Open Journal of Radiology
Vol. 2 No. 3 (2012) , Article ID: 23309 , 4 pages DOI:10.4236/ojrad.2012.23012
Renal Pleomorphic Sarcoma in Polycystic Kidney Disease: Case Report
1Department of Radiology, University Hospital Mohammed VI, University Cadi Ayyad, Marrakesh, Morocco
2Department of Urology, University Hospital Mohammed VI, University Cadi Ayyad, Marrakesh, Morocco
3Department of Pathology, University Hospital Mohammed VI, University Cadi Ayyad, Marrakesh, Morocco
Email: kjiddi@hotmail.com
Received June 4, 2012; revised July 6, 2012; accepted July 16, 2012
Keywords: Polycystic Kidney Disaese; Pleomorphic Sarcoma
ABSTRACT
We report a case of pleomorphic sarcoma in a 42-year-old man with adult polycystic kidney disease [APKD]. Abdominal ultrasonography, computed tomography and MRI scans have revealed heterogenous bilateral renal cysts with a voluminous mass in the upper pole left kidney. Radical left nephrectomy with histopathologic and immuno-histologic examination have confirmed the diagnosis of pleomorphic sarcoma. Sarcoma associated with adult polycystic kidney disease is extremely rare and does not have particular radiological or biological features when compared to primary renal sarcoma.
1. Introduction
Autosomal dominant polycystic kidney disease [APKD] occurs in approximately 1/500 to 1/1000 live births and is present in 10% - 12% of end-stage renal disease patients [1]. It is able, like other genetic diseases, of malignant transformation [2].
Renal cell carcinoma associated with APKD has already been reported in the literature, whereas only few cases of sarcoma have been reported.
We report a case of renal pleomorphic sarcoma in a patient with APKD.
2. Case Report
A 42-year-old male, without significant medical history presented with a pain of the left flank associated to constipation without urinary symptoms. Abdominal examination discovered a mass of left flank.
Ultrasonography revealed multiple and bilateral renal cystic masses of which some have heteroechoic content. Enhanced computed tomography [CT] shows bilateral polycystic kidney and a voluminous solid mass in the upper pole of the left kidney with heterogeneous enhancement and large central necrosis (Figure 1).
Abdominal MRI achieved in axial and coronal plans in T2 and T1 weighted sequences before and after gadolinium injection, confirmed the presence of multiple cysts and enhancing masses. These masses presented an isosignal on T1 and low signal on T2. Following intravenous gadolinium injection, the lesions showed intense and heterogeneous enhancement (Figures 2 and 3).
The most voluminous lesion measured 15cm and was located in the upper pole of left kidney with large central necrosis in low signal on T1 and high signal on T2. It involved the tail of the pancreas without adenopathy or vascular extension. The diagnosis of bilateral renal cancer associated with polycystic kidney disease was considered. A bilateral nephrectomy was decided.
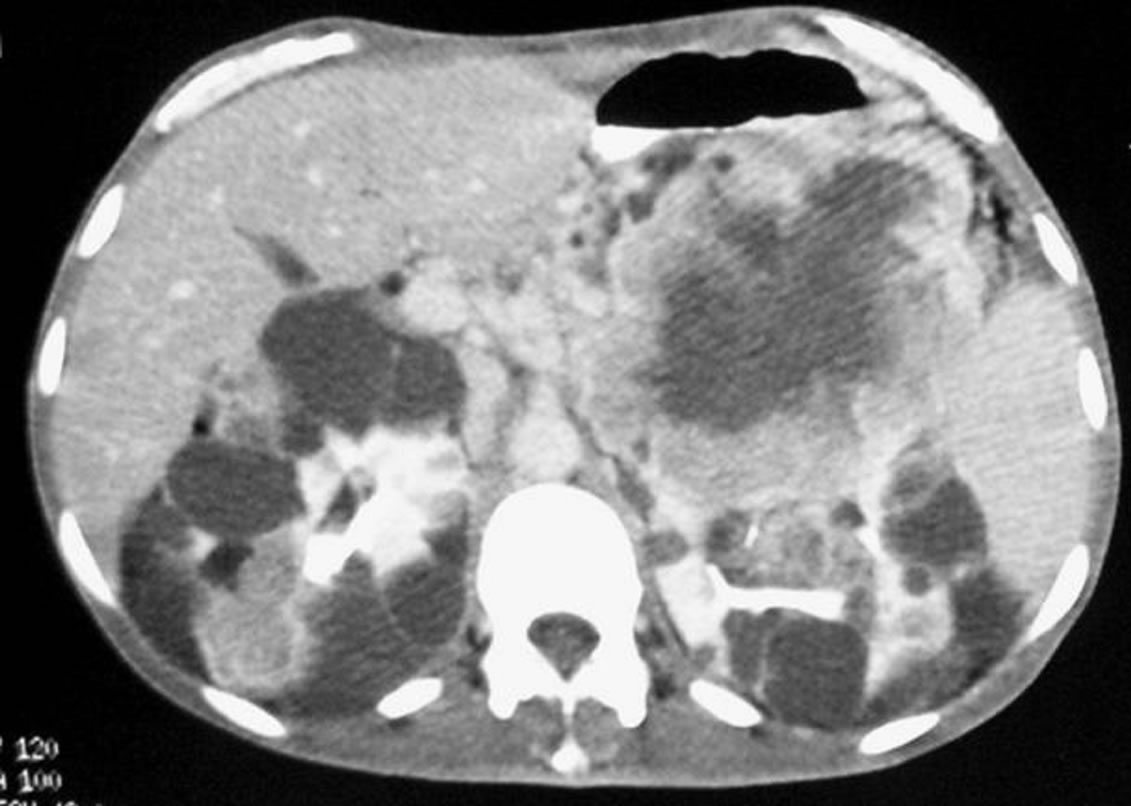
Figure 1. Abdominal CT: bilateral polycystic kidney with heterogeneous solid content of multiple cysts enhanced after contrast and voluminous tumor of the upper pole of left kidney.
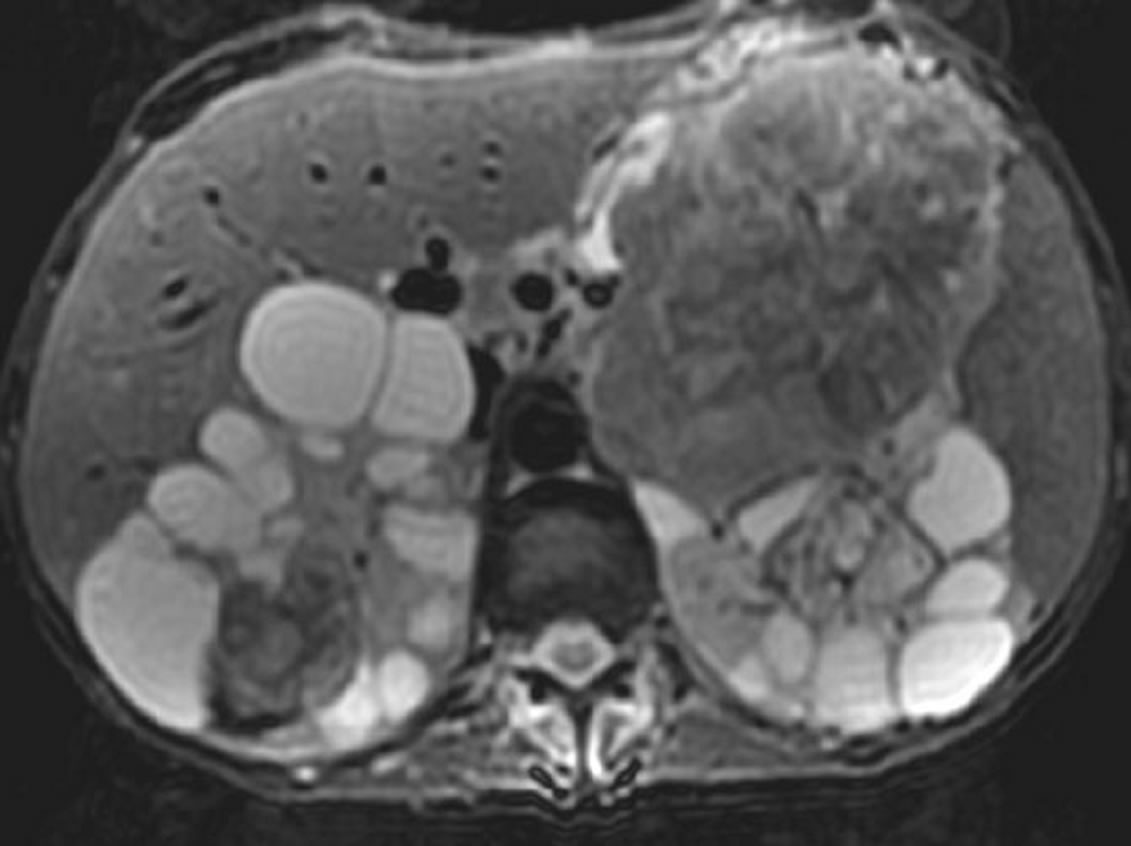
Figure 2. Abdominal MRI (axial T2): kidneys with multiple cystic lesions in high signal and bilateral solid masses in low signal.
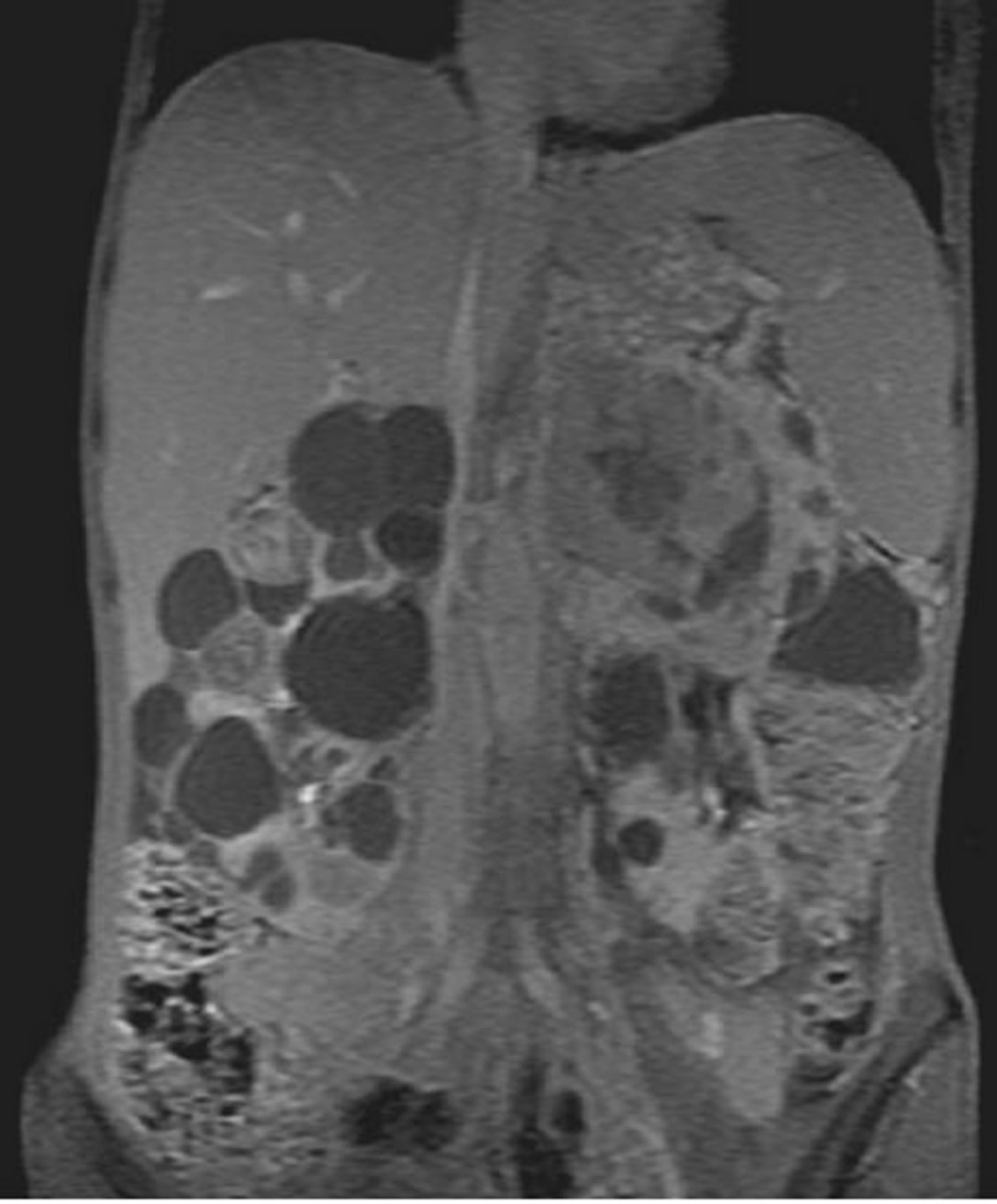
Figure 3. Abdominal MRI (coronal T1 with gadolinium): intense and heterogeneous enhancement of the solid masses transplanted on renal cysts.
The first surgical exploration revealed locally advanced tumor with invasion of transverse mesocolon and pancreas tail, with ascites. A radical left nephrectomy was performed (Figure 4).
At histopathologic examination, the left kidney was 1 Kg 500 in weight and 20 cm in length. The renal parenchyma was multicystic with a tumor of 8 cm containing hemorrhagic and necrosis components. Histologic examination showed a fusiform cells proliferation with cytonuclear atypies and elevated mitotic index infiltrating the perirenal fat tissue, renal capsule, pancreas and left mesocolon (Figure 5).
Immunohistochemical examination showed an intense and positive staining with vimentine (Figure 6). The staining with actine, desmine, cytokeratin and myogenine was negative. The diagnosis of high-grade pleomorphic sarcoma was confirmed. Right nephrectomy could not be performed.
3. Discussion
The association between adult polycystic kidney disease and renal cell carcinoma has been reported in about thirteen cases [3-5], whereas the association with sarcoma is a very uncommon situation. To our knowledge, it has been reported in only three cases [1,6,7].
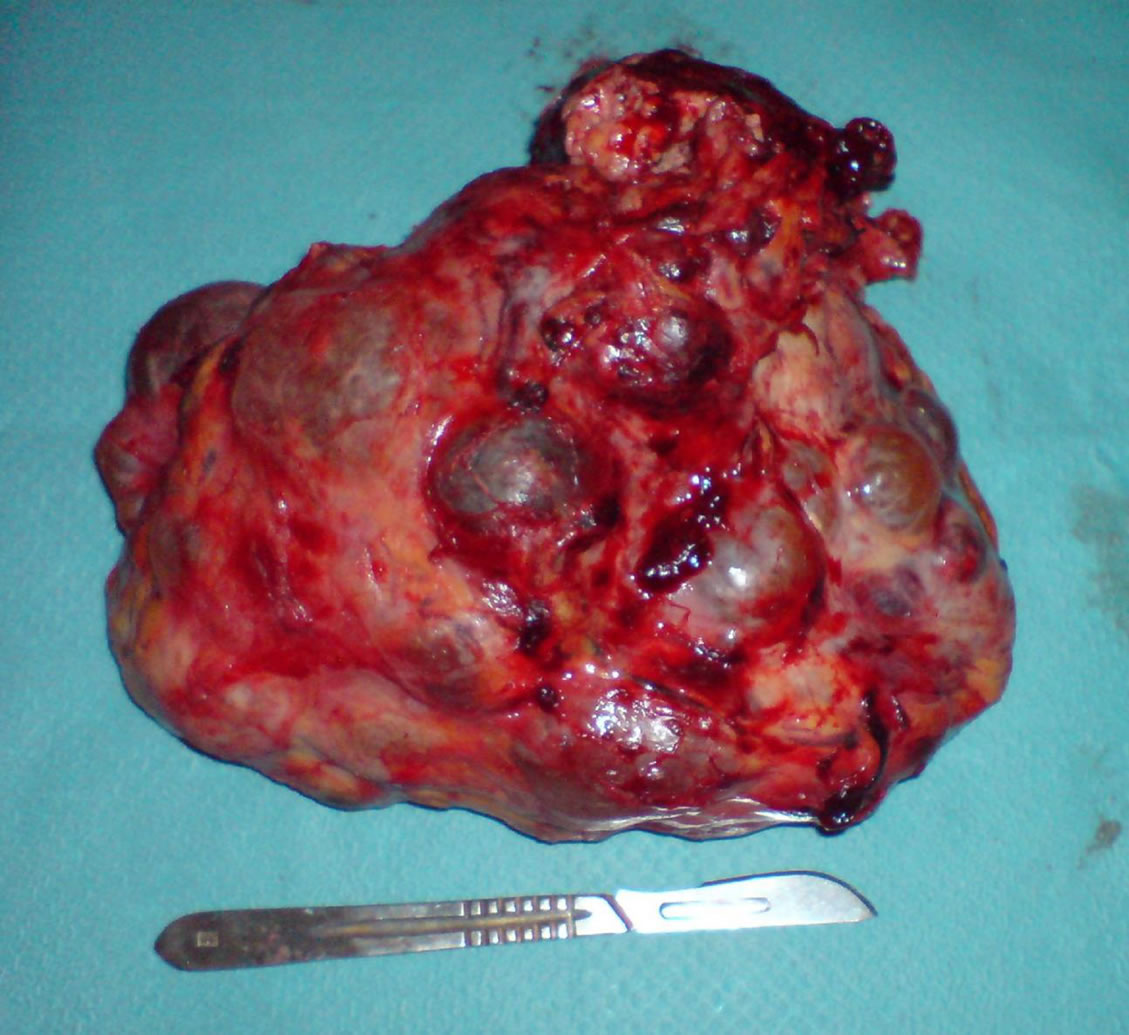
Figure 4. Macroscopic view of the left kidney once removed.
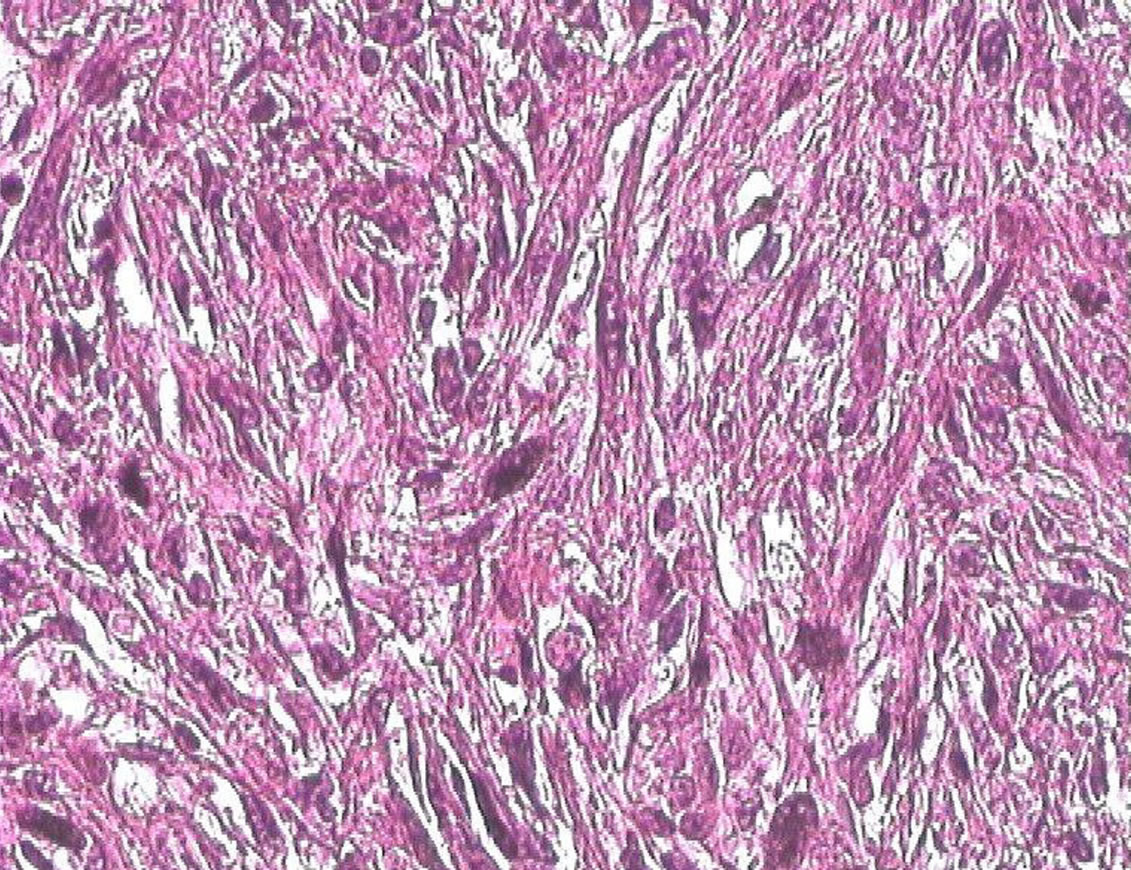
Figure 5. Pleomorphic fusocellular proliferation: cytonuclear atypies with elevated mitotic index (HE × 200).
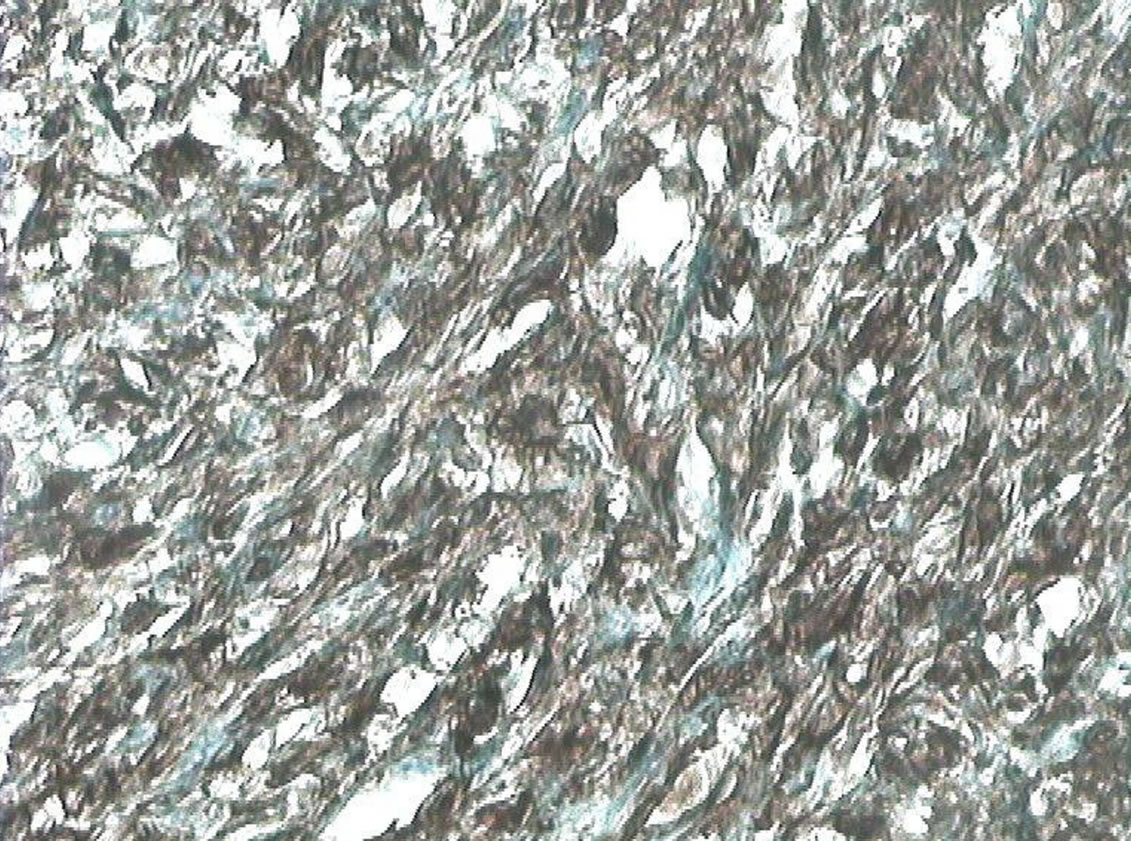
Figure 6. Pleomorphic fusocellular proliferation with positive staining for vimentine (G × 400).
Due to increased survival of patients with APKD undergoing dialysis and renal transplantation, coexisting malignant tumor should be considered when complex cysts are discovered within the kidney on imaging performed for various symptoms or for screening [1,4,5].
Any asymmetry in size and shape of the kidneys, a definite palpable mass, hematuria and unexplained fever or weight loss, all indicate the need for a careful further investigation in patients with polycystic disease [5].
Imaging of these patients is further made complex by the marked distortion of the renal architecture due to the underlying polycystic disease. A combination of various imaging modalities is necessary to make a definite preoperative diagnosis [5].
A complex cyst on ultrasound examination, any solid mass on CT and/or MR should be investigated by fine needle aspiration cytology or biopsy [5].
Sarcomas of the kidney are uncommon; they represent 1% to 3% of all malignant renal tumors. Macroscopically, the tumors are voluminous [mean size = 19.5 cm]. Like other genitourinary sarcomas, most of these lesions are histologically high-grade (86%) and are greater than 5 cm in size at diagnosis (56%) [1,8].
Primary renal sarcomas have imaging features of malignant tumor without histological specificity. They cannot be differentiated from other renal cancers or retroperitoneal sarcoma [9].
Ultrasonography shows an echoic solid mass involving the renal parenchyma.
On enhanced CT series, the tumor is often voluminous without capsule and is heterogeneous with central necrosis. After contrast injection, the enhancement is variable [9].
CT scan is also useful in evaluating loco-regional extension, venous invasion and permits to diagnose thoracic or hepatic secondary localizations [9].
There is no specific signal for this tumor in MRI but it permits to detect local extension and venous invasion like in other malignant tumor of kidney.
The primary treatment for renal sarcoma is complete resection with histologically negative margins [1].
Complete tumor resection is possible only in 72% of patients with renal sarcomas and 86.6% of them die within 23 months after surgery [1].
4. Conclusion
Sarcoma associated with APKD is very rare and have similar radiological features as primary renal sarcoma or carcinoma which require histological evaluation for definitive diagnosis.
REFERENCES
- D. Minardi, P. Mantovani, M. Dellabella, L. Dell’Atti, R. Mazzucchelli, A. Santinelli, et al., “Renal Sarcoma Associated with Adult Polycystic Kidney Disease. A Case Report and Literature Review,” Archivio Italiano di Urologia, Andrologia, Vol. 76, No. 2, 2004, pp. 94-96.
- R. Kalifat and F. Sellami, “Polysystic Renal Disease and Bilateral Renal Cancer,” Annales d Urologie, Vol. 21, No. 1, 1987, pp. 3-6.
- E. K. Lang and R. Davis, “Autosomal Dominant Polycystic Disease with Renal Cell Carcinoma,” Journal of Urology, Vol. 173, No. 3, 2005, p. 987. doi:10.1097/01.ju.0000153739.20733.6a
- K. El Khader, A. Koutani, M. El Mamoun, A. Ibn Attya, M. Hachimi and A. Lakrissa, “Renal Cell Carcinoma Associated with Polycystic Kidney. A Case Report,” Progres en Urologie, Vol. 7, No. 3, 1997, pp. 464-467.
- A. Kapoor, S. Gamanagatti and R. Sharma, “Renal Cell Carcinoma in Autosomal Dominant Polycystic Kidney Disease,” European Journal of Radiology, Vol. 51, No. 2, 2004, pp. 87-89. doi:10.1016/j.ejrex.2004.05.003
- R. A. Nazirov, “Angiosarcoma of a Duplicate Polycystic Kidney in a Girl,” Urologiia a Nefrologiia, Vol. 35, No. 3, 1970, pp. 9-58.
- M. Hayakawa, H. Kinoshita, S. Jitsukawa, H. Ishikawa, M. Mukai and H. Tazaki, “Rhabdomyosarcoma Associated with Polycystic Kidney: A Case Report with Light and Electron Microscopic Observation,” Nippon Jinzo Gakkai Shi, Vol. 23, No. 11, 1981, pp. 1359-1367.
- M. De Fromont and C. Coulange, “Rare Kidney Tumors in an Adult,” Annales d Urologie, Vol. 38, No. 1, 2004, pp. 15-23. doi:10.1016/j.anuro.2003.10.004
- A. Adnani, R. Latib, S. Bouklata, A. Ajana, L. Hammani and F. Imani, “Clear Cell Sarcoma of the Kidney in an Adult: A Case Report,” Journal de Radiologie, Vol. 87, No. 2, 2006, pp. 136-138. doi:10.1016/S0221-0363(06)73985-3

