivatives of thiazole, imidazole, benzimidazole owing to the marked bathochromic effect of such groups [6] - [12] . A majority of acid dyestuffs are sulphonic acid derivatives of azo dyes. The free dye acids are difficult to isolate and are hydroscopic in nature making it difficult to pack and store them. These dyes are invariably isolated as sodium salts. Coumarins are attractive and versatile molecules that find applications in various fields like medicine, perfumery, dyes, pigments, optical brighteners, lasers, optical data storage devices, solar cells [13] - [19] . The coumarin is not fluorescent, but the introduction of an electron-withdrawing group such as a diazotized aromatic amine or an acetyl group makes it highly fluorescent. Coumarin establishes a family of dyes [20] - [24] that are applicable in different fields of science and technology [25] - [27] . The present work was carried out with the following objectives, synthesis and identification of some newly acid dyes based on coumarin derivatives, and the possibility of its use in dyeing of wool and silk fabrics.
2. Results and Discussion
Chemistry
The present investigation deals with the synthesis of novel coumarin compounds to use as acid dyes by using compounds 1-4 as a starting materials, which were prepared by interaction of 2-hydroxybenzaldehyde with ethyl 3-oxobutanoate, diethylmalonate, 4-nitrobenzenediazonium chloride and 4-sulfobenzenediazonium chloride, respectively (Scheme 1). Structure of compound 3 was established on the basis of its elemental analysis and spectral data. Thus, IR spectrum of 3indicated absorption bands at vmax = 3370 cm−1 for hydroxyl group, 1653 cm−1 for carbonyl group and 1456, 1290 cm−1 for nitro group. 1H NMR spectrum showed three singlet signals at δ = 8.05, 10.30 and 11.78 ppm corresponding to H6 of hyroxyphenyl ring, hydroxyl and formyl protons, respectively, two doublet signals, each doublet for two protons at δ = 7.95 and 8.32 ppm for H2,6 and H3,5of nitrophenyl ring, respectively, two doublet signals, each doublet for one proton at δ = 7.17 and 8.14 ppm due to H3 and H4 of hyroxyphenyl ring, respectively. IR spectrum of compound 4 showed absorption bands at vmax = 3462 cm−1 for hydroxyl group, vmax = 1658 cm−1 for carbonyl group and vmax = 1386, 1150 cm−1 for sulphate group. 1H NMR spectrum showed three singlet signals at δ = 7.19, 10.33 and 11.56 ppm corresponding to H6 of hyroxyphenyl-
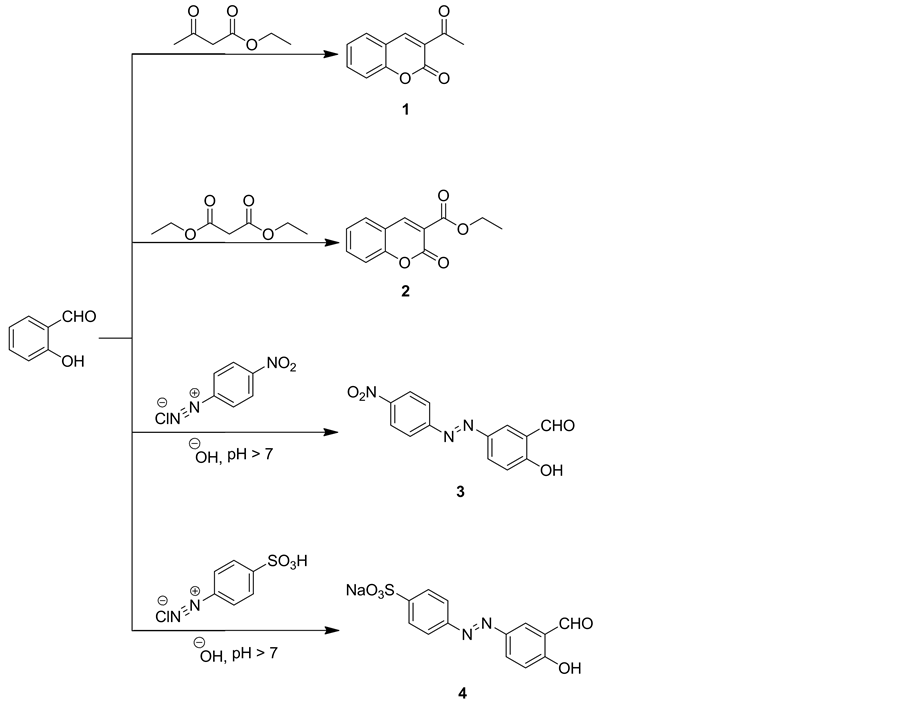
Scheme 1. Synthetic pathways for compounds 1-4.
ring, hydroxyl and formyl protons, respectively, two doublet signals, each doublet for two protons at δ = 7.76 - 7.79 and 8.15 ppm for H2,6 andH3,5 of benzenesulfonate ring, respectively, two doublet signals, each doublet for one proton at δ = 7.17 and 8.06 - 8.08 ppm due to H3 and H4 of hyroxyphenyl ring, respectively.
3-Acetyl-2H-chromen-2-one (1) reacted with bromine and 2-cyanoaceto-hydrazide to give phenacyl bromide derivative 5and 2-cyanoacetohydrazone derivative 6, respectively. Phenacyl bromide derivative 5 reacted with potassium cyanide in refluxing ethanol to produce 3-oxo propanenitrile derivative 7 (Scheme 2). The structure of compound 7 was established on the basis of their elemental analysis and spectral data. Thus, IR spectrum of compound 7 revealed absorption bands at vmax = 2207 cm−1 for cyano group, vmax = 1703 and 1636 cm−1 for cyclic carbonyl of coumarin and acyclic carbonyl, respectively. The mass spectrum of compound 7 showed a molecular ion peak at m/z = 213 and a base peak at m/z = 101.The methylene group in compound 6 proved to be highly reactive. Thus, 2-cyanoacetohydrazone derivative 6 underwent coupling with equimolar amount of 2- sulfo-4-((4-sulfophenyl)-diazenyl)benzenediazonium chloride to give coumarin acid dye 8 (Scheme 2). The IR spectrum of coumarin acid dye 8 showed, two bi-forked characteristic absorption bands at vmax = 3421 and 3350 cm−1 assignable to 2NH groups, another absorption bands at vmax = 2212 cm−1 for cyano group and at vmax = 1755 and 1657 cm−1 for two carbonyl groups of coumarin ring and amide group, respectively. Mass spectrum of compound 8 showed a molecular ion peak at m/z = 681 and a base peak at m/z = 50.
3-Oxo-3-(2-oxo-2H-chromen-3-yl)propanenitrile (7) underwent coupling with equimolar amount of 8-hy- droxy-6-sulfonaphthalene-2-diazonium chloride and 8-hydroxy-3,6-disulfonaphthalene-1-diazonium chloride to give coumarin acid dyes 9 and 10,respectively (Scheme 3). The structure of coumarin acid dyes 9 and 10 were established on the basis of their elemental analysis and spectral data. Thus, IR spectrum of compound 9 revealed absorption bands at vmax = 3424 and 3320 cm−1 for OH and NH groups, respectively, vmax = 2248 cm−1 for cyano group and at vmax = 1710 and 1632 cm−1 for cyclic carbonyl of coumarin and acyclic carbonyl, respectively.
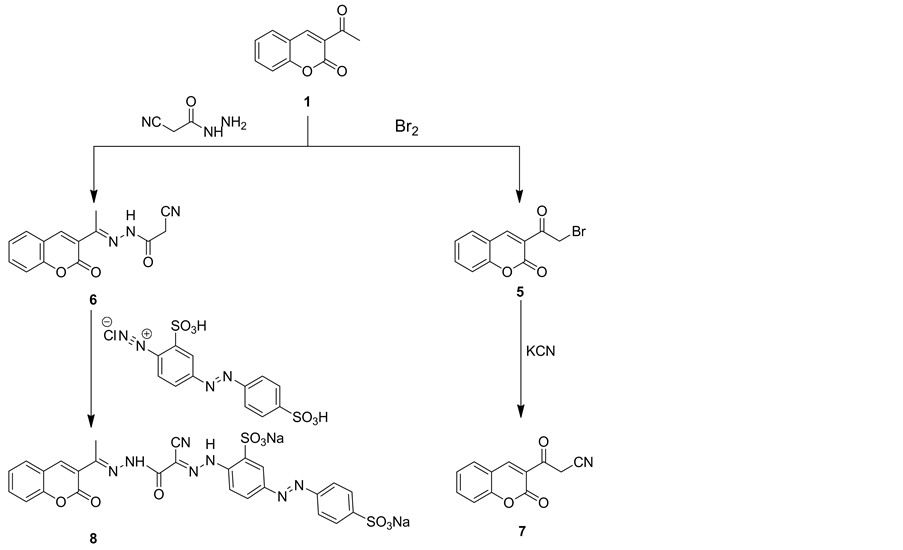
Scheme 2. Synthetic pathways for compounds 5-8.
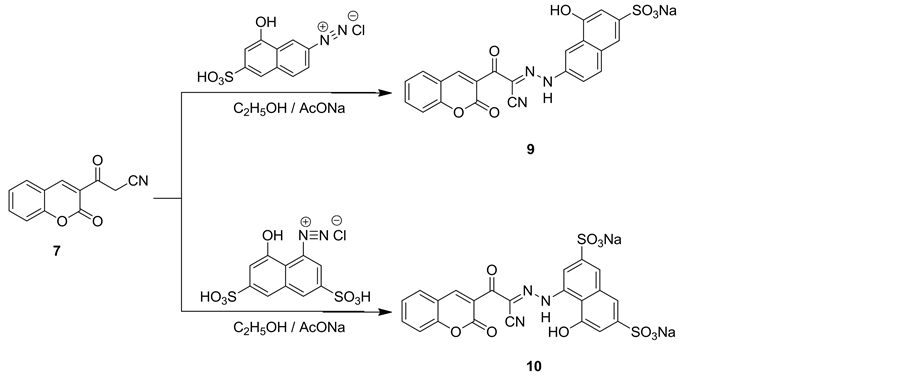
Scheme 3. Synthetic pathways for compounds 9 and 10.
1H NMR spectrum of compound 9 showed two singlet signals at δ = 12.71 and 12.80 ppm corresponding to protons of OH and NH groups. IR spectrum of compound 10 revealed broad absorption band from vmax = 3486 to 3380 cm−1 for OH and NH groups, vmax = 2225 cm−1 for cyano group and at vmax = 1710 and 1632 cm−1 for cyclic carbonyl of coumarin and acyclic carbonyl, respectively. Mass spectrum of compound 10 showed a molecular ion peak at m/z = 587 and a base peak at m/z = 219.
Interaction of ethyl 2-oxo-2H-chromene-3-carboxylate (2) with 2-amino-5-((4-sulfophenyl)diazenyl) benzene-sulfonic acid in refluxing ethanol afforded coumarin acid dye 11 (Equation 1). The structure of coumarin acid dye 11 was established on the basis of their elemental analysis and spectral data. Thus, IR spectrum of compound 11 revealed absorption bands at vmax = 3325 cm−1 for NH group, vmax = 1736 and 1617 cm−1 for two carbonyl groups of coumarin ring and amide group, respectively. Mass spectrum of compound 11 showed a molecular ion peak at m/z = 573 and a base peak at m/z = 394.
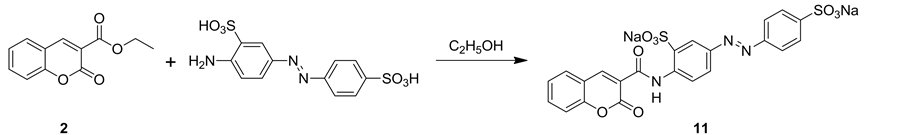
Equation 1. Synthetic pathway for compound 11.
Interaction of compound 2 with benzene-1,4-diamine in refluxing ethanol afforded a single product identified as N-(4-aminophenyl)-2-oxo-2H-chromene-3-carboxamide (12) on the basis of elemental analysis and spectral data. Thus, IR spectrum of compound 12 showed, two bi-forked characteristic absorption bands at vmax = 3458 and 3360 cm−1 assignable to amino group, vmax = 3320 cm−1 for NH group and vmax = 1702 and 1650 cm−1 for two carbonyl groups of coumarin ring and amide group, respectively. Mass spectrum of compound 12 showed a molecular ion peak at m/z = 280 and showed other peaks at m/z = 173 corresponding to fragment (M+-C6H7N2), m/z = 107as a base peak for fragment (C6H7N2+) (Chart 1).
Compounds 12 was suspended with stirring in concentrated sulfuric acid and cooled to 0˚C - 5˚C then diazotized by adding sodium nitrite. After stirring at 0˚C for 1 h, the diazonium sulphate solution of compound 12 was added to dissolved coupler compound of 4-amino-5-hydroxynaphthalene-2,7-disulfonic acid in 20% sodium hydroxide solution at 0˚C to give compound 13 (Scheme 4). IR spectrum of compound 13 revealed broad absorption band from vmax = 3505 to 3273 cm−1 for hydroxyl, amino and NH groups and at vmax = 1700 and 1648 cm−1 for two carbonyl groups of coumarin ring and amide group, respectively. Mass spectrum of compound 13 showed a molecular ion peak at m/z = 654 and a base peak at m/z = 107.
In the same manner, compound 2 underwent condensation with 3,3’-dimethoxy-[1,1’-biphenyl]-4,4’-diamine to produce N-(4’-amino-3,3’-dimethoxy-[1,1'-biphenyl]-4-yl)-2-oxo-2H-chromene-3-carboxamide (14). The elemental analysis and spectral data of the latter structure were in agreement with its assigned structure. Thus, IR spectrum of compound 14 revealed a broad absorption band from vmax = 3400 to 3270 cm−1 due to amino and NH groups and vmax = 1718 and 1658 cm−1 for two carbonyl groups of coumarin ring and amide group, respectively. Besides, the mass spectrum was compatible with the molecular formula C24H20N2O5, m/z =416 confirmed structure 14. Diazonium sulphate of compound 14 coupling with 4-amino-5-hydroxynaphthalene-2,7-disulfonic acid to give compound 15 (Scheme 5). IR spectrum of compound 15 revealed a broad absorption band from vmax = 3509 to 3380 cm−1 due to hydroxyl, amino and NH groups, vmax = 1726 and 1661 cm−1 for two carbonyl groups of coumarin ring and amide group, respectively and vmax = 1449 cm−1 for N=N group. Mass spectrum of compound 15 showed a molecular ion peak at m/z = 791 and a base peak at m/z = 64.
Cyclocondensation of 2-hydroxy-5-((4-nitrophenyl)diazenyl)benzaldehyde (3) with ethyl 3-oxobutanoate, diethyl malonate and/or malononitrile afforded derivatives of 3-acetyl-2H-chromen-2-one 16, ethyl 2-oxo-2H- chromene-3-carboxylate17 and 2-imino-2H-chromene-3-carbonitrile 18, respectively (Scheme 6). The structures of compounds 16-18 were established on the basis of their elemental analysis and spectral data. Thus, IR spectrum of compound 16 revealed an absorption bands atvmax = 1745 and 1676 cm−1 for two carbonyl groups of coumarin ring and acetyl group, respectively, vmax = 1522 cm−1 corresponding to N=N group and two absorption bands at vmax = 1338 and 1231 cm−1 for nitro group.1H NMR spectrum of 16 revealed three singlet signals at δ= 2.53, 8.42 and 8.80 ppm for methyl, H5and H4 of coumarin, respectively, two doublet signals each doublet signal for one proton at δ= 7.65, 8.25 ppm corresponding to H8 and H7 of coumarin, respectively, besides two doublet signals each doublet signal for two protons at δ= 8.08 and 8.59 ppm corresponding to H2,6 and H3,5 of nitrophenyl ring, respectively.IR spectrum of compound 17 revealed an absorption bands at vmax = 1741 and 1705 cm−1 for two carbonyl groups of coumarin ring and ester group, respectively, vmax = 1521 cm−1 corresponding to N=N group and two absorption bands at vmax = 1338 and 1251 cm−1 for nitro group. 1H NMR spectrum of 17 revealed triplet and quartet signals at δ= 1.30 and 4.30 ppm, respectively corresponding to protons of methyl and methylene of ester group, respectively, two singlet signals at δ= 8.40 and 8.89 ppm for H5 and H4 of coumarin, respectively, two doublet signals each doublet signal for one proton at δ = 7.60, 8.19 ppm corresponding to H8 and H7 of coumarin, respectively, besides two doublet signals each doublet signal for two protons at δ = 8.03 and 8.52 ppm corresponding to H2,6 and H3,5 of nitrophenyl ring, respectively.
IR spectrum of compound 18 revealed an absorption band at vmax = 3337 for NH group, vmax = 2205 for cyano group, vmax = 1556 cm−1 corresponding to N=N group and two absorption bands at vmax = 1338 and 1251 cm−1
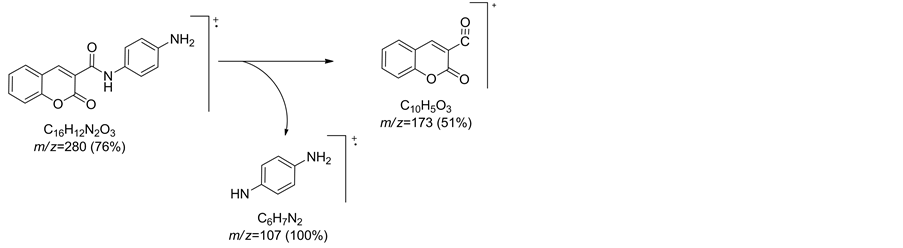
Chart 1. Fragmentation pattern for compound 12.
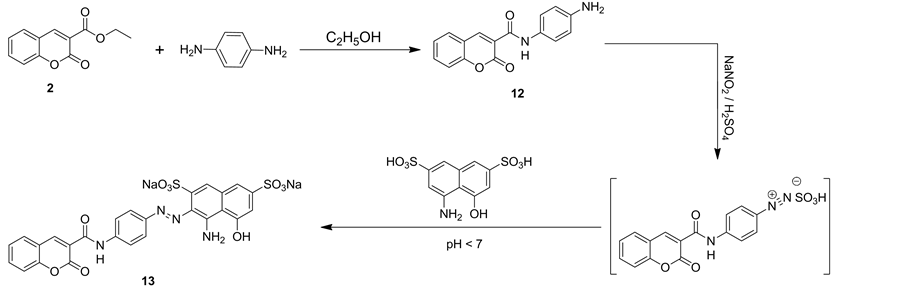
Scheme 4. Synthetic pathways for compounds 12 and 13.
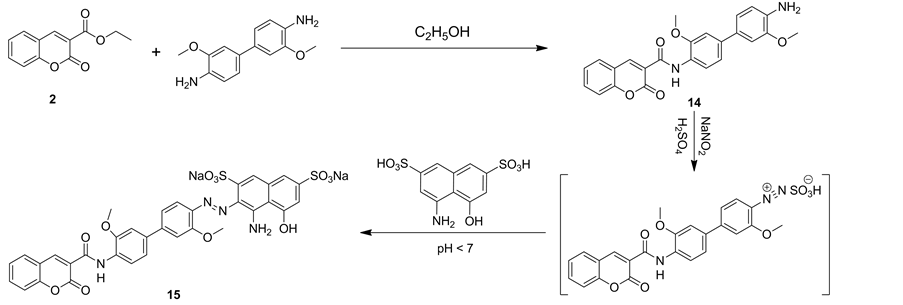
Scheme 5. Synthetic pathways for compounds 14 and 15.
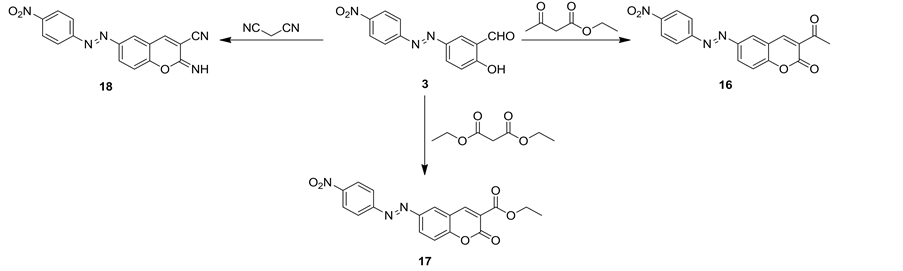
Scheme 6. Synthetic pathways for compounds 16-18.
for nitro group.Mass spectrum of compound 18 showed a molecular ion peak at m/z = 321 (M++2) and showed other peaks at m/z = 319 (M+), m/z = 170 corresponding to fragment (M+-C6H3N3O2), m/z = 150 for fragment (M+-C10H5N2O), m/z = 106 for fragment (C7H6O+), m/z = 77 for fragment (C6H5+), m/z = 65 for fragment (C5H5+) and m/z = 52 for fragment (C4H4+) (Chart 2).
Reaction of sodium benzenesulfonate derivative 4 with ethyl 3-oxobutanoate and/or hydrazine hydrate gave compounds 19 and 20, respectively (Scheme 7). IR spectrum of compound 19 revealed an absorption bands at vmax = 1754 and 1672 cm−1 for two carbonyl groups of coumarin ring and acetyl group, respectively, vmax = 1564 cm−1 corresponding to N=N group and two absorption bands at vmax = 1382 and 1122 cm−1 for SO2 group. 1H NMR spectrum of 19 revealed triplet signal at δ = 2.57 ppm corresponding to protons of methyl group, two singlet signals at δ = 8.19 and 8.80 ppm for H5 and H4 of coumarin, respectively, two doublet signals each
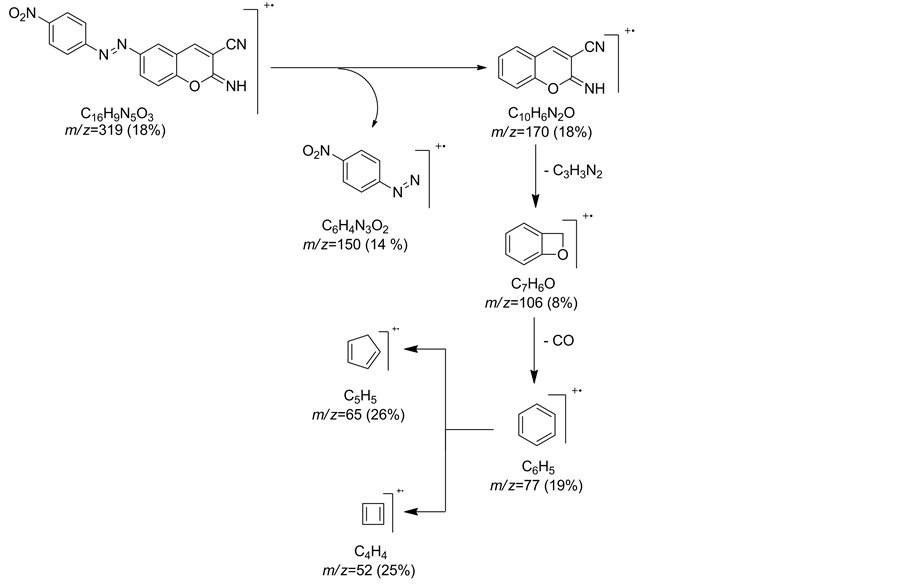
Chart 2. Fragmentation pattern for compound 18.
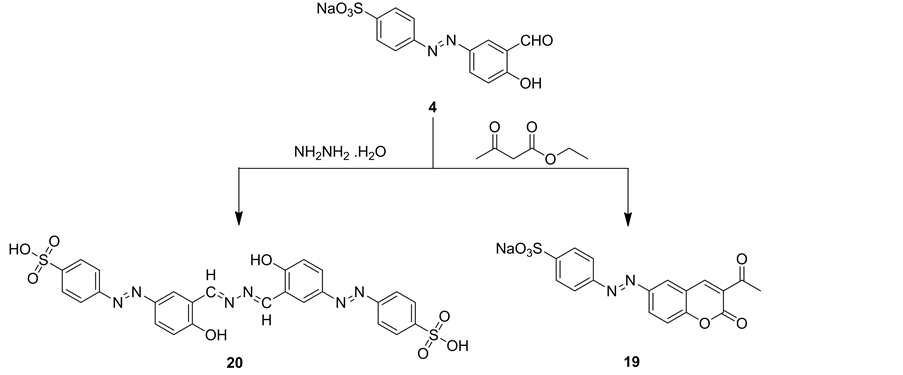
Scheme 7. Synthetic pathways for compounds 19 and 20.
doublet signal for one proton at δ = 7.62, 7.85 ppm corresponding to H8 and H7 of coumarin, respectively, besides two doublet signals each doublet signal for two protons at δ = 7.78 and 8.52 ppm corresponding to H2,6 and H3,5 of benzenesulfonate ring, respectively.
IR spectrum of compound 20 revealed an absorption broad band at vmax = 3384 for OH groups, vmax = 1486 cm−1 corresponding to N=N group and two absorption bands at vmax = 1384 and 1132 cm−1 for SO2 group. Mass spectrum of compound 20 showed a molecular ion peak at m/z = 552 (M+-2N2) and showed other peaks at m/z = 450 corresponding to fragment (C20H15N6O5S+) and m/z = 295 for fragment (C14H10N6O2+) (Chart 3).
3. Experimental
3.1. Methods and Materials
3.1.1. Wool Fabric
Wool fabric of 310 g/m2, supplied by Golden Tex Co., Tenth of Ramadan-Egypt, was initially treated in an aqueous solution with a liquor ratio 50:1 containing 0.5 g/L sodium carbonate and 2 g/L nonionic detergent at 60˚C for 30 min, then thoroughly washed, and air dried at room temperature.
3.1.2. Silk Fabric
Degummed and bleached silk fabric (El-Khateib Co., Egypt) weighing 90 g/m2 was used throughout this work. Before dyeing, the fabric was treated in an aqueous solution containing 2 g/l non-ionic detergent for 1 h at 90˚C and at a liquor ratio 50:1, then washed thoroughly in water and air dried at room temperature.
3.1.3. Chemicals
H-acid, γ-acid and 4-aminoazobenzene-3,4’-disulphonic acid, 1-amino-3-bromo-5,10-dioxoanthracene-2-sul- phonic acid were obtained from Fluka Chemie AG. All other chemicals used in the study were of reagent gradeand applied without further purification.
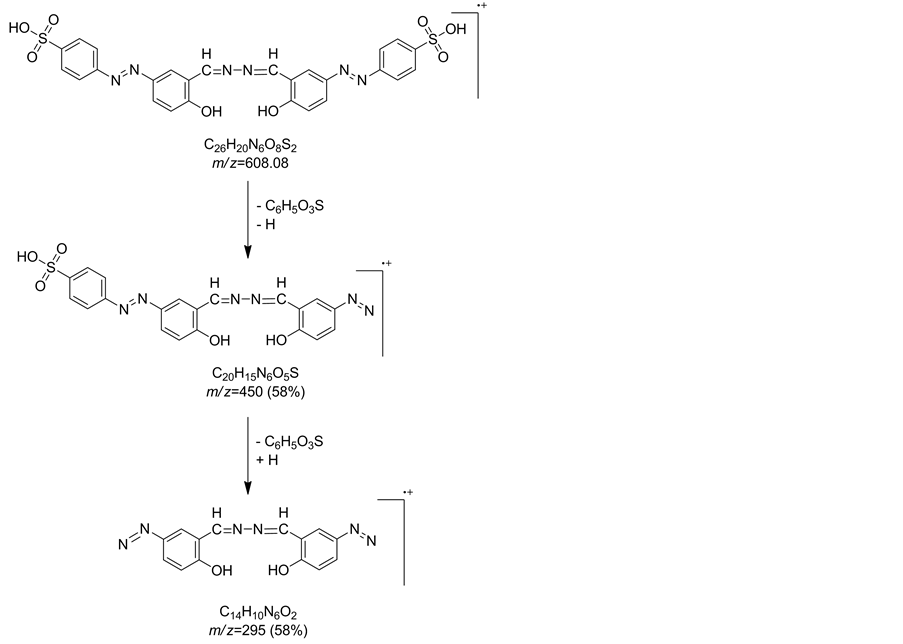
Chart 3. Fragmentation pattern for compound 20.
3.2. Chemistry
Melting points (˚C, uncorrected) were determined in open capillaries on a Gallen Kemp melting point apparatus (Sanyo Gallen Kemp, Southborough, UK). IR spectra (KBr) were recorded on FT-IR 5300 spectrometer and Perkin Elmer spectrum RXIFT-IR system (ν, cm−1). Pre-coated silica gel plates (silica gel 0.25 mm, 60 G F 254; Merck, Germany) were used for thin layer chromatography. The NMR spectra in (DMSO-d6) were recorded at 400 MHz on a Varian Gemini NMR spectrometer (δ, ppm). Mass spectra were obtained on GC Ms-QP 1000 EX mass spectrometer at 70 ev. Elemental analyses were performed on Carlo Erba 1108 Elemental Analyzer (Heraeus, Hanau, Germany). All compounds were within ±0.4% of the theoretical values. Analyses were carried out by the Micro analytical Research Center, Faculty of Science, Cairo University and Al-Azhar University.
3.2.1. 3-Acetyl-2H-Chromen-2-One (1)
Compound 1 was synthesized according to the literature procedure [28] .
3.2.2. Ethyl 2-Oxo-2H-Chromene-3-Carboxylate (2)
Compound 2 was synthesized according to the literature procedure [29] .
3.2.3. 2-Hydroxy-5-((4-Nitrophenyl)Diazenyl)Benzaldehyde (3)
2-Hydroxybenzaldehyde (1.22 g, 0.01 mol) was dissolved in water (20 mL) containing (0.4 g, 0.01 mol) of sodium hydroxide and (4.24 g, 0.04 mol) of sodium carbonate during the period of 30 min at 0˚C. The resulting solution was added slowly to a solution of diazonium chloride of 4-nitroaniline (1.38 g, 0.01 mol) in water at 0˚C - 5˚C. The reaction mixture was stirred for 1 h at 0˚C and then allowed to warm slowly to room temperature. The product was collected by filtration and washed with 100 mL of NaCl solution (10%) under vacuum. The obtained solid was dried under vacuum at 80˚C overnight to give 3. Brown crystals, yield, 92%; mp 185-188˚C. IR (KBr, cm−1): vmax = 3370 (OH), 1653 (C=O), 1578 (C=C), 1526 (N=N), 1456, 1290 cm−1 (NO2). 1H NMR (DMSO-d6): δ = 7.17 (1H, d, Ar-H3), 7.95 (2H, d, nitrophenyl-H2,6), 8.05 (1H, s, Ar-H6), 8.14 (1H, d, Ar-H4), 8.32 (2H, d, nitrophenyl-H3,5), 10.30 ppm (1H, s, OH), 11.78 (1H, s, CHO). Anal. Calcd. for C13H9N3O4 (271.23): C, 57.57; H, 3.34; N, 15.49. Found: C, 57.29; H, 3.56; N, 15.28%.
3.2.4. Sodium 4-((3-Formyl-4-Hydroxyphenyl)Diazenyl)Benzenesulfonate (4)
2-Hydroxybenzaldehyde (1.22 g, 0.01 mol) was dissolved in water (20 mL) containing (0.40 g, 0.01 mol) of sodium hydroxide and (4.24 g, 0.04 mol) of sodium carbonate during the period of 30 min at 0˚C. The resulting solution was added slowly to a solution of 4-sulfobenzenediazonium chloride (2.2 g, 0.01 mol) in water at 0˚C - 5˚C. The reaction mixture was stirred for 1 h at 0˚C and then allowed to warm slowly to room temperature. The product was collected by filtration and washed with 100 mL of NaCl solution (10%) under vacuum. The obtained solid was dried under vacuum at 80˚C overnight to give 4. Yellow crystals, yield: 61%; mp 350˚C - 351˚C (dec.). IR (KBr, cm−1): vmax = 3462 (OH), 1658 (C=O), 1558 (C=C), 1478 (N=N), 1386, 1150 cm−1 (SO2). 1H NMR (DMSO-d6): δ =7.17 (1H, d, Ar-H3), 7.19 (1H, s, Ar-H6), 7.76 - 7.79 (2H, d, benzenesulfonate-H2,6), 8.06-8.08 (1H, d, Ar-H4), 8.15 (2H, d, benzenesulfonate-H3,5), 10.33 (1H, s, OH), 11.56 ppm (1H, s, CHO). Anal. Calcd. for C13H9N2NaO5S (328.28): C, 47.56; H, 2.76; N, 8.53; S, 9.77. Found: C, 47.21; H, 3.03; N, 8.18; S, 10.02%.
3.2.5. 3-(2-Bromoacetyl)-2H-Chromen-2-One (5)
Compound 1 was synthesized according to the literature procedure [30] .
3.2.6. 2-Cyano-N'-(1-(2-Oxo-2H-Chromen-3-Yl)Ethylidene)Acetohydrazide (6)
Compound 1 was synthesized according to the literature procedure [31] .
3.2.7. 3-Oxo-3-(2-Oxo-2H-Chromen-3-yl)Propanenitrile (7)
A mixture of 3-(2-bromoacetyl)-2H-chromen-2-one (5; 2.66 g, 0.01 mol) and potassium cyanide (0.65 g, 0.01 mol) in ethanol (20 mL) was heated under reflux for 4 h. during the reflux period, a brown crystalline solid was separated. The separated solid was filtered off, washed with ethanol and recrystallized from ethanol to give 7. Brown crystals, yield 62%; mp 180˚C - 182˚C. IR (KBr, cm−1): vmax = 2931 (CH-aliphatic), 2207 (C≡N), 1703 (O-C=O), 1636 (C=O). MS m/z (%): 213 [M+] (99), 175 (27), 134 (47), 101 (100). Anal. Calcd. for C12H7NO3 (213.19): C, 67.61; H, 3.31; N, 6.57. Found: C, 67.09; H, 3.13; N, 7.02%.
3.2.8. Sodium 2-(2-(1-Cyano-2-Oxo-2-(2-(1-(2-oxo-2H-Chromen-3-yl)Ethylidene)Hydr-Azinyl) Ethylidene)Hydrazinyl)-5-((4-Sulfonatophenyl)Diazenyl)Benzenesulfonate (8)
To a stirred solution of (6; 2.69 g, 0.01 mol) in ethanol (50 mL) containing, sodium acetate (3 g), 2-sulfo-4- ((4-sulfophenyl)diazenyl)benzenediazonium chloride (prepared by adding sodium nitrite (0.69 g, 0.01 mol) to 2-amino-5-((4-sulfophenyl)diazenyl)benzenesulfonic acid (3.57 g, 0.01 mol) in conc. HCl (6 mL) at (0˚C - 5˚C) under stirring) was added drop wise while cooling to (0˚C -5˚C) and stirring. The reaction mixture was then left at room temperature for 2 h and the solid product formed was collected by filtration and recrystallized from DMF/acetone to give 8.Orange crystals, yield, 82%; mp >300˚C. lmax (H2O) 518 nm. IR (KBr, cm−1): vmax = 3421, 3350 (2NH), 2929 (CH-aliphatic), 2212 (C≡N), 1755 (O-C=O), 1657 cm−1 (C=O). MS m/z (%): 681 [M+] (5), 356 (77), 310 (32), 267 (78), 50 (100). Anal. Calcd. for C26H17N7Na2O9S2 (681.56): C, 45.82; H, 2.51; N, 14.39; S, 9.41. Found: C, 45.25; H, 2.69; N, 15.35; Na, 6.09; S, 8.98, O, 21.60%.
3.2.9. General Procedure for Preparation of Compounds 9 and 10
To a stirred solution of (7; 2.13 g, 0.01 mol) in ethanol (50 mL) containing, sodium acetate (3 g) 8-hydroxy- 6-sulfonaphthalene-2-diazonium chloride and/or 8-hydroxy-3,6-disulfonaphthalene-1-diazonium chloride (prepared by adding sodium nitrite (0.69 g, 0.01 mol) to 6-amino-4-hydroxynaphthalene-2-sulfonic acid and/or 4-amino-5-hydroxynaphthalene-2,7-disulfonic acid (0.01 mol) in conc. HCl (6 mL) at (0˚C - 5˚C) under stirring) was added drop wise while cooling to (0˚C - 5˚C) and stirring. The reaction mixture was then left at room temperature for 2 h and the solid product formed was collected by filtration and recrystallized from the appropriate solvents to afford colored products9and 10,respectively.
Violet crystals, yield, 88%; mp >300˚C (DMF/acetone), lmax(H2O) 545 nm. IR (KBr, cm−1): vmax = 3424(OH), 3320 (NH), 2907 (CH-aliphatic), 2248 (C≡N), 1710 (O-C=O), 1632 cm−1 (C=O). 1H NMR (DMSO-d6): δ = 7.22 - 8.81 (10H, m, 2Ar-H + coumarin-H4-8), 12.71, 12.80 ppm (2H, 2s, OH, NH). Anal. Calcd. for C22H12N3NaO7S (485.40): C, 54.44; H, 2.49; N, 8.66; S, 6.61. Found: C, 54.84; H, 2.56; N, 8.32; S, 6.07%.
Violet crystals, yield, 88%; mp >300˚C (DMF/acetone), lmax(H2O) 520 nm. IR (KBr, cm−1): vmax = 3486 - 3380 (OH, NH), 2225 (C≡N), 1710 (O-C=O), 1632 cm−1 (C=O). MS m/z (%): 587 [M+] (23), 537 (52), 506 (18), 358 (71), 219 (100). Anal. Calcd. for C22H11N3Na2O10S2 (587.45): C, 44.98; H, 1.89; N, 7.15; S, 10.92. Found: C, 45.04; H, 2.02; N, 6.96; S, 11.06%.
3.2.10. General Procedure for Preparation of Compounds 11, 12, 14
Interaction of ethyl 2-oxo-2H-chromene-3-carboxylate(2; 2.18 g, 0.01 mol) with aromatic aryl amine derivatives namely, 2-amino-5-((4-sulfophenyl)diazenyl)-benzenesulfonic acid, benzene-1,4-diamine and/or 3,3'-dime- thoxy-[1,1'-biphenyl]-4,4'-diamine (0.01 mol) in ethanol (20 mL) was heated under reflux for 4 h. The separated solid wazxzs filtered off, washed with ethanol and recrystallized from the appropriate solvents to give the compounds 11, 12 and 14, respectively.
Yellow crystals, yield, 91%; mp >300˚C (DMF/acetone), lmax(H2O) 440 nm. IR (KBr, cm−1): vmax = 3325 (NH), 3060 (CH-aromatic), 1736 (O-C=O), 1617 (C=O), 1509 cm−1 (N=N). MS m/z (%): 573 [M+] (2), 448 (99), 394 (100), 358 (78), 246 (86). Anal. Calcd. for C22H13N3Na2O9S2 (573.46): C, 46.08; H, 2.28; N, 7.33; S, 11.18. Found: C, 45.89; H, 2.12; N, 7.96; S, 11.36%.
Bright orange crystals, yield, 65%; mp 233˚C - 235˚C (AcOH). IR (KBr, cm−1): vmax = 3458, 3360 (NH2), 3320 (NH), 3020 (CH-aromatic), 1702 (O-C=O), 1650 cm−1 (C=O). MS m/z (%): 280 [M+] (76), 173 [M+-C6H7N2] (51), 107 [C6H7N2+] (100) (Chart 1). Anal. Calcd. for C16H12N2O3 (280.28): C, 68.56; H, 4.32; N, 9.99. Found: C, 68.25; H, 4.51; N, 10.06%.
Brownish-yellow crystals, yield, 70%; mp 110˚C - 112˚C (DMF/acetone). IR (KBr, cm−1): vmax = 3400-3270 (NH2, NH), 3183 (CH-aromatic), 2935 (CH-aliphatic), 1718 (O-C=O), 1658 cm−1 (C=O). MS m/z (%): 416 [M+] (1), 244 (74), 201 (100), 186 (35), 158 (36). Anal. Calcd. for C24H20N2O5 (416.43): C, 69.22; H, 4.84; N, 6.73. Found: C, 68.97; H, 5.01; N, 6.86%.
3.2.11. Sodium 4-Amino-5-Hydroxy-3-((4-(2-Oxo-2h-Chromene-3-Carboxamido)Phenyl)-Diazenyl) Naphthalene-2,7-Disulfonate (13)
Compounds 12 (2.80 g, 0.01 mol) was suspended with stirring in concentrated H2SO4 (3 mL) and cooled to 0˚C - 5˚C then diazotized by adding NaNO2 (0.9 g, 0.013 mol) drop wise at 0˚C - 5˚C. After stirring at 0˚C for 1h, the diazonium sulphate solution of compound 12 were checked with starch paper for the presence of HNO2. There were a colour change from white to brown, sulfamic acid was added to destroy excess HNO2. Coupler compound 4-amino-5-hydroxynaphthalene-2,7-disulfonic acid (3.19 g, 0.01 mol) was dissolved in NaOH solution (3 mL, 20%) at 0˚C. The cold solution of diazo compound 12 that were prepared above were added to this solution, keeping the temperature at 0˚C - 5˚C and maintaining pH 4 by adding AcONa. After stirring for 1 h at 0˚C, the dissert 13dyes was collected by filtration then recrystallized from DMF/acetone. Violet crystals, yield, 89%; mp >300˚C, lmax(H2O) 570 nm. IR (KBr, cm−1): vmax = 3505 - 3273 (OH, NH2, NH), 3073 (CH-aromatic), 1700 (O-C=O), 1648 (C=O), 1502 cm−1 (N=N). MS m/z (%): 654 [M+] (4), 486 (5), 280 (71), 173 (50), 107 (100). Anal. Calcd. for C26H16N4Na2O10S2 (654.54): C, 47.71; H, 2.46; N, 8.56; S, 9.80. Found: C, 47.29; H, 3.01; N, 8.86; S, 10.16%.
3.2.12. Sodium 4-Amino-3-((3,3'-Dimethoxy-4'-(2-Oxo-2h-Chromene-3-Carboxamido)-[1,1'-Bi- Phenyl]-4-Yl)Diazenyl)-5-Hydroxynaphthalene-2,7-Disulfonate (15)
Compounds 14 (4.16 g, 0.01 mol) was suspended with stirring in concentrated H2SO4 (3 mL) and cooled to 0˚C - 5˚C then diazotized by adding NaNO2 (0.9 g, 0.013 mol) drop wise at 0˚C - 5˚C. After stirring at 0˚C for 1h, the diazonium sulphate solution of compound 14 were checked with starch paper for the presence of HNO2. There were a colour change from white to brown, sulfamic acid was added to destroy excess HNO2. Coupler compound 4-amino-5-hydroxynaphthalene-2,7-disulfonic acid (3.19 g, 0.01 mol) was dissolved in NaOH solution (3 mL, 20%) at 0˚C. The cold solution of diazo compound 14 that were prepared above were added to this solution, keeping the temperature at 0-5˚C and maintaining pH 4 by adding AcONa. After stirring for 1h at 0˚C, the dissert dye 15 was collected by filtration then recrystallized from DMF/acetone. Violet crystals, yield, 89%; mp >300˚C, lmax(H2O) 405 nm. IR (KBr, cm−1): vmax = 3509 - 3380 (OH, NH2, NH), 1726 (O-C=O), 1661 (C=O), 1449 cm−1 (N=N). MS m/z (%): 791 [M++1] (5), 744 (8), 472 (9), 146 (52), 118 (63), 64 (100). Anal. Calcd. for C34H24N4Na2O12S2 (790.68): C, 51.65; H, 3.06; N, 7.09; S, 8.11. Found: C, 51.29; H, 2.96; N, 6.86; S, 7.92%.
3.2.13. General Procedure for Preparation of Compounds 16 - 18
To a mixture of 2-hydroxy-5-((4-nitrophenyl)diazenyl)benzaldehyde (3, 2.71 g, 0.01 mol) and ethyl 3-oxobuta- noate, diethyl malonate and/or malononitrile (0.01 mol) in ethanol (20 mL), glacial AcOH (0.3 mL) and piperidine (0.3 mL) were added under rapid stirring. The reaction mixture was heated under reflux for 5 h and left to cool, the solid formed after cooling filtered off and recrystallized from a suitable solvent to afford the pure products 16-18, respectively.
Brown crystals, yield, 59%; mp 225-227˚C (DMF).IR (KBr, cm−1): vmax = 3050 (CH-aromatic), 2928 (CH-ali- phatic), 1745 (O-C=O), 1676 (C=O), 1600 (C=C), 1522 (N=N), 1338, 1231 cm−1 (NO2). 1H NMR (DMSO-d6): δ = 2.53 (3H, s, CH3), 7.65(1H, d, coumarin-H8), 8.08 (2H, d, nitrophenyl-H2,6), 8.25 (1H, d, coumarin-H7), 8.42 (1H, s, coumarin-H5), 8.59 (2H, d, nitrophenyl-H3,5), 8.80 ppm (1H, s, coumarin-H4). Anal. Calcd. for C17H11N3O5 (337.29): C, 60.54; H, 3.29; N, 12.46. Found: C, 60.30; H, 3.48; N, 12.28%.
Brown crystals, yield, 56%; mp 245˚C - 246˚C (DMF). IR (KBr, cm−1): vmax = 3097 (CH-aromatic), 2960 (CH-aliphatic), 1741 (coumarin C=O), 1705 (ester C=O), 1521(N=N), 1338, 1251 cm−1 (NO2). 1H NMR (DMSO-d6): δ = 1.30 (3H, t, CH3), 4.30 (2H, q, CH2), 7.60 (1H, d, coumarin-H8), 8.03 (2H, d, nitrophenyl-H2,6), 8.19 (1H, d, coumarin-H7), 8.40 (1H, s, coumarin-H5), 8.52 (2H, d, nitrophenyl-H3,5), 8.89 ppm (1H, s, coumarin-H4). Anal. Calcd. for C18H13N3O6 (367.31): C, 58.86; H, 3.57; N, 11.44. Found: C, 60.01; H, 3.34; N, 11.68%.
Brown crystals, yield, 56%; mp 250-252˚C (dec.) (DMF). IR (KBr, cm−1): vmax = 3337 (NH), 2205 (C≡N), 1605 (C=N), 1556 (N=N), 1336, 1246 cm−1 (NO2). MS m/z (%): 321 [M++2] (11), 319 [M+] (18), 300 (100), 170 [M+-C6H3N3O2] (18), 150 [M+-C10H5N2O] (14), 106 [C7H6O+] (8), 77 [C6H5+] (19), 65 [C5H5+] (26), 52 [C4H4+] (25) (Chart 2). Anal. Calcd. for C16H9N5O3 (319.27): C, 60.19; H, 2.84; N, 21.94. Found: C, 59.94; H, 3.01; N, 22.05%.
3.2.14. Sodium 4-((3-Acetyl-2-Oxo-2h-Chromen-6-yl)Diazenyl)Benzenesulfonate (19)
To a mixture of sodium 4-((3-formyl-4-hydroxyphenyl)diazenyl)benzene-sulfonate (4, 3.28 g, 0.01 mol) and ethyl 3-oxobutanoate (1.30 g, 0.01 mol) in ethanol (20 mL), glacial AcOH (0.3 mL) and piperidine (0.3 mL) were added under rapid stirring. The reaction mixture was heated under reflux for 5 h and, after cooling, the solid was filtered and recrystallized from DMF/acetone to afford the pure product 19. Bright yellow crystals, yield, 63%; mp > 360˚C (dec.). IR (KBr, cm−1): vmax = 3058 (CH-aromatic), 2922 (CH-aliphatic), 1754 (O-C=O), 1672 (C=O), 1620 (C=C), 1564 (N=N), 1382, 1122 cm−1 (SO2). 1H NMR (DMSO-d6): δ = 2.57 (3H, t, CH3), 7.62(1H, d, coumarin-H8), 7.78 (2H, d, Ar-H2,6), 7.85 (1H, d, coumarin-H7), 8.19 (1H, s, coumarin-H5), 8.52 (2H, d, Ar-H3,5), 8.80 ppm (1H, s, coumarin-H4). Anal. Calcd. for C17H11N2NaO6S (394.33): C, 51.78; H, 2.81; N, 7.10; S, 8.13. Found: C, 52.01; H, 3.05; N, 6.94; S, 7.95%.
3.2.15. 4,4'-(((Hydrazine-1,2-Diylidenebis(Methanylylidene))Bis(4-Hydroxy-3,1-Phenyl-Ene)) Bis(Diazene-2,1-diyl))Dibenzenesulfonic Acid (20)
A mixture of sodium 4-((3-formyl-4-hydroxyphenyl)diazenyl)benzenesulfonate (4, 6.56 g, 0.02 mol) and hydrazine hydrate (0.5 g, 0.01 mol) in ethanol (30 mL) were refluxed for 2 h. The reaction mixture was concentrated and left to cool then recrystallized from DMF/acetone to give 20. Bright yellow crystals, yield, 63%; mp 320˚C - 322˚C (dec.). IR (KBr, cm−1): vmax = 3384 (OH), 3108 (CH-aromatic), 2934 (CH-aliphatic), 1600 (C=N), 1486 (N=N), 1384, 1132 cm−1 (SO2). MS m/z (%): 552 [M+-2N2] (60), 450 [C20H15N6O5S+] (58), 295 [C14H10N6O2+] (58), 80 (100) (Chart 3). Anal. Calcd. for C26H20N6O8S2 (608.60): C, 51.31; H, 3.31; N, 13.81; S, 10.54. Found: C, 51.61; H, 2.95; N, 14.02; S, 10.15%.
4. Dyeing of Coumarin Compounds 8-11
4.1. Dyeing of Coumarin Compounds 8-11 on Wool
4.1.1. Effect of pH and Dyeing Temperature on Percent of Dyeing Exhaustion
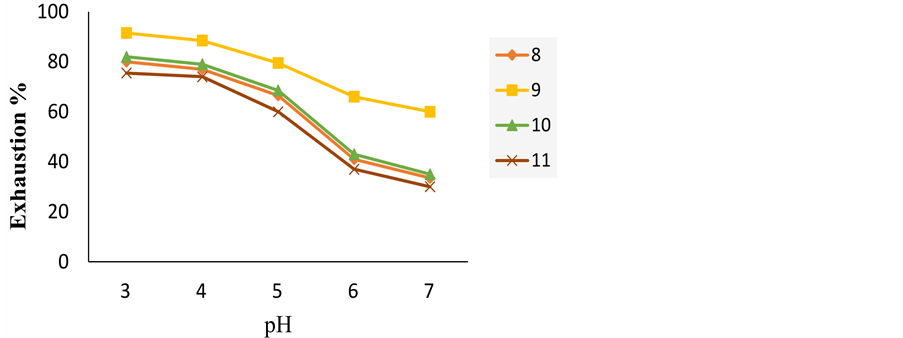
Table 1 and Figure 1 show the effect of pH on the exhaustion of acid dyes on wool fibre. The data revealed that at lower dyeing pH values (pH 4), the substantivity of the acid dyes on wool was virtually high. From these results, we can deduce that the dye 9 shows the most exhaustion value because this dye has lower molecular weight than others. The low substantivety of acid dye 8 is due to high molecular weight Although the dye 8 has higher molecular weight than dye 11, it is more substantivety, due to it contains diazo component.
Figure 1. Exhaustion of acid dyes 8-11 on wool at various pH values at 2% o.w.f.
Table 1. Exhaustion of acid dyes 8-11 on wool at various pH values.
4.1.2. Effect of Dye Concentration on Percent of Dyeing Exhaustion
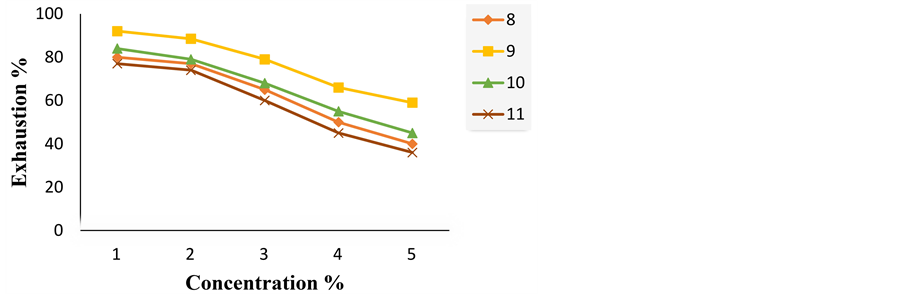
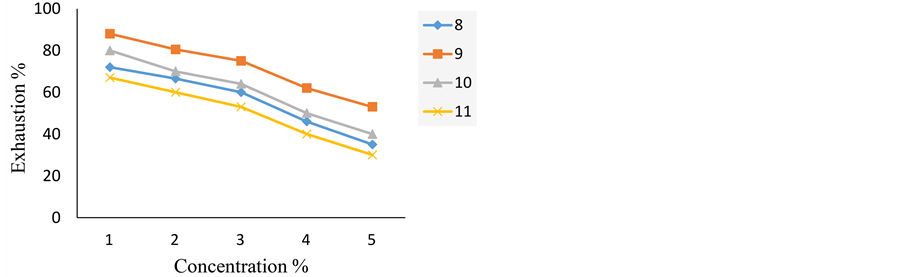
Figure 2 shows the exhaustion of the dyes on wool using different depth of shades (1% - 5% o.w.f.) at pH 4 and 100˚C. Increasing the dye concentration reduces the exhaustion on wool. From these results, we can deduce that the dye 9 exhibits the most exhaustion value while the dye 11 shows the lowest one.
4.1.3. Effect of Dyeing Time on Percent of Dyeing Exhaustion
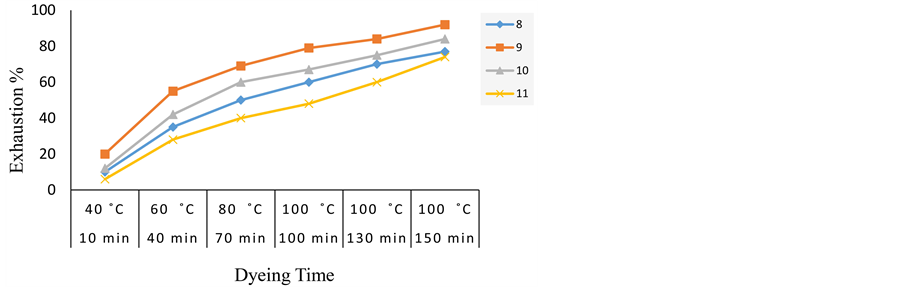
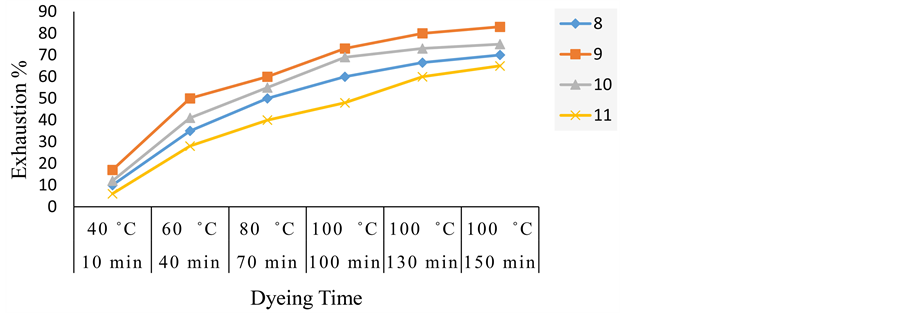
Figure 3 indicates the variation of dyeing time with temperature at pH 4 and (2% o.w.f.) dye concentration. It is apparent that the dye-fibre reaction is characterized by fast initial rate followed by slower rate, which levels off within the last 30 min of dyeing process. From these results, we can deduce that the dye 9 shows the most exhaustion value while the dye 11 shows the lowest one.
4.2. Dyeing of Coumarin Compounds 8-11 on Silk
4.2.1. Effect of pH on Percent of Dyeing Exhaustion
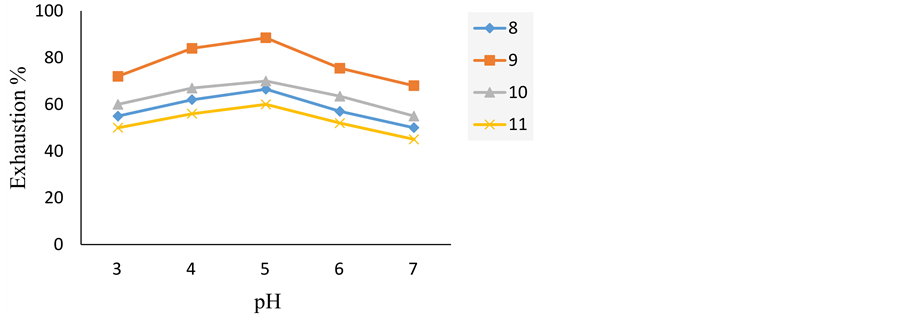
Table 2 and Figure 4 show the effect of pH on the exhaustion of acid dyes on silk fibre. The data revealed that at lower dyeing pH values (pH 5), the substantivity of the acid dyes on silk is virtually high. From these results, we can deduce the acid dye 9 exhibits the most exhaustion value because this dye has lower molecular weight than others. The low substantivety of acid dye 10 is due to high molecular weight, which decreases substantivety. Although the dye 10 has higher molecular weight than dye 11, it is more substantivety, because it contains diazo component.
4.2.2. Effect of Dye Concentration on Percent of Dyeing Exhaustion
Figure 5 shows the exhaustion of the dyes on silk using different depth of shades (1% - 5% o.w.f.) at pH 5 and 100˚C. Increasing the dye concentration reduces the exhaustion on silk. From these results, we can deduce the dye 9 is the most exhaustion value while the dye 11 is the lowest one.
4.2.3. Effect of Dyeing Time on Percent of Dyeing Exhaustion
Figure 6 indicates the variation of dyeing time with temperature at pH 5 and (2% o.w.f.) dye concentration. It is apparent that the dye-fibre reaction is characterized by fast initial rate followed by slower rate, which levels off within the last 30 min of dyeing process. From these results, we can deduce the acid dye 9 shows the most exhaustion value while the dye 11 is the lowest one i.e. increasing the time of dying, this leads to the increasing of the exhaustion values.
4.3. Colorimetric and Fastness Properties for All Dyeings
The colorimetric CIE L * a * b * C * h˚ data of the dyed wool and silk using the dye are shown in Table 3. The colour parameters were evaluated by means of the Cielab system and the modified CIEL * C * H˚ (D65/10˚) system. The following colour parameters for the dyed samples were obtained by the digital Cielab system: L*― lightness, a*―redness if positive coordinate, or greenness if negative coordinate, b*―yellowness if positive coordinate, or blueness if negative coordinate, C*―chromaticity, Ho―hue of the colour, X―coordinate x, Y―coordinate y, Z―coordinate z [32] . As shown in Table 4, the fastness to washing, rubbing and perspiration of all samples dyed with the dye was excellent to very good irrespective to the fabric used. Chromatic parameters were determined in comparison for samples dyed with all dyes. The light fastness of the dyes was found to
Figure 2. Exhaustion of acid dyes 8-11 on wool at various concentrations at pH 4.
Figure 3. Exhaustion of acid dyes 8-11 on wool at pH 4 (2% o.w.f.) with varying of dyeing time.
Figure 4. Exhaustion of acid dyes 8-11 on silk at various pH values.
Table 2. Exhaustion of acid dyes 8-11 on silk at various pH values.
Figure 5. Exhaustion of acid dyes 8-11 on silk at various concentrations at pH 5.
Figure 6. Exhaustion of acid dyes 8-11 on silk at pH 5 (2% o.w.f.) with varying of dyeing time.
Table 3. Colorimetric data of the dyed wool and silk fabrics using acid dyes 8-11 (2% o.w.f.) at 100˚C and at pH 4.
K/S: colour strength; L*: lightness; C*: chromaticity; Ho: hue of the colour; E*: total colour; a*: redness if positive coordinate, or greenness if negative coordinate; b*: yellowness if positive coordinate, or blueness if negative coordinate.
depend on the mobility of electrons through conjugated system from conjugated system from thiazole ring with coupler compounds afforded a good value of light fastness. The visible absorption spectra of some dyes showed that colours of dyes in the range are blue-purple, in addition, others in range are yellow-brown. The fastness of dyed fabrics to water, washing, alkaline and acid perspirations and rubbing was found to be very high irrespective of degree of sulphonation in the coupling component.
Table 4. Fastness properties of dyed wool and silk fabrics using acid dyes 8-11 (2% o.w.f.) at 100˚C and at pH 4.
Alt: colour change of dyed sample; SC: staining on cotton; SW: staining on wool, wash-scale (1-5) and light-scale (1-8).
5. Conclusion
This article shows that: the synthesis of some novel coumarin compounds is to be used as acid dyes. Dye 9 named sodium 6-(2-(1-cyano-2-oxo-2-(2-oxo-2H-chromen-3-yl)ethylidene)hydrazinyl)-4-hydroxynaphthalene- 2-sulfonate shows the most exhaustion value on wool and silk fibres.
Cite this paper
Mahmoud S.Bashandy,Fatma A.Mohamed,Mohamed M.El-Molla,Mahmoud B.Sheier,Ahmed H.Bedair,11, (2016) Synthesis of Novel Acid Dyes with Coumarin Moiety and Their Utilization for Dyeing Wool and Silk Fabrics. Open Journal of Medicinal Chemistry,06,18-35. doi: 10.4236/ojmc.2016.61002
References
- 1. AL-Adilee, K.J., Abass, A.K. and Taher, A.M. (2016) Synthesis of Some Transition Metal Complexes with New Heterocyclic Thiazolyl Azo Dye and Their Uses as Sensitizers in Photoreactions. Journal of Molecular Structure, 1108, 378-397.
http://dx.doi.org/10.1016/j.molstruc.2015.11.038 - 2. Ozkutuk, M., Ipek, E., Aydiner, B., Mamas, S. and Seferoglu, Z. (2016) Synthesis, Spectroscopic, Thermal and Electrochemical Studies on Thiazolyl Azo Based Disperse Dyes Bearing Coumarin. Journal of Molecular Structure, 1108, 521-532.
http://dx.doi.org/10.1016/j.molstruc.2015.12.032 - 3. Rufchahi, E.O.M., Gilani, A.G., Taghvaei, V., Karimi, R. and Ramezanzade, N. (2016) Synthesis, Structural Elucidation, Solvatochromism and Spectroscopic Properties of Some Azo Dyes Derived from 6-Chloro-4-hydroxyquino-line-2(1H)-one. Journal of Molecular Structure, 1108, 623-630.
http://dx.doi.org/10.1016/j.molstruc.2015.12.001 - 4. Geng, J., Dai, Y., Qian, H., Wang, N. and Huang, W. (2015) 2-Amino-4-chloro-5-formylthiophene-3-carbonitrile Derived Azo Dyes. Dyes and Pigments, 117, 133-140.
http://dx.doi.org/10.1016/j.dyepig.2015.02.010 - 5. Megarajan, S., Ahmed, K.B.A., Reddy, G.R.K., Kumar, P.S. and Anbazhagan, V. (2016) Phytoproteins in Green Leaves as Building Blocks for Photosynthesis of Gold Nanoparticles: An Efficient Electrocatalyst towards the Oxidation of Ascorbic Acid and the Reduction of Hydrogen Peroxide. Journal of Photochemistry and Photobiology B: Biology, 155, 7-12.
http://dx.doi.org/10.1016/j.jphotobiol.2015.12.009 - 6. Raposo, M.M., Castro, M.C., Belsley, M. and Fonseca, A.M. (2011) Push-Pull Bithiophene Azo-Chromophores Bearing Thiazole and Benzothiazole Acceptor Moieties: Synthesis and Evaluation of Their Redox and Nonlinear Optical Properties. Dyes and Pigments, 91, 454-465.
http://dx.doi.org/10.1016/j.dyepig.2011.05.007 - 7. Kaur, N., Dhaka, G. and Singh, J. (2015) Simple Naked-Eye Ratiometric and Colorimetric Receptor for Anions Based on Azo Dye Featuring with Benzimidazole Unit. Tetrahedron Letters, 56, 1162-1165.
http://dx.doi.org/10.1016/j.tetlet.2015.01.128 - 8. Kaur, N. and Kumar, S. (2011) Colorimetric Metal Ion Sensors. Tetrahedron, 67, 9233-9264.
http://dx.doi.org/10.1016/j.tet.2011.09.003 - 9. Patel, D.R., Patel, N.B., Patel, B.M. and Patel, K.C. (2014) Synthesis and Dyeing Properties of Some New Monoazo Disperse Dyes Derived from 2-Amino-4-(2’,4’-dichlorophenyl)-1,3 Thiazole. Journal of Saudi Chemical Society, 18, 902-913.
http://dx.doi.org/10.1016/j.jscs.2011.11.012 - 10. Mohammadi, A., Khalili, B. and Tahavor, M. (2015) Novel Push-Pull Heterocyclic Azo Disperse Dyes Containing Piperazine Moiety: Synthesis, Spectral Properties, Antioxidant Activity and Dyeing Performance on Polyester Fibers. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 150, 799-805.
http://dx.doi.org/10.1016/j.saa.2015.06.024 - 11. Saylam, A., Seferoglu, Z. and Ertan, N. (2014) Azo-8-hydroxyquinoline Dyes: The Synthesis, Characterizations and Determination of Tautomeric Properties of Some New Phenyl-and Heteroarylazo-8-hydroxyquinolines. Journal of Molecular Liquids, 195, 267-276.
http://dx.doi.org/10.1016/j.molliq.2014.02.027 - 12. Sahoo, J., Mekap, S.K. and Kumar, P.S. (2015) Synthesis, Spectral Characterization of Some New 3-Heteroaryl Azo 4-Hydroxy Coumarin Derivatives and Their Antimicrobial Evaluation. Journal of Taibah University for Science, 9, 187-195.
http://dx.doi.org/10.1016/j.jtusci.2014.08.001 - 13. Sunanda, B.P., Krishna, J.J. and Ganapati, S.S. (2013) Greener Protocol for One Pot Synthesis of Coumarin Styryl Dyes. Dyes and Pigments, 97, 105-112.
http://dx.doi.org/10.1016/j.dyepig.2012.12.001 - 14. Maryam, A. and Farahnaz, N. (2015) Producing Fluorescent Digital Printing Ink: Investigating the Effect of Type and Amount of Coumarin Derivative Dyes on the Quality of Ink. Journal of Luminescence, 167, 254-260.
http://dx.doi.org/10.1016/j.jlumin.2015.06.042 - 15. Simon, B., Azzam, C., Miroslav, M., Danie, T.G. and Denis, J. (2016) Optical Properties of V-Shaped Bis-Coumarins: Ab Initio Insights. Computational and Theoretical Chemistry, 1076, 57-64.
http://dx.doi.org/10.1016/j.comptc.2015.12.001 - 16. Anita, K.S., Kailas, K.S. and Ganapati, S.S. (2015) Synthesis and Photophysical Study of Novel Coumarin Based Styryl Dyes. Dyes and Pigments, 120, 190-199.
http://dx.doi.org/10.1016/j.dyepig.2015.04.018 - 17. Krishnaiah, V., Rajesh, K.K., Karnewar, S., Rajeswar, R.V., Srigiridhar, K. and Murali, K.T. (2015) Synthesis, Biological Activity Evaluation and Molecular Docking Studies of Novel Coumarin Substituted Thiazolyl-3-aryl-pyrazole-4-carbaldehydes. Bioorganic & Medicinal Chemistry Letters, 25, 5797-5803.
http://dx.doi.org/10.1016/j.bmcl.2015.10.042 - 18. Mahmoud, A.A. and Ali, E. (2015) Bismuth Triflate: A Highly Efficient Catalyst for the Synthesis of Bio-Active Coumarin Compounds via One-Pot Multi-Component Reaction. Chinese Journal of Catalysis, 36, 1124-1130.
http://dx.doi.org/10.1016/S1872-2067(14)60308-9 - 19. Raghavendra, U.P., Basanagouda, M., Melavanki, R.M., Fattepur, R.H. and Thipperudrappa, J. (2015) Solvatochromic Studies of Biologically Active Iodinated 4-Aryloxymethyl Coumarins and Estimation of Dipole Moments. Journal of Molecular Liquids, 202, 9-16.
http://dx.doi.org/10.1016/j.molliq.2014.12.003 - 20. Palita, K., Narid, P., Thitiya, S., Ruangchai, T., Supawadee, N., Taweesak, S., Tinnagon, K., Siriporn, J. and Vinich, P. (2015) N-Coumarin Derivatives as Hole-Transporting Emitters for High Efficiency Solution-Processed Pure Green Electroluminescent Devices. Dyes and Pigments, 112, 227-235.
http://dx.doi.org/10.1016/j.dyepig.2014.06.032 - 21. Tolga, E., Mustafa, B. and Meryem, C. (2015) Novel Phthalocyanines Bearing 7-Oxy-3-(3,5-difluorophenyl)coumarin Moieties: Synthesis, Characterization, Photophysical and Photochemical Properties. Journal of Photochemistry and Photobiology A: Chemistry, 300, 6-14.
http://dx.doi.org/10.1016/j.jphotochem.2014.12.005 - 22. He, X.W., Shang, Y.J., Zhou, Y., Yu, Z.Y., Han, G., Jin, W.J. and Chen, J.J. (2015) Synthesis of Coumarin-3-carboxylic Esters via FeCl3-Catalyzed Multicomponent Reaction of Salicylaldehydes, Meldrum’s Acid and Alcohols. Tetrahedron, 71, 863-868.
http://dx.doi.org/10.1016/j.tet.2014.12.042 - 23. Palita, K., Thitiya, S., Narid, P., Supawadee, N., Tinnagon, K., Siriporn, J., Taweesak, S. and Vinich, P. (2014) Synthesis, Characterization, and Properties of Novel Bis(aryl)carbazole-containing N-Coumarin Derivatives. Tetrahedron Letters, 55, 6689-6693.
http://dx.doi.org/10.1016/j.tetlet.2014.10.056 - 24. Mei, M.P., Pushparaj, H., Mani, G., Muthiahpillai, P. and Hyun, T.J. (2014) Solvent Free Synthesis of Coumarin Derivative by the Use of AlSBA-1 Molecular Sieves. Journal of Industrial and Engineering Chemistry, 20, 953-960.
http://dx.doi.org/10.1016/j.jiec.2013.06.028 - 25. Margaret, S., Tarik, J.D. and Okenwa, O. (2016) Progress towards Self-Healing Polymers for Composite Structural Applications. Polymer, 83, 260-282.
http://dx.doi.org/10.1016/j.polymer.2015.11.008 - 26. Ashis, M., Kotni, S., Ajoy, B. and Sumanta, B. (2014) Role of Charge Transfer Interaction and the Chemical Physics behind Effective Fulleropyrrolidine/Porphyrin Non-Covalent Interaction in Solution. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 121, 559-568.
http://dx.doi.org/10.1016/j.saa.2013.07.054 - 27. Matthew, V.D., Samer, D., Yi, H. and Ulrich, J.K. (2014) Lanthanide Upconversion Nanoparticles and Applications in Bioassays and Bioimaging. Analytica Chimica Acta, 832, 1-33.
http://dx.doi.org/10.1016/j.aca.2014.04.030 - 28. Gursoy, A. and Karali, N. (2003) Synthesis, Characterization and Primary Antituberculosis Activity Evaluation of 4-(3-Coumarinyl)-3-benzyl-4-thiazolin-2-one Benzylidenehydrazones. Turkish Journal of Chemistry, 27, 545-551.
- 29. Bhat, M.A., Siddiqui, N. and Khan, S.A. (2008) Synthesis of Novel 3-(4-Acetyl-5H/methyl-5-substituted phenyl-4,5-dihydro-1,3,4-oxadiazol-2-yl)-2H-chromen-2-ones as Potential Anticonvulsant Agents. Acta Poloniae Pharmaceutica. Drug Research, 65, 235-239.
- 30. Siddiqui, N., Arshad, M.F. and Khan, S.A. (2009) Synthesis of Some New Coumarin Incorporated Thiazolyl Semicarbazones as Anticonvulsants. Acta Poloniae Pharmaceutica, 66, 161-167.
- 31. Gomha, S.M. and Khalil, K.D. (2012)A Convenient Ultrasound-Promoted Synthesis of Some New Thiazole Derivatives Bearing a Coumarin Nucleus and Their Cytotoxic Activity. Molecules, 17, 9335-9347.
http://dx.doi.org/10.3390/molecules17089335 - 32. McDonald, R. (1997) Color Physics for Industry. 2nd Edition, Society of Dyes and Colorists, Bradford.
NOTES
*Corresponding author.