Open Journal of Medicinal Chemistry
Vol.4 No.2(2014), Article
ID:47128,9
pages
DOI:10.4236/ojmc.2014.42004
Resveratrol Reverses the Impaired Vasodilation Observed in 2K-1C Hypertension through Endothelial Function Improvement
B. F. M. Pereira, A. C. Scalabrini, T. S. Marinho, C. R. K. Antonietto, C. B. A. Restini*
School of Medicine, University of Ribeirão Preto (UNAERP), São Paulo, Brazil
Email: brunapereira2092@gmail.com, Angelikinha-cristina@hotmail.com, talitysm@gmail.com, carla_kita@hotmail.com, joyce.coliveira@hotmail.com, *carolbaraldi@hotmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 20 April 2014; revised 19 May 2014; accepted 18 June 2014
ABSTRACT
Background: The production of endothelial-derived factors induces either vasoconstriction or vasodilation; nitric oxide (NO) is the most distinguished relaxing factor. Endothelial dysfunction is associated with hypertension. The partial loss in the NO-promoted vasodilation is due to its decreased bioavailability and/or to an activity reduction of endothelium NO synthase (eNOS). Reactive oxygen species (ROS), present in oxidative stress, seize NO and diminish its bioavailability. Trans-resveratrol (RESV) has been proved to increase NO and eNOS levels. Thus, RESV could be capable of improving NO dependent vascular relaxation on aortic rings isolated from treated 2K-1C animals through ROS damage reduction. Aim: Evaluate the effects of RESV treatment on the relaxation of aortic rings isolated from treated 2K-1C rats while focusing on the effects of the treatment on systolic blood pressure. Methods: Male Wistar rats (180 g) were grouped: two 2K-1C and two Sham groups, one of each was treated with RESV (20 mg/kg, gavage) dissolved in Tween 80 and one of each was treated with water plus Tween 80 (control) for six weeks. The rats had their systolic blood pressure (SBP) measured before and after the treatments. Vascular reactivity studies were conducted in order to observe and compare acetylcholine (ACh)-induced relaxations in the presence and absence of the NOS inhibitor L-NAME (10−4 mol/L). Results: SBP for 2K-1C was significantly reduced in the treated group (179.13 ± 4.90 mmHg, n = 23) when compared to the untreated group (196.66 ± 6.06 mmHg, n = 15, p < 0.01). The maximum relaxation of aortic rings isolated from the 2K-1C treated group showed a higher efficacy (116.63% ± 1.72%, n = 12) than that from the untreated group (85.97% ± 0.69%, n = 6, p < 0.001); L-NAME exposure was responsible for a significant decrease in each group’s maximum relaxation efficacy. Conclusions: SBP reduction observed after RESV treatment in rat renal hypertension could be due to the reestablishment of vascular relaxation depend of NO.
Keywords:Resveratrol, Renal Hypertension, Nitric Oxide (NO), Vasodilation, Reactive Oxygen Species (ROS), Endothelium, Vascular Smooth Muscle

1. Introduction
According to the World Health Organization, hypertension is one of the main risk factors for death among noncommunicable diseases. Its complications account for 9.4 million deaths worldwide every year [1] being responsible for at least 45% of deaths due to ischemic heart disease and 51% of deaths due to cerebrovascular disease [2] . In 2008, approximately 40% of the global adult population aged 25 and above had been diagnosed with hypertension [2] . It is, therefore, considered a critical public health matter with elevated medical, social, and economic costs.
The Framingham Study has explained that antihypertensive treatments serve to lower blood pressure (BP) levels as well as to decrease cardiovascular morbidity and mortality [3] . Despite the cruciality of an ideal BP, a study done in 2006 revealed that only 34.5% of hypertensive individuals receiving treatment had theirs under control, and only 29.6% were considered adherent when using the Morisky-Green scale [4] . More recent studies, published in 2013, confirm the ongoing issue; one found 64.6% of low adherence and another found 42.3% of non-adherence [5] [6] . The numbers are similar in the US, where the CDC states that only 49% of patients comply with their long-term treatment [7] . Many factors prevent the ideal constancy of pharmacological intervention including drug side effects, and which, consequently, becomes one of the main issues concerning hypertension [8] -[10] .
Vascular endothelial cells have been proved essential for controlling vascular tone [11] ; given that the endothelium is sensitive to alterations in blood pressure, blood flow, chemical mediators and the metabolism, it can generate efficient responses in order to maintain homeostasis [12] . This is achieved through the production of endothelial-derived factors that induce either vasoconstriction or vasodilation—nitric oxide (NO) is the most distinguished relaxing factor [13] .
Although the vasoactive factors are physiologically in equilibrium, in pathological conditions such as hypertension, oxidative stress creates a disproportion [14] that impairs vasodilation and accounts for endothelial dysfunction [15] . Evidence suggests the latter is of fundamental importance to the physiopathology of hypertension, because it increases peripheral resistance and favors cardiovascular complications [16] . Human studies have confirmed a role of endothelial dysfunction in hypertension: in 1992, experimental results indicated abnormal nitric oxide-mediated dilation in the forearm arteriolar bed of untreated hypertensives [17] , and more recently, in 2001, endothelial dysfunction in resistance arteries was demonstrated in most hypertensives subjects [18] .
A partial loss in the vasodilation promoted by NO is one of the mechanisms involved in endothelial dysfunction associated with hypertension. It may result from decreased bioavailability and/or reduced activity of endothelium NO synthase (eNOS) [15] . Reactive oxygen species (ROS), present in oxidative stress, are known to quench NO and diminish its bioavailability due to the formation of peroxynitrite [19] . Additionally, peroxynitrite “uncouples” eNOS [20] and changes its function from NO production to ROS formation [21] . It is still unclear, however, if an excessive production of ROS is the cause of hypertension or a consequence of the vascular damage observed in this pathology [22] .
Dietary polyphenols are noted for possessing many biologically significant functions, such as protection against oxidative stress, which is attributed to their intrinsic reducing capabilities [23] . Trans-resveratrol (RESV), from the stilbenes subdivision, has been proved to increase NO and eNOS levels in cultured rat cardiac fibroblasts in vitro [23] [24] .
Goldblatt’s method can be used to induce renovascular hypertension [25] ; in the two-kidney one clip model (2K-1C), one renal artery is constricted while the other is left untouched. The stenosis activates peripheral RAAS and sympathetic nervous system [26] . Since the intact kidney maintains normal salt and water output for about six weeks, the hypertension is initially renin-angiotensin dependent (animal models). Increased plasma renin activity has been correlated to increased systemic oxidative stress [27] -[30] , and endothelium-dependent vasodilation has been showed impaired in aortic segments from 2K-1C animals [31] .
In arterial hypertension, oxidative stress due to ROS formation is one of the causes of endothelium-dependent relaxation impairment. Given that ROS oxidize NO, consequently decrease its bioavailability and production, and that RESV has antioxidant properties, the hypothesis is that RESV could be capable of improving NO dependent vascular relaxation on aortic rings isolated from treated 2K-1C animals by reducing the damage done by ROS. The goal is to evaluate the effects of RESV treatment on the relaxation of aortic rings isolated from treated 2K-1C rats focusing the effects of RESV treatment on systolic blood pressure.
2. Materials and Methods
2.1. Experimental Animals and Ethic Procedures
The male Wistar rats (180 - 200 g of body weight) used to develop this study were obtained from the bioterium of the University of Ribeirão Preto—UNAERP, Sao Paulo State, Brazil.
The animals were kept at a constant temperature of 22˚C, and they were fed ad libitum on standard chow while a 12 h light/dark cycle was maintained.
All procedures were performed in compliance with the international ethical standards of animal testing to avoid any distress and/or harm, and with the authorization of Ribeirão Preto University Ethics Committee (protocol no. 007/2010).
2.2. Drugs and Chemicals
The salts were obtained from Lab. Synth. Phenylephrine, acetylcholine and resveratrol came from Sigma Chemicals Co. (St. Louis, MO, EUA). The anesthetics chloridrate ketamine, isoflurane, and Dopaser® (Xilasina) were from União Química Farmacêutica Nacional (Embu-Guaçu, SP, Brazil), Baxter Healthcare Co. (Deerfield, IL, USA), Lab. Calier (Barcelona, Spain), respectively; and the antibiotic oxytetracycline from Pfizer (New York, NY, USA).
2.3. Instrumentals
The polygraph was from PowerLab 2/20, 415—ADInstruments (Paraíso, São Paulo, SP); the tail plethysmography from Bonter Ensino e Pesquisa (Ribeirão Preto, SP, Bra); and the isotonic transducer from Letica Scientific Instruments (Barcelona, Spain).
2.4. Surgery and Experimental Groups
Surgery to induce renal hypertension: the animals were anesthetizes with, 0.2 mL of Ketamine® (Chloridrate Ketamine) and 0.1 mL of Dopaser® (Xilasina), prior to a midline laparotomy. The control, normotensive, group was submitted to the laparotomy only, whereas the hypertensive group had a silver clip (internal diameter of 0.20 mm) placed around the left renal artery according to the 2K-1C Goldblatt model [25] . The incision was then sutured and each animal received a single antibiotic intramuscular injection (0.2 g/Kg) of oxytetracycline [32] .
2.5. Systolic Blood Pressure Measurement
Systolic blood pressure (SBP) was measured by an indirect tail-cuff method. This procedure was developed twice: before the surgery and one day prior to the sacrifices done to develop the reactivity studies. SBP values greater than or equal to 160 mmHg were considered demonstrative of hypertension In order to improve the detection of tail artery pulsations and to achieve a steady pulse level, the rats were warmed for 30 min at 28˚C before the SBP measurements.
2.6. Treatment Groups
Before beginning the treatments, the rats were randomly distributed into four named groups: hypertensive (2K-1C) treated and untreated, and normotensive (sham) treated and untreated.
Both named treated groups received a solution containing resveratrol (20 mg/Kg) diluted in Tween 80, while the two named untreated groups received 0.3 mL of tap water and Tween 80 instead. These procedures began the day after the surgery through gavage administration and were carried out three times a week for six weeks.
2.7. Vascular Reactivity Study
Six weeks post-op, all rats were anesthetized with isoflurane and decapitated in order to allow the thoracic aorta to be isolated, dissected, and lastly cut into rings of 4 mm in length. Two stainless steel holders were carefully placed through the rings’ lumen; the bottom holder was fixed to the chamber and the top holder was connected to a force-displacement transducer. The rings were then placed in a bath chamber for isolated organs containing 5.0 mL of physiologic Krebs solution modified with the following composition (in mmol/L): NaCl 130.0; KCl 4.7; KH2PO4 1.2; CaCl2 1.6; MgSO4 1.2; NaHCO3 14.9; glucose 5.5; pH 7.4, continuously bubbled with 95% O2 and 5% CO2 at 37˚C. The preparations sat for 60 min to stabilize under the previously determined optimal basal tension of 1.5 g. Vessels were tested with KCl 60 mmol/L to check their functional integrity. Since it was essential for the vascular endothelium to remain intact, an analysis of the percentage of relaxation as a response to ACh (EC50 of 1 μmol/L) after Phenylephrine pre-contraction (10−7 mmol/L) tested its conservation. The endothelia were considered intact if at least 95% of relaxation was observed. Next, the rings were pre-contracted with Phenylephrine (10−7 mmol/L), and then suffered cumulative ACh stimulation, which were graphed as concentration effect curves (1 nmol/L - 1 mmol/L) before and after L-NAME (10−4 mol/L).
3. Data Analysis
The results are expressed as means with their standard error of mean (M ± SEM). Sample numbers are indicated by “n”. Relaxation percentages express responses to ACh stimulation in relation to the phenylephrine pre-contractions. The pharmacological parameters analyzed from concentration-effect curves to ACh are represented by pD2 (−logEC50: negative logarithm of the molar concentration of the drug required to promote 50% of the maximal effect) and Emax (maximal relaxation) values. EC50 values were calculated by non-linear regression analysis from complete concentration-effect curves in individual experiments. Statistical analyses were developed by appropriately applying either Student’s t-test (paired or unpaired) or one-way ANOVA followed by Newman-Keuls post test. p values less than 0.05 were considered significant. Graphics and statistical analysis were done through GraphPad Prism (Software Corporation, version 5.0, San Diego, CA, USA).
4. Results
4.1. Systolic Blood Pressure
The mean SBP value before the surgery (119.38 ± 2.25 mmHg, n = 59) was found to be significantly different than averages from both 2K-1C groups, treated (179.13 ± 4.90 mmHg, n = 23, p < 0.001) and untreated (196.66 ± 6.06 mmHg, n = 15, p < 0.001), but not different than either values from the sham groups, treated (121.81 ± 5.53, n = 11, p > 0.05) and untreated (121.00 ± 3.48, n = 10, p > 0.05). The 2K-1C treated group had different SBP values than both sham groups, treated (p < 0.001) and untreated (p < 0.001); and the 2K-1C untreated group also had different SBP values than both sham groups, treated (p < 0.001) and untreated (p < 0.001). While the mean SBP for 2K-1C treated and untreated was significantly different (p < 0.01), there was no difference between sham treated and untreated (p > 0.05). The data can be seen in Figure 1.
4.2. Vascular Relaxation
The results presented in Figure 2 compares ACh-induced relaxations between the 2K-1C treated and untreated groups. The maximum relaxation has a significantly higher efficacy in the treated group (116.63% ± 1.72%, n = 12) when compared to the untreated (85.97% ± 0.69%, n = 6, p < 0.001). The pD2 values, however, do not show different potencies (p > 0.05) between the treated (7.71 ± 0.30, n = 20) and untreated (7.56 ± 0.33, n = 9) groups.
4.3. L-NAME
The relaxation stimulated by ACh was studied in both absence and presence of L-NAME, a NOS inhibitor. The
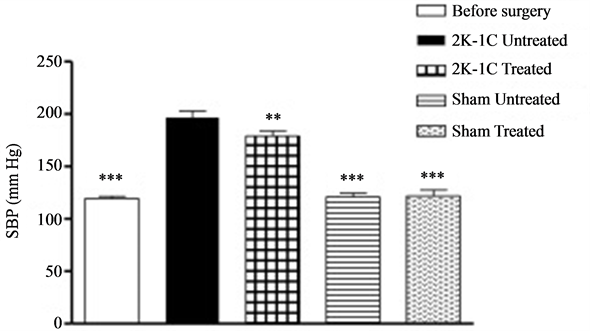
Figure 1. Systolic blood pressure (SBP) values of male Wistar rats before and after 2K-1C Goldblatt surgery. The values were obtained through tail cuff plethysmography and the statistical differences were analyzed with ANOVA followed by Newman-Keuls’s post-test. The symbol ***indicates groups with significant differences to 2K-1C treated and untreated (p < 0.001), and **denotes the difference between 2K-1C treated and untreated (p < 0.01).
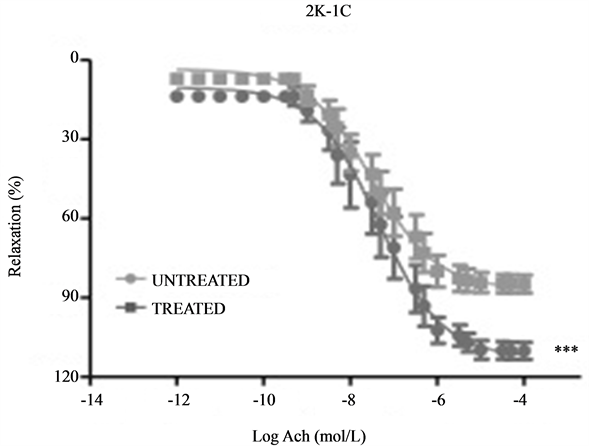
Figure 2. Vascular relaxation induced by ACh (1 nmol/L - 1 mmol/L) under phenylephrine pre-contraction. Concentration-response curves for ACh done on endothelium-intact aortic rings isolated from 2K-1C treated (RESV; 20 mg/Kg) and untreated. ***Denotes a significantly higher relaxation percentage (p < 0.001, unpaired Student’s t-test).
graphical results are shown in Figure 3 and the Emax and pD2 values are described in Table1 The exposure to the L-NAME (10−4 mol/L) was responsible for a significant decrease in the relaxation in each group’s efficacy and for different potencies in both sham groups (p < 0.05). Different potencies were not seen in either 2K-1C groups, however (p > 0.05). Table 2 presents statistical analysis through two by two group comparisons of Emax and pD2 values for both before and after L-NAME exposure.
5. Discussion
Arterial hypertension has been associated with endothelial dysfunction, with changes in vascular reactivity to contractile agents [33] , and with oxidative stress. It can be observed in the latter a reduction in the endothelium
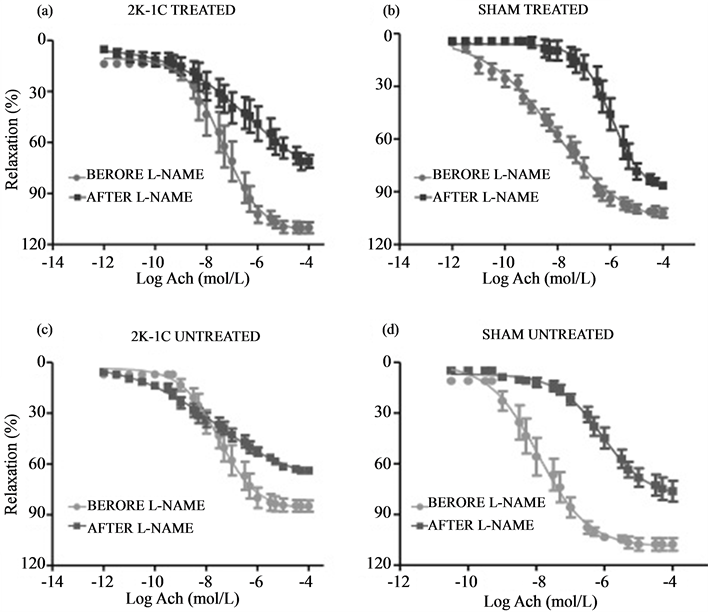
Figure 3. Effect of NOS inhibitor (L-NAME 10−4 mol/L) on vascular relaxation induced by ACh (1 nmol/L - 1 mmol/L) on endothelium-intact aortic rings isolated from 2K-1C rats treated (a) and untreated (c), and Sham treated (b) and untreated (d) with RESV (20 mg/Kg). Each pair of curves quantifies the ACh-induced relaxation as a percentage of phenylephrine (10−7 mol/L) pre-contraction performed on the same sample before and 20 min after L-NAME incubation.
Table 2. Two by two comparison for ACh-induced relaxation before and after incubation with L-NAME. (ANOVA, one way, Newman-Keuls post test).
dependent relaxation stimulated by ACh in hypertensive rats’ arteries, including in the 2K-1C renal hypertension model [34] . The reduction can be associated with a decrease in NO [35] , and with an increase of 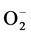 production [36] .
production [36] .
Figure 1 shows an increase in systolic blood pressure (SBP) as a result of the Goldblatt model surgery. The activation of the renin-angiotensin-aldosterone system initially increases systemic blood pressure due to increased serum levels of Ang-II, and maintains it due to liquid retention [37] -[39] .
Endothelium-produced NO is extremely important to the relaxation of blood vessels. The vasodilation activated by endothelial receptors, through ACh for example, or by sheer stress is responsible for activating eNOS, concluding with NO production [11] . As seen in Figure 3, all groups’ production of NO was inhibited by L-NAME. The difference between before and after relaxations is most likely caused by a decrease in NO’s production and availability.
The ACh induced relaxation in 2K-1C untreated animals was lower than the control groups’ average relaxation values, suggesting that an excessive production of ROS in hypertension blocks NO’s action. In other words, our results confirm Kojda and Harrison’s hypothesis [40] that pathological conditions, like hypertension, decrease the bioavailability of NO by oxidative inactivation due to an excessive reactive oxygen species in the endothelium. The interaction of NO with 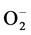 forms ONOO− [41] , a potent free radical.
forms ONOO− [41] , a potent free radical.
According to Dell’agli et al. [42] polyphenols present in red wine and grapes, like RESV, can induce vascular dependent relaxation through an increase in NO generation. In fact, our work shows that RESV treatment interferes with the vascular relaxation, since it was observed that the treated hypertensive group had a higher maximum relaxation than the untreated hypertensive group, as seen in Figure 2. Once again this can be explained and reinforced by the antioxidant action of RESV [43] -[45] . In fact, confocal microscopy results published by our lab using the same animal hypertensive model, and schedule of RESV treatment and doses, showed through ROS sensitive to DHE that the treatment locally exerted its protective effect of anti-oxidation by reducing the local bioavailability of ROS. Soon after their isolation, arterial samples were immediately loaded with dihydroethidium (DHE), a dye used to measure ROS, and analyzed in a confocal microscope, therefore the antioxidant effect of RESV was promptly observed in the ex vivo samples [43] .
6. Conclusion
This work tests a natural substance with the future intention of optimizing anti-hypertensive treatment by possibly decreasing pharmaceutical doses in order to reduce side effects that prevent adherence. There is, however, a limitation to our work in the sense that we used aortas, not resistance vessels like mesenteric arteries. The caliber of the latter has more influences on systemic blood pressure. Since, the relaxation in 2K-1C aortic rings improved with RESV treatment, one could infer the possibility of similar results in mesenteric rings. This inference can be used to explain the decreased average SBP in the treated animals. It can be validated based on the work from Dolinsky et al. [46] , which used mesenteric arteries from SHRs and Ang-II infused mice, and demonstrated that RESV increased endothelial NO synthase (eNOS) phosphorylation by enhancing the LKB1/adenosine monophosphate (AMP)-activated protein kinase (AMPK) signal transduction pathway [46] . Finally, this work concludes that RESV treatment, due to its antioxidant property, has potential to protect the cardiovascular system and enhance the current anti-hypertensive conduct.
Acknowledgements
Authors thank to CNPq and UNAERP for the financial support.
References
- Lim, S.S., et al. (2012) A Comparative Risk Assessment of Burden of Disease and Injury Attributable to 67 Risk Factors and Risk Factor Clusters in 21 Regions, 1990-2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet, 380, 2224-2260. http://dx.doi.org/10.1016/S0140-6736(12)61766-8
- World Health Organization (2009) WHO/UNICEF Estimates of National Immunization Coverage. World Health Organization, Geneva. http://www.who.int/immunization_monitoring/routine/immunization_coverage/en/index.html
- Kannel, W.B. (1996) Blood Pressure as a Cardiovascular Risk Factor: Prevention and Treatment. The Journal of the American Medical Association, 273, 1571-1576. http://dx.doi.org/10.1001/jama.1996.03530440051036
- Dórea, E.L. and Lotufo, P.A. (2004) Epidemiologia da Hipertensao Arterial Sistêmica. Revista da Sociedade Brasileira de Hipertensao, 7, 86-93.
- Santos, J.C., Faria Jr., M. and Restini, C.B.A. (2012) Potenciais interacoes medicamentosas identificadas em prescricoes a pacientes hipertensos. Rev Bras Clin Med., 10, 1-10.
- Hashmi, S.K., Afridi, M.B., Abbas, K., Sajwani, R.A., Saleheen, D., et al. (2007) Factors Associated with Adherence to Anti-Hypertensive Treatment in Pakistan. PLoS ONE, 2, 1-8. http://dx.doi.org/10.1371/journal.pone.0000280
- American College of Preventive Medicine (2011) Medication Adherence Time Tool: Improving Health Outcomes. http://www.acpm.org/?MedAdherTT_ClinRef#
- Sarquis, L.M.M., et al. (1998) A Adesao ao Tratamento na Hipertensao Arterial: Analise da Producao Científica. Revista da Escola de Enfermagem da USP, 32, 353-355.
- Clark, L.T., et al. (1991) Improving Compliance and Increasing Control of Hypertension: Needs of Special Hypertensive Populations. American Heart Journal, 121, 664-669. http://dx.doi.org/10.1016/0002-8703(91)90443-L
- Jin, J., Sklar, G.E., Oh, V.M.S. and Li, S.C. (2008) Factors Affecting Therapeutic Compliance: A Review from the Patient’s Perspective. Therapeutics and Clinical Risk Management, 4, 269-286.
- Furchgott, R. and Zawadzki, J.V.B. (1980) The Obligatory Role of Endothelial Cells in The Relaxation of Arterial Smooth Muscle by Acetylcholine. Nature, 288, 373-376. http://dx.doi.org/10.1038/288373a0
- Verma, S. and Anderson, T.J. (2002) Fundamentals of Endothelial Function for the Clinical Cardiologist. Circulation, 105, 546-549. http://dx.doi.org/10.1161/hc0502.104540
- Moncada, S. and Higgs, A. (2012) The L-Arginine-Nitric Oxide Pathway. The New England Journal of Medicine, 1993, 329.
- Dzau, V.J. (1989) Significance of Endothelial Derived Vasoactive Substances. Journal of Vascular Medicine and Biology, I, 43-55.
- Endemann, D. and Schiffrin, E. (2004) Endothelial Dysfunction. Frontiers in Nephrology, 15, 1983-1992.
- Sotomayor, M.A., Perez-Guerrero, C., Herrera, M.D. and Marhuenda, E. (1999) Effects of Chronic Treatment with Simvastatin on Endothelial Dysfunction in Spontaneously Hypertensive Rats. Journal of Hypertension, 17, 769-776. http://dx.doi.org/10.1097/00004872-199917060-00008
- Calver, A., Collier, J., Moncada, S. and Vallance, P. (1992) Effect of Local Intra-Arterial NG-Monomethil-L-Arginine in Patients with Hypertension: The Nitric Oxide Dilator Mechanism Appears Abnormal. Journal of Hypertension, 10, 1025-1031. http://dx.doi.org/10.1097/00004872-199209000-00017
- Schiffrin, E.L. (2001) A Critical Review of the Role of Endothelial Factors in the Pathogenesis of Hypertension. Journal of Cardiovascular Pharmacology, 38, S3-S6. http://dx.doi.org/10.1097/00005344-200111002-00002
- Koppenol, W.H., Moreno, J.J., Pryor, W., Ischiropoulos, H. and Beckman, J.S. (1992) Peroxynitrite, a Cloaked Oxidant Formed by Nitric Oxide and Superoxide. Chemical Research in Toxicology, 5, 834-842. http://dx.doi.org/10.1021/tx00030a017
- Milstien, S. and Katusic, Z. (1999) Oxidation of Tetrahydrobiopterin by Peroxynitrite: Implications for Vascular Endothelial Function. Biochemical and Biophysical Research Communications, 263, 681-684. http://dx.doi.org/10.1006/bbrc.1999.1422
- Landmesser, U. and Harrison, D.G. (2001) Oxidative Stress and Vascular Damage in Hypertension. Coronary Artery Disease, 12, 455-461. http://dx.doi.org/10.1097/00019501-200109000-00004
- Grossman, E. (2008) Does Increased Oxidative Stress Cause Hypertension? Diabetes Care, 31, S185-S189. http://dx.doi.org/10.2337/dc08-s246
- Han, X.Z., Shen, T. and Lou, H.X. (2007) Dietary Polyphenols and Their Biological Significance. International Journal of Molecular Sciences, 8, 950-988. http://dx.doi.org/10.3390/i8090950
- Wang, S.J., Wang, X.X., Yan, J., Xie, X.D., Fan, F.H., Zhou, X.H., Han, L. and Chen, J.Z. (2007) Resveratrol Inhibits Proliferation of Cultured Rat Cardiac Fibroblasts: Correlated With NO-cGMP Signaling Pathway. European Journal of Pharmacology, 567, 26-35. http://dx.doi.org/10.1016/j.ejphar.2007.04.023
- Goldblatt, H., Lynch, J., Hamzal, R. and Summerville, W.W. (1934) Studies on Experimental Hypertension, I: The Production of Persistent Elevation of Systolic Blood Pressure by Means of Renal Ischemia. Journal of Experimental Medicine, 59, 347-379. http://dx.doi.org/10.1084/jem.59.3.347
- Mok, J., Kong, M. and Hutchinson, J. (1985) Cardiovascular Effects of Central and Peripheral Administration of Dopamine in Hypertensive and Normotensive Rats. Indian Journal of Pharmacology, 17, 192-196.
- Boulanger, C. and Lüscher, T.F. (1990) Release of Endothelin from the Porcine Aorta: Inhibition by Endothelium-Derived Nitric Oxide. Journal of Clinical Investigation, 85, 587-590. http://dx.doi.org/10.1172/JCI114477
- Fazan, J.R.R., Silva, V.J.D. and Salgado, H.C. (2001) Modelos De Hipertensao Arterial. Revista Brasileira De Hipertensao, 8, 19-29.
- Simone, G., Devereux, R.B., Chinali, M., Roman, M.J., Best, L.G., Welty, T.K., Lee, E.T., Howard, B.V. for the Strong Heart Study Investigators (2006) Risk Factors for Arterial Hypertension in Adults with Initial Optimal Blood Pressure: The Strong Heart Study. Hypertension, 47, 162-167. http://dx.doi.org/10.1161/01.HYP.0000199103.40105.b5
- Usui, M., Egashira, K., Kitamoto, S., Koyanagi, M., Katoh, M., Kataoka, C., Shimokawa, H. and Takeshita, A. (1999) Pathogenic Role of Oxidative Stress in Vascular Angiotensin-Converting Enzyme Activation in Long-Term Blockade of Nitric Oxide Synthesis in Rats. Hypertension, 34, 546-551. http://dx.doi.org/10.1161/01.HYP.34.4.546
- Callera, G.E., Varanda, W.A. and Bendhack, L.M. (2000) Impaired Relaxation to Acetylcholine in 2K-1C Hypertensive Rat Aortas Involves Changes in Membrane Hyperpolarization Instead of an Abnormal Contribution of Endothelial Factors. General Pharmacology, 34, 379-389. http://dx.doi.org/10.1016/S0306-3623(01)00075-1
- Callera, G.E., Varanda, W.A. and Bendhack, L.M. (2001) Ca2+ Influx Is Increased in 2-Kidney, 1-Clip Hypertensive Rat Aorta. Hypertension, 38, 592-596.http://dx.doi.org/10.1161/hy09t1.096248
- Lunardi, C.N., da Silva, R.S. and Bendhack, L.M. (2009) New Nitric Oxide Donors Based on Ruthenium Complexes. Brazilian Journal of Medical and Biological Research, 42, 87-93. http://dx.doi.org/10.1590/S0100-879X2009000100013
- Callera, G.E., Varanda, W.A. and Bendhack, L.M. (2000) Impaired Relaxation to Acetylcholine in 2K-1C Hypertensive Rat Aortas Involves Changes in Membrane Hyperpolarization Instead of an Abnormal Contribution of Endothelial Factors. General Pharmacology, 34, 379-389. http://dx.doi.org/10.1016/S0306-3623(01)00075-1
- Mantelli, L., Amerini, S. and Ledda, F. (1995) Roles of Nitric Oxide and Endothelium-Derived Hyperpolarizing Factor in Vasorelaxant Effect of Acetylcholine as Influenced by Aging and Hypertension. Journal of Cardiovascular Pharmacology, 25, 595-602. http://dx.doi.org/10.1097/00005344-199504000-00013
- Heitzer, T., Wenzel, U., Hink, U., Krollner, D., Skatchkov, M., Stahl, R.A.K., Macharzina, R., Brasen, J.H., Meinertz, T. and Münze, T. (1999) Increased NAD(P)H Oxidase-Mediated Superoxide Production in Renovascular Hypertension: Evidence for an Involvement of Protein Kinase C. Kidney International, 55, 252-260. http://dx.doi.org/10.1046/j.1523-1755.1999.00229.x
- Okamura, T., Miyazaki, M., Inagami, T. and Toda, N. (1986) Vascular Renin-Angiotensin System in Two Kidney, One Clip Hypertensive Rats. Hypertension, 8, 560-565. http://dx.doi.org/10.1161/01.HYP.8.7.560
- Martinez-Maldonado, M. (1991) Pathophysiology of Renovascular Hypertension. Hypertension, 17, 707-719. http://dx.doi.org/10.1161/01.HYP.17.5.707
- Guan, S., Fox, J., Mitchell, K.D. and Navar, L.G. (1992) Angiotensin and Angiotensin Converting Enzyme Tissue Levels in Two-Kidney, One Clip Hypertensive Rats. Hypertension, 20, 763-767. http://dx.doi.org/10.1161/01.HYP.20.6.763
- Kojda, G. and Harrison, D. (1999) Interactions between NO and Reactive Oxygen Species: Pathophysiological Importance in Atherosclerosis, Hypertension, Diabetes and Heart Failure. Cardiovascular Research, 43, 562-571. http://dx.doi.org/10.1016/S0008-6363(99)00169-8
- Rubany, G.M. and Vanhoutte, P.M. (1986) Superoxide Anions and Hyperoxia Inactivate Endothelium-Derived Relaxing Factor. American Journal of Physiology, 250, H822-H827.
- Dell'agli, M., Busciala, A. and Bosisio, E. (2004) Vascular Effects of Wine Polyphenols. Cardiovascular Research, 63, 593-602.
- de Oliveira, J.C., Antonietto, C.R.K., Scalabrini, A.C., Marinho, T.S., Pernomian, L. and Corrêa, J.W.N. (2012) Antioxidant Protective Effects of the Resveratrol on the Cardiac and Vascular Tissues from Renal Hypertensive Rats. Open Journal of Medicinal Chemistry, 2, 61-71. http://dx.doi.org/10.4236/ojmc.2012.23008
- Sobey, C.G. (2001) Potassium Channel Function in Vascular Disease. Arteriosclerosis, Thrombosis and Vascular Biology, 21, 28-38. http://dx.doi.org/10.1161/01.ATV.21.1.28
- Lima, M.S., de Souza Veras Silani, I., Toaldo, I.M., Corrêa, L.C., Telles Biasoto, A.C., Pereira, G.E., Bordignon-Luiz, M.T. and Ninow, J.L. (2014) Phenolic Compounds, Organic Acids and Antioxidant Activity of Grape Juices Produced from New Brazilian Varieties Planted in the Northeast Region of Brazil. Food Chemistry, 161, 94-103. http://dx.doi.org/10.1016/j.foodchem.2014.03.109
- Dolinsky, V., Chakrabarti, S., Pereira, T.J., Oka, T., Levasseur, J., Beker, D., Zordoky, B.N., Morton, J.S., Nagendran, J., Lopaschuk, G.D., Davidge, S.T. and Dyck, J.R.B. (2013) Resveratrol Prevents Hypertension and Cardiac Hypertrophy in Hypertensive Rats and Mice. Biochimica et Biophysica Acta, 1832, 1723-1733. http://dx.doi.org/10.1016/j.bbadis.2013.05.018
NOTES

*Corresponding author.


