Open Journal of Emergency Medicine
Vol.03 No.03(2015), Article ID:59426,4 pages
10.4236/ojem.2015.33004
Bilateral Pneumonia and Pleural Effusions Subsequent to Electronic Cigarette Use
Kendall Moore, Henry Young II, Matthew F. Ryan*
Department of Emergency Medicine, University of Florida, Gainesville, FL, USA
Email: kendalldmoore@ufl.edu, hyoungii@ufl.edu, *mfryan@ufl.edu
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 8 July 2015; accepted 1 September 2015; published 7 September 2015
ABSTRACT
Electronic nicotine delivery systems also known as electronic cigarettes (or e-cigarettes) are marketed by their manufactures as a safer alternative to tobacco cigarettes because of potentially reduced delivery of toxins. However, the scientific evidence and the long-term health effects of e-cigarettes are limited. We describe a case of a 43-year-old man who had been smoking electronic cigarettes excessively for three days and presented with acute dyspnea, increased work of breathing and tachycardia. Subsequent chest x-ray revealed bilateral pleural effusions. In addition, the patient had a new oxygen requirement and was thus admitted with a diagnosis of pneumonia and bilateral pleural effusions. The case and the potential harmful effects of electronic cigarettes are discussed herein.
Keywords:
Electronic Cigarettes, E-Cigarettes, Pneumonia, Pleural Effusion, Vaping

1. Introduction
Electronic nicotine delivery systems also known as electronic cigarettes (or e-cigarettes) are marketed by their manufactures as a safer alternative to tobacco cigarettes because of the potentially reduced delivery of toxins. Electronic cigarettes are made to look like a tobacco cigarette but have a completely different mechanism of operation. For example, e-cigarettes contain a liquid mixture of nicotine, flavoring and a delivery agent which is typically propylene glycol but they do not contain nor burn tobacco. Instead, the liquid mixture is heated with a coil when inhaled and delivers a heated vapor of the mixture [1] . The chemical composition and pyrolysis products of cigarettes and cigarette smoke are complex with more than 4000 compounds identified in cigarette smoke [2] including polyaromatic hydrocarbons and other known carcinogens. Electronic cigarettes do not burn tobacco and therefore the number of toxins is reported to be less than in regular cigarettes. However, the number of products in e-cigarette vapor has not been fully elucidated and recent Food and Drug Administration (FDA) analysis has indicated that e-cigarettes indeed contain a number of toxins and established carcinogens [3] .
Because e-cigarettes are made to feel and look like cigarettes yet still deliver nicotine, their popularity has increased dramatically. Reasons for this are varied and users see them as safe alternatives to tobacco, a bridge to tobacco cessation and a method to curb withdrawal from conventional cigarettes [4] . Yet, the overall long-term health effects of electronic cigarettes are unclear and to date there is limited data regarding the toxicity, carcinogenicity, pharmacology and public health effects of electronic cigarettes [5] . A review of the FDA Adverse Events Reports from 2009 through 2013 regarding electronic cigarettes reveals dozens of reports concerning untoward outcomes possibly linked to electronic cigarettes including throat irritation, exacerbations of asthma, chronic pulmonary disease, congestive heart failure and nicotine toxicity [6] . Herein we report an illustrative case regarding a previously healthy man with a remote history of tobacco use who presented with dyspnea, chest pain and hypoxia. The patient recently purchased an electronic cigarette kit and was using it “non-stop” for 3 days. We discuss the case as well as review key aspects of electronic cigarettes.
2. Case Report
A 43-year old man with a past medical history of hypertension presented to the emergency department (ED) complaining of one day of worsening shortness of breath and pleuritic chest pain made worse when recumbent and improved when seated. The patient was a prior smoker but stated he quit smoking seven years ago. However, three days ago he purchased an electronic cigarette kit and stated he had been smoking “non-stop” since its purchase. When queried how many puffs he took, he said he had taken hundreds each day, and that he had been using the electronic cigarette all day long without stopping until bedtime. Even when he began to feel ill, he continued to use the electronic cigarette until he called for emergency medical service and was then brought to our facility with complaints of chest pain and feeling short of breath. His history was negative for ill contacts, recent travel, or novel exposures. The patient had no known allergies and took no medications. He denied fever, chills, upper respiratory symptoms, abdominal symptoms or musculoskeletal complaints.
In the emergency department, the patient was in moderate distress and diaphoretic, sitting up in the stretcher. His presenting vital signs were temperature of 37.4˚C (oral), blood pressure of 128/90 with a pulse of 95. Of considerable concern was the patients increased work of breathing with a respiratory rate of 22 and pulse oximetry reading of 91% on room air representing worrisome hypoxia. The patient was placed on 2 L supplemental oxygen via nasal cannula with improvement of his oxygen saturation to 96% and lessened his air hunger.
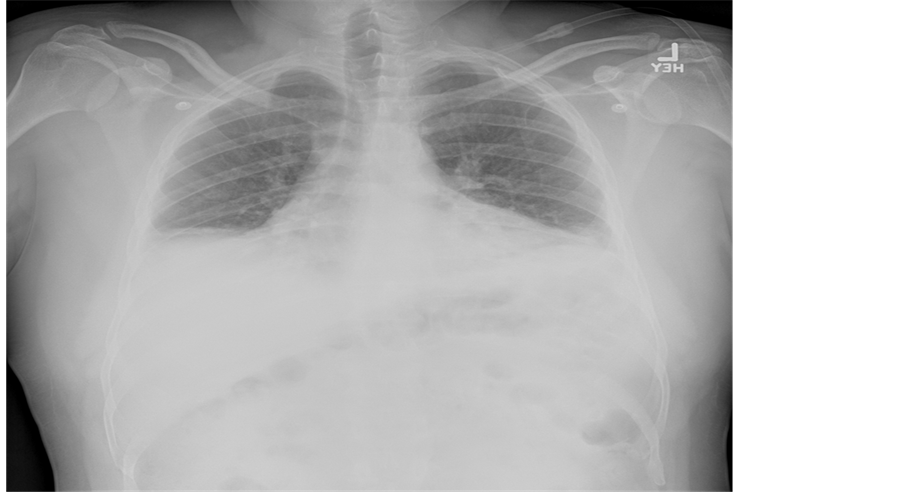
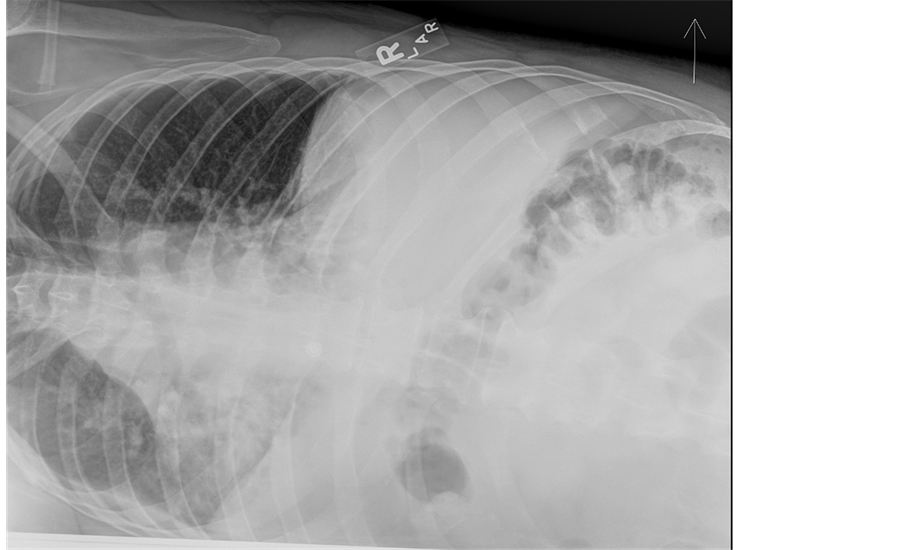
In addition to the increased work of breathing, his pulmonary exam was remarkable for bibasilar crackles. An electrocardiogram showed normal sinus rhythm with normal axis and intervals. Basic chemistry labs including a cardiac panel were with normal values save a serum creatinine of 1.22 mg/dL. Inflammatory markers were expectedly elevated with a sedimentation rate of 31 mm/h and a C-reactive protein value of 33 mg/L. A complete blood count with differential was normal. An influenza panel was negative for influenza A and B (later verified by antigen testing). An anterior chest ray revealed hypoinflated lungs with bibasilar parenchymal consolidation and associated bilateral pleural effusions (Figure 1) which were confirmed by a lateral decubitus image (Figure 2). Note, the presence of an effusion is evident by increased opacification of the compressed left lung with concomitant aeration of the right lung.
The patient was given several nebulized albuterol-ipratropium breathing treatments and started on antibiotics for community acquired pneumonia. Because of his concerning respiratory status including a new supplemental oxygen requirement, the patient was admitted for continued medical management. A subsequent echocardiogram (to assess his cardiac function and for heart failure) and chest computed tomography (to assess other causes of his pulmonary disease including pulmonary embolism) were both unremarkable. The patient was discharged after two days in the hospital and has had no issues since.
3. Discussion
Electronic nicotine delivery systems resemble conventional tobacco cigarettes but do not burn tobacco and thus do not produce pyrolytic or micro particles as tobacco does. Depending on the brand, most electronic cigarettes contain a limited number of ingredients which includes nicotine, a humectant (typically propylene glycol or glycerine) and flavoring.
Figure 1. The figure is an erect anterior chest x-ray of the patient showing bilateral pleural effusions and lung opacification. Also noted is hypo aerated lung fields with costophrenic blunting.
Figure 2. The figure is a left lateral decubitus radiograph. Decubiti radiographs are useful in assessing the volume of an effusion. The presence of an effusion is evident by increased opacification of the compressed left lung with concomitant aeration of the right lung.
Electronic cigarettes are battery operated and use a heated coil to vaporize their ingredients which the user then inhales delivering nicotine to the lungs [1] . The user exhales a vapor of the humectant and trace amounts of the remaining ingredients. For this reason, people refer to the process of using e-cigarettes as vaping. Of concern and what is currently unknown is the exact composition of the inhaled and exhaled vapor. For example, it is established that the components of conventional cigarettes and cigarette smoke are numerous in part because cigarette smoke undergoes time and temperature dependent chemical and physical changes and thus the inhaled components of smoke differ from the smoke exhaled back into the environment [7] .
Some users may be more susceptible to complications from e-cigarettes for a variety of reasons. First, we consider that complications and long and short-term responses can depend on an e-cigarette user’s current medical conditions and medications, family and social history as well as their pulmonary physiology and function. Also a user’s puff topography is to be considered, which assesses the features of smoking or vaping such as number of puffs per cigarette, puff volume, total volume drawn per cigarette, puff flow rate, puff duration, and inter-puff interval [8] . Additionally, the type of device used is a factor that may influence a person’s response to e-cigarettes including the ingredients contained therein, their concentration and purity and thus any chemical or physical changes to the ingredients during use and types contaminants contained in the cigarettes. For example, a recent study found unsafe levels of acetaldehyde in a brand of e-cigarettes [9] . Further, electronic cigarettes have been shown to require stronger and stronger vacuums (increase puff strength) to create vapor as the amount of fluid decreases with use [10] . The variability of the puff strength varied with the brand of electronic cigarette tested.
Existing clinical studies regarding e-cigarette use show varied results. Recently, Romagna and his group report that in vitro, e-cigarette vapor is significantly less toxic than tobacco smoke [11] . This is not unexpected given the thousands of known chemical and toxins known to be in tobacco smoke. Vardavas and coworkers reported that after five minutes of electronic cigarette use, pulmonary function was compromised as manifested in decreased FENO. This was a measure of lung inflammation, oxidative stress, airway impedance, and increased lung flow resistance. These changes are consistent with the use of tobacco cigarettes [12] . For example, in the test group, there was a 16% decrease in FENO as compared to the control group (0% change). As this was after only five minutes, the negative impacts of e-cigarettes would likely be compounded after repeated and prolonged use. Heavy use can impact pulmonary function as was the case herein.
4. Conclusion
A previously health man precipitously developed pneumonia and pleural effusions from heavy use of an electronic cigarette. Manufactures have recommend stopping for 30 minutes after taking up to 16 puffs to avoid complications from excessive use. Nevertheless, as these devices continue to gain popularity, the specter electronic cigarettes raise in both long-term and short-term health effects is real. Emergency medicine physicians should recognize electronic cigarettes as a cause of acute pulmonary disease.
Cite this paper
KendallMoore,HenryYoung II,Matthew F.Ryan, (2015) Bilateral Pneumonia and Pleural Effusions Subsequent to Electronic Cigarette Use. Open Journal of Emergency Medicine,03,18-22. doi: 10.4236/ojem.2015.33004
References
- 1. Trtchounian, A. and Talbot, P. (2011) Electronic Nicotine Delivery Systems: Is There a Need for Regulation? Tobacco Control, 20, 47-52.
http://dx.doi.org/10.1136/tc.2010.037259 - 2. Green, C.R. and Rodgman, A. (1996) Methods of Collection of Smoke or Analytical Purposes. Recent Advances in Tobacco Science, 22, 131-133.
- 3. Summary of Results: Laboratory Analysis of Electronic Cigarettes Conducted by FDA.
http://www.fda.gov/newsevents/publichealthfocus/ucm173146.htm - 4. Caponnetto, P., Campagna, D., Cibella, F., Morjaria, J.B. and Caruso M. (2014) Efficiency and Safety of an Electronic Cigarette (ECLAT) as Tobacco Cigarettes Substitute: A Prospective 12-Month Randomized Control Design Study. PLoS ONE, 8, e66317.
http://dx.doi.org/10.1371/annotation/e12c22d3-a42b-455d-9100-6c7ee45d58d0 - 5. Etter, J.F., Bullen, C., Flouris, A.D., Laugesen, M. and Eissenberg, T. (2001) Electronic Nicotine Delivery Systems: A Research Agenda. Tobacco Control, 20, 243-248.
http://dx.doi.org/10.1136/tc.2010.042168 - 6. http://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/AbouttheCenterforTobaccoProducts/UCM379475.pdf
- 7. Jenkins, R.A., Tomkins, B. and Guerin, M.R. (2000) The Chemistry of Environmental Tobacco Smoke: Composition and Measurement, Ch. 4. CRC Press, Boca Raton.
- 8. Frederiksen, L.W., Miller, P.M. and Peterson, G.L. (1977) Topographical Components of Smoking Behavior. Addictive Behaviors, 2, 55-61.
- 9. Uchiyama, S., Iraba, Y. and Kumugta, N. (2010) Determination of Acrolein and Other Carbonyls in Cigarette Smoke Using Coupled Silica Cartridges Impregnated with Hydroquinone and 2,4-Dinitrophenylhydrazine. Journal of Chromatography A, 1217, 4383-4388.
http://dx.doi.org/10.1016/j.chroma.2010.04.056 - 10. Trtchounian, A., Williams, W. and Talbot, P. (2010) Conventional and Electronic Cigarettes (E-Cigarettes) Have Different Smoking Characteristics. Nicotine & Tobacco Research, 12, 905-912.
http://dx.doi.org/10.1093/ntr/ntq114 - 11. Romagna, G., Allifranchini, E., Bocchietto, E., Todeschi, S., Esposito, M. and Farsalinos, K.E. (2013) Cytotoxicity Evaluation of Electronic Cigarette Vapor Extract on Cultured Mammalian Fibroblasts (ClearStream-LIFE): Comparison with Tobacco Cigarette Smoke. Inhalation Toxicology, 25, 354-361.
- 12. Vardavas, C., Anagnostopoulos, N., Kougias, M., Evangelapoulus, V., Connelly, G. and Behrakis, P. (2012) Short- Term Pulmonary Effects of Using Electronic Cigarettes: Impact on Respiratory Flow Resistance, Impedance, and Exhaled Nitric Oxide. Chest, 141, 1400-1406.
http://dx.doi.org/10.1378/chest.11-2443
NOTES

*Corresponding author.