Open Journal of Medicinal Chemistry
Vol.2 No.2(2012), Article ID:19680,6 pages DOI:10.4236/ojmc.2012.22004
Potent Anticonvulsant 1H-Imidazol-5(4H)-One Derivatives with Low Neurotoxicity
1Pharmaceutical Organic Chemistry Department, Faculty of Pharmacy, Helwan University, Cairo, Egypt
2Pharmacology and Toxicology Department, Faculty of Pharmacy, Helwan University, Cairo, Egypt
Email: *alamka@yahoo.com
Received October 27, 2011; revised February 24, 2012; accepted March 10, 2012
Keywords: Imidazole; Imidazolone; Anticonvulsant; Antiepileptic; Valproic Acid; Neurotoxicity
ABSTRACT
we report here the synthesis and in vivo anticonvulsant/neurotoxicity activities of a series of compounds belonging to 2-aryl-4-arylidene-1-phenyl-1H-imidazol-5(4H)-one. The scaffold is based on the commonality of 5-membered lactam ring structures as successful anticonvulsant agents. The present compounds exhibited a range of anticonvulsant activity in pentylenetetrazole (PTZ)-induced seizure test. In particular, the protection was excellent by compounds bearing furylmethylidene on C4, possibly due to good pharmacokinetic properties. It was found that high lipophilicity and/or electron deficient aryl ring substitution at C4 compromised the anticonvulsant activities. For example, chloro analogues were found much less active than unsubstituted phenyl or furyl derivatives. Regarding side effects, active compounds exerted no observable neurotoxic effect at their therapeutic doses in Chimney test.
1. Introduction
Interest in developing new anticonvulsant medicines is still high after a century of introducing phenobarbital in 1912 [1]. This can be attributed to variety of reasons related to the epilepsy as a disease and to the anticonvulsants as drugs. Epilepsy is a serious disease that does not only devastate the welfare of the affected person and people around, but also it threatens the patient’s life [2]. The estimated proportion of the general population with active epilepsy at a given time is between 4 and 10 per 1000 people [3]. This ratio is doubled in developing countries as they harbor more than 90% of active epileptic cases. This is possibly due to increased risk of incidents leading to permanent brain damages [4]. Regardless the fact that there are at least 20 FDA approved antiepileptic drugs (Examples, Figure 1(a)) [5], the control of epileptic seizures stays around 70% of active epileptic cases only [6]. This underscores the need for continuing the research aiming at developing new antiepileptic agents to help patients not responding to current medicines [7].
On the other side, anticonvulsant agents are never been limited to the treatment of epilepsy only. Indeed, the anticonvulsants are versatile agents that have plethora of indications such as bipolar disorders, fibromyalgia, neuropathic pain, idiopathic pain, prophylaxis of migraine, and others [8]. This explains the rapid growth of the anticonvulsant market from $8 billion in 2003 to approximately $14 billion in 2008 [9].
Chemically, anticonvulsants have very diverse structures even among those sharing similar mechanism of actions.
Nonetheless, a five-membered ring having at least an amide group (lactam) is a common feature in several established anticonvulsant agents (Figure 1(b)) [10]. In this
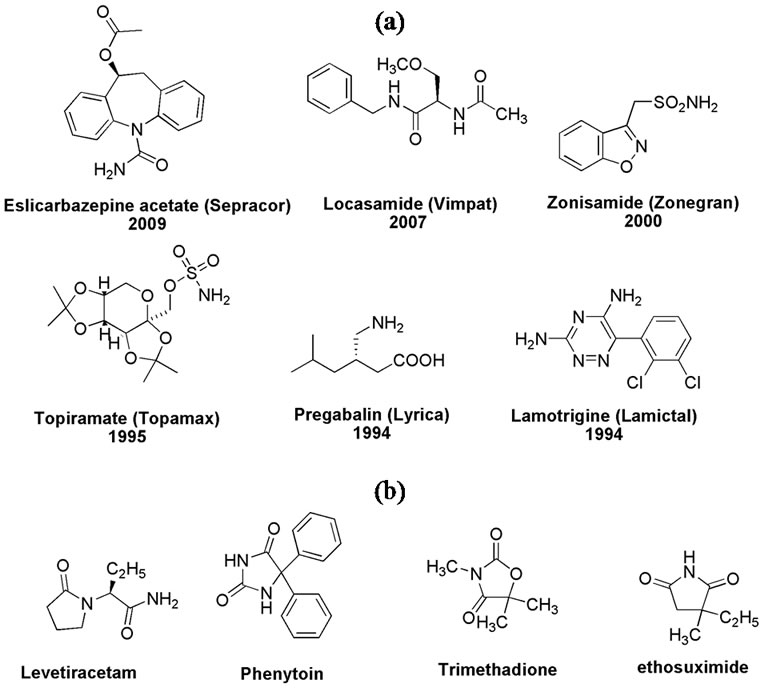
Figure 1. (a) Some FDA approved anticonvulsants since 1990. (b) Anticonvulsants having five-membered lactam core.
direction, imidazolone derivatives have been explored for anticonvulsant activity. For instance, compounds of chemotype I-IV (Figure 2) were found to have weak to moderate activities in pentylenetetrazole (PTZ)-induced seizure test. However, compound I showed neurotoxicity which is a common adverse action accompanying anticonvulsant activities [11-13]. Accordingly, we decided to investigate the potentiality of imidazolone derivatives as anticonvulsants against their neurotoxic side effects.
2. Chemistry
The target 2-aryl-4-arylidene-1-phenyl-1H-imidazol-5(4H)- one derivatives 3a-x were synthesized by the condensation of a series of aryl amines with 4-arylidene-2- phenyl-5-(4H)-oxazolones (2a-d). The intermediate oxazolones (2a-d) were prepared by the condensation of aryl aldehyde with benzoylglycine in the presence of sodium acetate and acetic anhydride (Erlenmeyer oxazolone condensation) as illustrated in scheme 1 [13]. The structure and purity of the compounds 3a-x were established by melting point, elemental analysis and spectral data.
3. Pharmacological Screening
In vivo anticonvulsant activities of orally administered compounds 3a, f, g, l, m, r, s, x at 147.8 mmol/kg dose were determined using pentylenetetrazole (PTZ)-induced
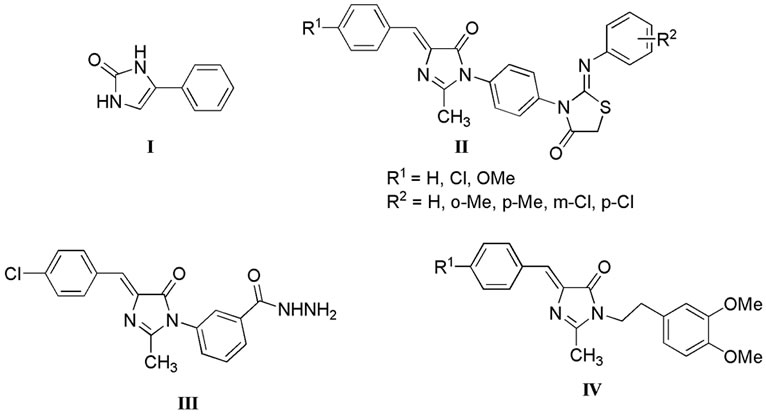
Figure 2. Representatives of literature imidazolone derivatives with anticonvulsant activities.
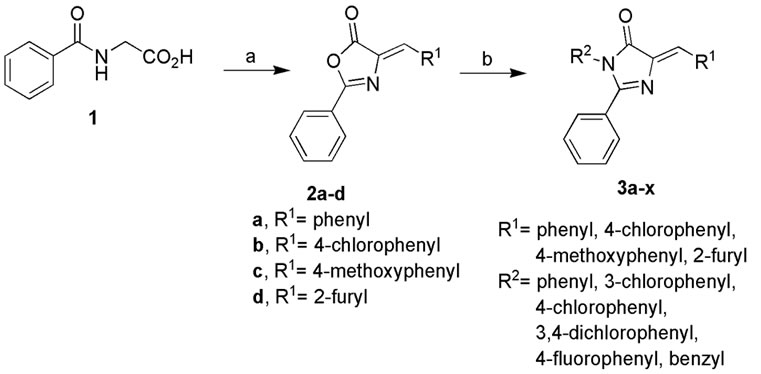
Scheme 1 . Reagents and conditions: (a) R1CHO, Ac2O, NaOAc (freshly fused), 100˚C, 2 h; (b) R2NH2, AcOH NaOAc (freshly fused), boiling water bath, 2 - 6 h.
seizure test and compared to those obtained for sodium valproate as a reference (established broad spectrum an-tiepileptic drug with wide safety margin) [14]. The preliminary results revealed that compounds 3a, f, s and 3x produced significant anticonvulsant activity (Table 1).
ED50 measurement of the four active compounds confirmed the high potency of compounds 3s and 3x compared to that of the reference compound (Table 2). Noteworthy, none of the active compounds showed neurontoxic activity at their ED50 molar concentration in Chimney test for measuring locomotor impairment [15].
4. Results and Discussion
The design of the compounds which was based on precedent five-membered lacatm anticonvulsants (Figures 1(b) and 2) met our expectation of promising anticonvulsant activities.
The excellent potencies of some of imidazolone compounds (compared to the reference) constitute strong evidence of inherence of anticonvulsant activity to the presented scaffold [13]. From our point of view, the higher activity of our compounds can be attributed to the aryl substituent at C2 (phenyl group) while some of previously reported compound lacked this group. In addi
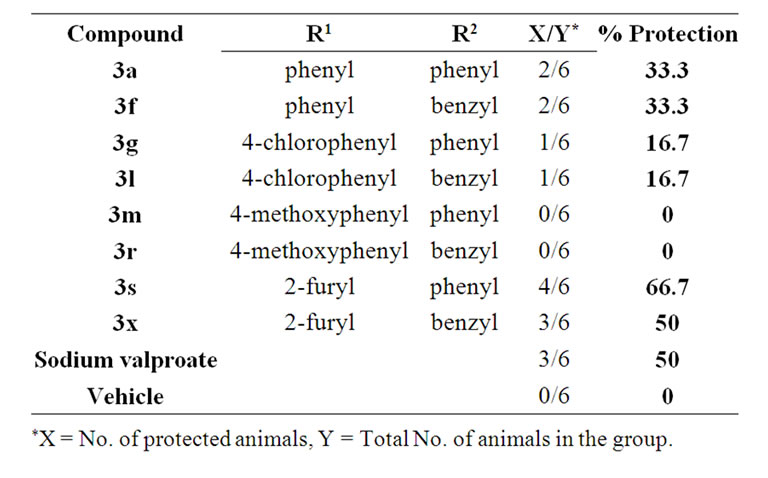
Table 1. Preliminary anticonvulsant activity at 147.8 mmol/kg dose (the dose which produced 50% protection for reference compound).
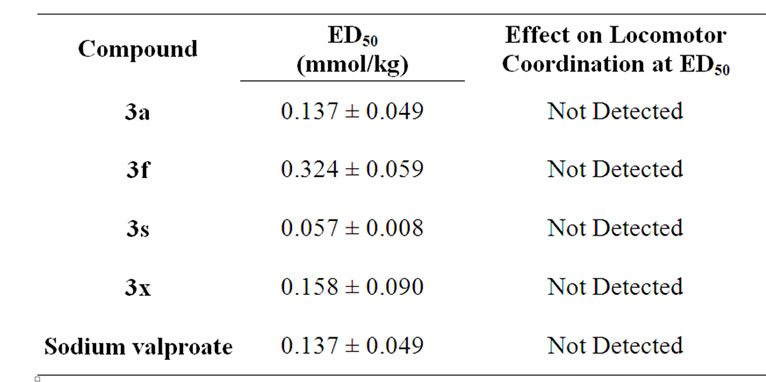
Table 2. Anticonvulsant activity (ED50, mol/kg orally) and locomotor coordination (test of neurotoxicity) of compounds 3a, 3f, 3s and 3x using PTZ induced seizure test.
tion, results showed clearly that the presence of benzylidene and furfurylidene groups at R1 is contributing significantly to the protection against seizures. The benzyl 3f and 3x showed one fold decrease in potency than phenyl derivatives 3a and 3s. Therefore, anticonvulsant action deemed less sensitive to variations at R2 group. There is more than one possible explanation to this SAR profile. The R1 site may only tolerate small electron rich ring such as 2-furyl or phenyl. Substituted phenyl might pose steric conflicts inside the binding site of these compounds. Second possible reason may relate the potency to the global molecular properties. The miLogP of the furfurylidene compounds (especially 3s) are optimum (3.6) and for benzylidene (4.48) while other Lipinski’s Ro5 parameters are well controlled [16]. Although methoxybenzylidene derivatives (example compound 3m) have similar miLogP to that of phenyl analogue 3a (4.43) and the ring has high electron density, it exhibited no anticonvulsant activity. It is notable that the methoxy group is the bulkiest among all substitution in the presented series while the furyl is the smallest. Another molecular descriptor might be interfering with potency, is the fractional polar surface area of the molecule. It is well known that the anticonvulsant compounds must achieve maximum penetration through the blood-brain barrier, therefore, the bioavailability are dependent on fine balance of the molecular structure and its global physical properties [17]. In this regard, it is noted that the furyl derivative 3s has significantly higher polar surface area than that of the other phenyl analogs such as compound 3a.
5. Conclusion
This work did not only introduce compounds with higher in vivo potency than that of reference compound but also they caused no impairment of locomotor coordination at therapeutic doses. Moreover, the furylmethylidene at R1 proved ideal for potency as research should continue to explore more compounds of this chemotype and their isosters.
6. Experimental
6.1. Synthetic Procedures
All melting points are uncorrected and measured using Electro-thermal IA 9100 apparatus (Shimadzu, Japan). IR spectra were recorded as potassium bromide pellets on a Perkin-Elmer 1650 spectrophotometer (USA), 1H NMR spectra were determined on a Varian Mercury (300 MHz) spectrometer (Varian, UK) and chemical shifts were expressed as ppm against TMS as internal reference. Mass spectra were recorded on 70 eV EI Ms-QP 1000 EX (Shimadzu). Microanalyses were operated using Vario, Elementar apparatus (Shimadzu) and the results were within the accepted range (±0.40) of the calculated values.
6.1.1. General Procedure for the Synthesis of 4-Arylidene-2-Phenyl-5-(4H)-Oxazolones (2a-d)
A mixture of benzoylglycine 1 (1.77 g, 0.01 mol), aldehyde (0.01 mol) and freshly fused sodium acetate (0.5 g) in acetic anhydride (20 mL) was heated at 100˚C for 2 h. and cooled. The obtained crystalline product was washed with water then aqueous ethanol and crystallized from ethanol to give white crystalline solid of the oxazolone derivative. Compounds 2a-d were characterized and confirmed by comparison with literature data [18,19].
6.1.2. General Procedure for the Synthesis of 3,5-Dihydro-Imidazol-4-Ones (3a-x)
An appropriate oxazolone 2a (2.49 g, 0.01 mol), 2b (2.83 g, 0.01 mol) or 2c (2.79 g, 0.01 mol) or 2d (2.39 g, 0.01 mol) and the appropriate aromatic amine [aniline (0.93 g, 0.01 mol), 3-chloroaniline or 4-chloroaniline (1.28 g, 0.01 mol) or 3,4-dichloroaniline (1.62 g, 0.01 mol) or 4-fluoroaniline (1.1 g, 0.01 mol) or benzyl amine (1.07 g, 0.01 mol)] in glacial acetic acid (5 mL) containing freshly fused sodium acetate (0.5 g) was heated on boiling water bath with constant stirring for 2 - 6 h. The separated product was filtered, washed with aqueous ethanol then crystallized from ethanol to give 3a-y, respectively. Some physical and spectroscopic data for 3a-y are listed below.
Compounds 3a, 3c, 3d, 3f, 3g, 3i, 3l, 3m, 3o, 3r, 3s and 3x [20-28] were prepared according to the general method and structures were confirmed via comparison with literature data.
(4Z)-4-Benzylidene-1-(3-Chlorophenyl)-2-Phenyl-1H-Imidazol-5(4H)-One (3b)
Yield: 75%; yellowish white crystals; Mp: 253˚C - 255˚C; IR (ν, cm–1): 3076 (CH), 1712 (C=O), 1622 (C=C), 1563 (C=N); 1H NMR: (CDCl3) 7.2 (s, 1H, CH=C), 7.5 - 8.4 (m, 14H, Ar-H). Anal. Calcd for C22H15ClN2O: C, 73.64; H, 4.21; N, 7.81; Cl, 9.88. Found: C, 73.42; H, 4.44; N, 7.59; Cl, 9.70.
(4Z)-4-Benzylidene-1-(4-Fluorophenyl)-2-Phenyl-1H-Imidazol-5(4H)-One (3e)
Yield: 70%; yellowish white crystals; Mp: 188˚C - 190˚C; IR (ν, cm–1): 3055 (CH), 1712 (C=O), 1629 (C=C), 1585 (C=N); 1H NMR (CDCl3): 7.2 (s, 1H, CH=C), 7.4 - 8.2 (m, 14H, Ar-H). Anal. Calcd for C22H15FN2O: C, 77.18; H, 4.42; N, 8.18. Found: C, 77.41; H, 4.21; N, 7.95.
(4Z)-4-(4-Chlorobenzylidene)-1-(3-Chlorophenyl)-2-Phenyl-1H-Imidazol-5(4H)-One (3h)
Yield: 85%; yellowish white crystals; Mp: 280˚C - 282˚C; IR (ν, cm–1): 3064 (CH), 1709 (C=O), 1619 (C=C), 1569 (C=N); 1H NMR (CDCl3): 7.1 (s, 1H, CH=C), 7.3 - 8.2 (m, 13H, Ar-H). Anal. Calcd for C22H14Cl2N2O: C, 67.19; H, 3.59; N, 7.12; Cl, 18.03. Found: C, 67.32; H, 3.25; N, 7.23; Cl, 18.23.
(4Z)-4-(4-Chlorobenzylidene)-1-(3,4-Dichlorophenyl)-2-Phenyl-1H-Imidazol-5(4H)-One (3j)
Yield: 75%; pale yellow crystals; Mp: 300˚C - 302˚C; IR (ν, cm–1): 3105 (CH), 1717 (C=O), 1616 (C=C), 1585 (C=N); 1HNMR (CDCl3): 7.1 (s, 1H, CH=C), 7.3 - 8.2 (m, 12H, Ar-H). Anal. Calcd for C22H13Cl3N2O: C, 61.78; H, 3.06; N, 6.55; Cl, 24.87. Found: C, 61.52; H, 3.31; N, 6.73; Cl, 24.73.
(4Z)-4-(4-Chlorobenzylidene)-1-(4-Fluorophenyl)-2-Phenyl-1H-Imidazol-5(4H)-One (3k)
Yield: 70%; yellow crystals; Mp: 260˚C - 262˚C; IR (ν, cm–1): 3084 (CH), 1745 (C=O), 1622 (C=C), 1578 (C=N); 1H NMR (CDCl3) 7.0 (s, 1H, CH=C), 7.3 - 8.3 (m, 13H, Ar-H). Anal. Calcd for C22H14ClFN2O: C, 70.12; H, 3.74; N, 7.43; Cl, 9.41. Found: C, 70.41; H, 3.35; N, 7.22; Cl, 9.25.
(4Z)-4-(4-Methoxybenzylidene)-1-(3-Chlorophenyl)-2-Phenyl-1H-Imidazol-5(4H)-One (3n)
Yield: 73%; yellow crystals; Mp: 260˚C - 262˚C; IR (ν, cm–1): 3095-2813 (CH), 1670 (C=O), 1615 (C=C), 1576 (C=N), 1226 (C-O); 1H NMR (CDCl3) 3.8 (s, 3H, OCH3), 7.0 (s, 1H, CH=C), 7.2 - 8.0 (m, 13H, Ar-H). Anal. Calcd for C23H17ClN2O2: C, 71.04; H, 4.41; N, 7.20; Cl, 9.21. Found: C, 71.25; H, 4.58; N, 7.44; Cl, 9.01.
3-(3,4-Dichloro-Phenyl)-5-[1-(4-Methoxy-Phenyl)-Methylidene]-2-Phenyl-3,5-Dihydro-Imidazol-4-One (3p)
Yield: 79%; yellow crystals; Mp: 252˚C - 254˚C; IR (ν, cm–1): 3085-2809 (CH), 1682 (C=O), 1629 (C=C), 1579 (C=N), 1212 (C-O); 1HNMR (CDCl3): 3.8 (s, 3H, OCH3), 7.1 (s, 1H, CH=C), 7.3 - 8.4 (m, 12H, Ar-H). Anal. Calcd for C23H16Cl2N2O2: C, 65.26; H, 3.81; N, 6.61; Cl, 16.75. Found: C, 65.49; H, 3.61; N, 6.86; Cl, 6.86.
(4Z)-4-(4-Methoxybenzylidene)-1-(4-Fluorophenyl)-2-Phenyl-1H-Imidazol-5(4H)-One (3q)
Yield: 77%; brownish yellow crystals; Mp: 237˚C - 239˚C; IR (ν, cm–1): 3096-2822 (CH), 1665 (C=O), 1624 (C=C), 1585 (C=N), 1210 (C-O); 1H NMR (CDCl3): 4.0 (s, 3H, OCH3), 6.9 (s, 1H, CH=C), 7.2 - 8.2 (m, 13H, Ar-H). Anal. Calcd for C23H17FN2O2: C, 74.18; H, 4.60; N, 7.52. Found: C, 74.31; H, 4.79; N, 7.30.
(4Z)-1-(3-Chlorophenyl)-4-[(Furan-2-yl)Methylene]-2-Phenyl-1H-Imidazol-5(4H)-One (3t)
Yield: 72%; yellowish white crystals; Mp: 230˚C - 232˚C; IR (ν, cm–1): 3095 (CH), 1720 (C=O), 1617 (C=C), 1581 (C=N), 1220 (C-O); 1H NMR (CDCl3): 6.8 (s, 1H, CH=C), 7.0 - 8.2 (m, 12H, Ar-H). Anal. Calcd for C20H13ClN2O2: C, 68.87; H, 3.76; N, 8.03; Cl, 10.16. Found: C, 68.60; H, 3.92; N, 8.32; Cl, 10.27.
(4Z)-1-(4-Chlorophenyl)-4-[(Furan-2-yl)Methylene]-2-Phenyl-1H-Imidazol-5(4H)-One (3u)
Yield: 74%; yellow crystals; Mp: 320˚C - 322˚C; IR (ν, cm–1): 3095 (CH), 1720 (C=O), 1617 (C=C), 1581 (C=N), 1220 (C-O) cm; 1HNMR (CDCl3): 6.7 (s, 1H, CH=C), 6.9 - 8.0 (m, 12H, Ar-H). Anal. Calcd for C20H13ClN2O2: C, 68.87; H, 3.76; N, 8.03; Cl, 10.16. Found: C, 68.71; H, 3.57; N, 8.24; Cl, 10.01.
(4Z)-1-(3,4-Dichlorophenyl)-4-[(Furan-2-yl)Methylene]-2-Phenyl-1H-Imidazol-5(4H)-One (3v)
Yield: 74%; yellowish brown crystals; Mp: 208˚C - 210˚C; IR (ν, cm–1): 3088 (CH), 1706 (C=O), 1619 (C=C), 1590 (C=N), 1210 (C-O); 1H NMR (CDCl3): 6.9 (s, 1H, CH=C), 7.2 - 8.5 (m, 11H, Ar-H). Anal. Calcd for C20H12Cl2N2O2: C, 62.68; H, 3.16; N, 7.31; Cl, 18.50. Found: C, 62.85; H, 3.35; N, 7.06; Cl, 18.38.
(4Z)-1-(4-Fluorophenyl)-4-[(Furan-2-yl)Methylene]-2-Phenyl-1H-Imidazol-5(4H)-One (3w)
Yield: 78%; pale yellow crystals; Mp: 180˚C -182˚C; IR (ν, cm–1): 3089 (CH), 1718 (C=O), 1629 (C=C), 1587 (C=N), 1217 (C-O); 1H NMR (CDCl3) 6.7 (s, 1H, CH=C), 6.9 - 8.0 (m, 12H, Ar-H). Anal. Calcd for C20H13FN2O2: C, 72.28; H, 3.94; N, 8.43. Found: C, 72.45; H, 3.71; N, 8.69.
6.2. Anticonvulsant Assay
6.2.1. Animals
Male Swiss albino mice (The Nile company for pharmaceutical and chemical industries, Cairo, Egypt) weighing 20 - 30 g were housed in plastic cages in a temperature-controlled (25˚C ± 1˚C) environment under a 12 hr light/dark cycle. Standard rodent food pellet and tap water were available ad libitum. The mice acclimatized to these conditions for a minimum of 1 week before initiating experiments.
6.2.2. Determination of ED50
Convulsions were induced by using pentylenetetrazole (PTZ) (90 mg/kg, i.p). The mice were randomly divided into 21 experimental groups of 6 animals each.
They were treated as follows:
Group 1: vehicle (tween 80/saline) p.o. + PTZ i.p.
Group 2-5: Sodium valproate (four doses ranging from 59.2 289.5 mmol/kg) p.o. + PTZ i.p.
Group 6-9: Compound 3a (four equimolar doses to valproate) p.o. + PTZ i.p.
Group 10-13: Compound 3f (four equimolar doses to valproate) p.o. + PTZ i.p.
Group 14-17: Compound 3s (four equimolar doses to valproate) p.o. + PTZ i.p.
Group 18-21: Compound 3x (four equimolar doses to valproate) p.o. + PTZ i.p.
Intraperitoneal injection was given 30 min. after oral treatment. The animals were individually placed in plastic boxes and observed immediately after PTZ injection for a period of 15 min. The percentage of protection against incidence of seizure and mortality were recorded and ED50 for each compound. ED50 were calculated by log-probit analysis based on Finney method (1971) using StatPlus 2009 software (AnalystSoft Inc.).
6.2.3. Neurotoxicity Assessment
The synthesized and the reference sodium valproate were assessed for their neurotoxic (motor impairment) properties using Chimney test [15]. The animals had to climb backwards up a glass tube (3 cm inner diameter, 25 cm long). Motor impairment was evidenced by the inability of mice to climb backwards up the tube within 30 s. Mice were divided into 6 groups of 6 animals each. The first group received vehicle (tween 80/saline). The second group received sodium valproate. Group 3-6 received compounds 3a, f, s, y respectively. Drugs (ED50) were given orally 30 min before being tested.
7. Acknowledgements
Authors thank Dr. Abdelsattar M. Omar, Assistant Professor of Pharmaceutical Chemistry, Al-Azahr University, Cairo, for his valuable advices in chemical synthesis. We also thank Molinispiration Cheminformatics and AnalystSoft Inc. for giving free access to their software.
REFERENCES
- D. F. Scott, “The History of Epileptic Therapy: An Account of How Medication was Developed,” Taylor & Francis, London, 1993.
- N. F. Moran, K. Poole, G. Bell, J. Solomon, S. Kendall, J. Solomon, S. Kendall, M. McCarthy, D. McCormick, L. Nashef, J. Sander and S. D. Shorvon, “Epilepsy in the United Kingdom: Seizure Frequency and Severity, AntiEpileptic Drug Utilization and Impact on Life in 1652 People with Epilepsy,” Seizure, Vol. 13, No. 6, 2004, pp. 425-433. doi:10.1016/j.seizure.2003.10.002
- R. Sridharan, “Epidemiology of Epilepsy,” Current Science (India), Vol. 82, No. 6, 2002, pp. 664-670.
- N. Senanayake and G. C. Roman, “Epidemiology of Epilepsy in Developing Countries,” Bulletin of the World Health Organization, Vol. 71, No. 2, 1993, pp. 247-258.
- www.fda.gov
- P. Kwan and M. J. Brodie, “Early Identification of Refractory Epilepsy,” New England Journal of Medicine, Vol. 342, No. 5, 2000, pp. 314-319.
- W. Löscher and D. Schmidt, “Modern Antiepileptic Drug Development Has Failed to Deliver: Ways out of the Current Dilemma,” Epilepsia, Vol. 52, No. 4, 2011, pp. 657-678. doi:10.1111/j.1528-1167.2011.03024.x
- C. J. Landmark, “Antiepileptic Drugs in Non-Epilepsy Disorders: Relations between Mechanisms of Action and Clinical Efficacy,” CNS Drugs, Vol. 22, No. 1, 2008, pp. 27-47.
- W. Raasch, “Antiepileptic Drug Market,” 2011. http://www.wikinvest.com/wiki/Antiepileptic_Drug_Market
- http://www.drugbank.ca
- U. Calis, S. Dalkara and M. Ertan, “Synthesis and Anticonvulsant Activity of Some New 4-Aryl-1,3-Dihydro- 2H-Imidazol-2-One Derivatives,” Arzneimittel Forschung, Vol. 42, No. 5, 1992, pp. 592-594.
- M. Amir and E. Singh, “1-p-(2-Arylimino-thiazolidon-3- yl)phenyl-2-methyl-4-arylidene-5-imidazolones as anticonvulsants,” Acta Pharmaceutica, Vol. 42, No. 5, pp. 133- 137.
- H. Joshi, P. Upadhyay, D. Karia and A. J. Baxi, “Synthesis of Some Novel Imidazolinones as Potent Anticonvulsant Agents,” European Journal of Medicinal Chemistry, Vol. 38, No. 9, 2003, pp. 837-840. doi:10.1016/S0223-5234(03)00117-X
- H. S. White, J. H. Woodhead, M. R. Franklin, E. A. Swinyard and H. H. Wolf, “General Principles: Experimental Selection, Quantification, and Evaluation of Antiepileptic Drugs,” In: R. H. Levy, R. H. Mattson and B. S. Meldrum, Eds., Antiepileptic Drugs, 4th Edition, Raven Inc., New York, 1995, pp. 99-121.
- J. R. Boissier, J. Tardy and J. C. Diverres, “Une Nouvelle Methode Simple Pour Explorer l’Action Tranquilisante: Le Test de la Cheminee,” The Journal of Experimental Medicine, Vol. 3, 1960, pp. 81-84.
- Molinispiration Cheminformatics, “Calculation of Molecular Properties and Bioactivity Score,” 2011. http://www.molinspiration.com/cgi-bin/properties
- P. Ertl, B. Rohde and P. Selzer, “Fast Calculation of Molecular Polar Surface Area as a Sum of Fragment Based Contributions and Its Application to the Prediction of Drug Transport Properties,” Journal of Medicinal Chemistry, Vol. 43, No. 20, 2000, pp. 3714-3717. doi:10.1021/jm000942e
- P. Raghavendra, G. Veena, G. A. Kumar, E. R. Kumar, N. Sangeetha, B. Sirivennela, S. Smarani, H. Kumar, S. Praneeth and R. Suthakaran, “Microwave Synthesis and Anti-Inflammatory Evaluation of Some New Imidazolo Quinoline Analogs,” RASAYAN Journal of Chemistry, Vol. 4, No. 1, 2011, pp. 91-102.
- B. M. Trost and P. J. Morris, “Palladium-Catalyzed Diastereoand Enantioselective Synthesis of Substituted Cyclopentanes through a Dynamic Kinetic Asymmetric Formal [3 + 2]-Cycloaddition of Vinyl Cyclopropanes and Alkylidene Azlactones,” Angewandte Chemie International Edition, Vol. 50, No. 27, pp. 6167-6170. doi:10.1002/anie.201101684
- M. Kidwai and R. Mohan, “Solid Supported Reaction of Substituted 2-Oxazoline with Amines under Microwave Irradiation,” Journal of the Chinese Chemical Society, Vol. 50, No. 5, 2003, pp. 1075-1078.
- C. W. Bird and J. D. Twibell, “Rearrangement of 3-(acylamino)azetidinones,” Journal of the Chinese Chemical Society, Vol. 19, 1971, pp. 3155-3158.
- P. Kumar and K. A. Mukerjee, “Synthesis of 4-arylidene- 1,2-Disubstituted-Δ2-Imidazolin-5-Ones,” Indian Journal of Chemistry—Section B: Organic and Medicinal Chemistry Indian, Vol. 20B, No. 5, 1981, pp. 416-418.
- M. Z. A. Badr, H. A. H. El-Sherief and M. E. Tadros, “Synthesis of 1,2-Disubstituted-4-Benzylidene-2-Imidazolin-5-Ones and Their Thione Derivatives,” Indian Journal of Chemistry—Section B: Organic and Medicinal Chemistry Indian, Vol. 18, No. 3, pp. 240-242.
- A. S. Girgis, “Facile Regioselective Synthesis of 1,2,6,8- Tetraazaspiro[4.4]Nona-2,6-Dien-9-Ones,” Zeitschrift für Naturforschung B (Chemical Sciences), Vol. 31, No. 24, 2010, pp. 222-226.
- O. A. Miqdad, N. M. Abunada and H. M. Hassaneen, “Regioselectivity of Nitrilimines 1,3-Dipolar Cycloaddition: Novel Synthesis of Spiro[4,4] Nona-2,8-Dien-6-One Derivatives,” Heteroatom Chemistry, Vol. 22, No. 2, 2011, pp. 131-136. doi:10.1002/hc.20666
- M. A. Abdallah, M. E. Zayed and A. S. Shawali, “Reaction of Benzonitrilium N-Phenylimide with (Z)-4-Arylmethyleneimidazol-5(4H)-Ones,” Indian Journal of Chemistry—Section B: Organic and Medicinal Chemistry Indian, Vol. 40, No. 3, pp. 187-190.
- F. M. Ismail and N. G. Kandile, “Reaction of 4-(Arylmethylene)-2-Phenyl-2-Oxazolin-5-Ones with Schiff Bases and Benzalazine,” Asian Journal of Chemistry, Vol. 1, No. 3, 1989, pp. 254-260.
- G. V. S. R. Sarma and V. M. Reddy, “1,2-Disubstituted -4-[(Furan-2-yl)Methylene]Imidazolin-5(4H)-Ones,” Journal of the Institution of Chemists (India), Vol. 72, No. 3, 2000, pp. 106-110.
NOTES
*Corresponding author.

