Open Journal of Soil Science
Vol.3 No.4(2013), Article ID:34930,9 pages DOI:10.4236/ojss.2013.34021
A Comparison of the Solubilizing Potential of Some Aminopolycarboxylic Acids, Hortrilon® and Fetrilon® for Use in Phytoextraction
![]()
1Department of Soil Genesis, Survey and Classification, Soil Research Institute, Kumasi, Ghana; 2Department of Analytical Chemistry and Echo Chemistry, Faculty of BioScience Engineering, Ghent University, Gent, Belgium; 3Department of Chemistry, University of Cape Coast, Cape Coast, Ghana.
Email: *emmaamoakwah@yahoo.co.uk
Copyright © 2013 Emmanuel Amoakwah et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 13th, 2013; revised June 13th, 2013; accepted June 20th, 2013
Keywords: EDTA; EDDS; NTA; Hortrilon®; Fetrilon®; Soil Amendments; Phytoextraction
ABSTRACT
Phytoextraction has been introduced as a new technology to clean up soils contaminated with heavy metals as the use of conventional methods to clean up the soil is very expensive and destructive to the ecosystem. However, using plants to clean up contaminated soils takes a considerable period before the contaminants are removed from the soil by the plants. This has necessitated the use of amendments to enhance phytoextraction in order to shorten the period of contaminants removal by plants. In view of this, a pot experiment was conducted to study the effect of various aminopolycarboxylic acids (EDTA, EDDS, NTA dry and NTA liquid) and two commercial fertilizers which are Hortrilon® and Fetrilon® on their ability to solubilize Cd and Zn in contaminated soils. It was observed that the inducing effect of EDTA on the solubility of Cd and Zn persisted throughout the experimental period. Initially, EDDS enhanced the solubility Cd and Zn, however, its effect dissipated with time. The application of both NTA dry (powder) and NTA liquid had a significant effect on the solubility of Zn as a result of the formation of Zn-NTA soluble complexes. Hortrilon® and Fetrilon® solubilized significant concentrations of both Cd and Zn with Hortrilon® having the greatest inducing effect on the solubility of Cd and Zn.
1. Introduction
Heavy metals, natural components of the earth’s crust can become toxic to living things when their concentration exceeds acceptable levels [1]. At high concentrations heavy metals can induce oxidative stress in biological materials through free radical formation. In plants and some microorganisms, heavy metals can disrupt the normal function of pigments and enzymes by substituting for essential elements [2]. Heavy metals are also toxic to humans as they build up in tissues and organs which can disrupt their normal functions. Uncontrolled disposal of waste, accidental spillage, mining and smelting of metalliferous ores are some of the factors responsible for the migration of heavy metals into noncontaminated soils [2].
Phytoextraction, which is one of the remediation strategies employed to reduce heavy contamination, involves the use of plants to remove harmful elements from contaminated soils. A key requirement of phytoextraction is the ability of plants to produce chelating compounds to solubilize heavy metals making them available for uptake [3]. To enhance the phytoextraction process, synthetic chelating agents can be used to improve the solubility and bioavailability of heavy metals for uptake by plants [4,5]. In addition to the solubilizing ability, chelating agents prevent metals from forming insoluble precipitates and reduce their toxicity to plants by lowering their concentrations [6]. Among the most used chelating agents are ethylenediaminetetraacetic acid (EDTA) and nitrotriloacetate (NTA) [7].
In spite of their solubilizing potential, EDTA and NTA application have some drawbacks which have restricted their use. Firstly, the fate of the residual chelate after phytoextraction is unclear [8], with a possibility of leaching into deeper soil layers. Secondly, EDTA has the potential of persisting in the soil environment for a long period due to its low biodegradability [9]. Also EDTA-heavy metal complexes can be toxic to some plants and soil microorganisms [10,11]. Thus the use of EDTA requires more expensive conventional remediation methods for their removal after phytoextraction [12]. In view of these deficiencies, new chelating agents need to be found which have about the same solubilization potential as EDTA but have better biodegradability and shorter life span in the soil. Hence the objective of this research was to investigate the solubilization potential of some aminopolycarboxylic acids (APCAs) and commercial fertilizers on Cd and Zn using soil from a heavy metal polluted site in Flanders, Belgium. Among the APCA’s investigated were ethylenediaminetetraacetic acid (EDTA), nitrotriloacetate (NTA) and ethylenediamine-N, N’-disuccinic acid (EDDS) which have been found to have an estimated half-life of 2.5 days in natural soils [13], and the commercial fertilizers Hortrilon® and Fetrilon®.
2. Materials and Method
2.1. Soil Samples and Soil Characterization
Soil samples were taken from Lommel in the Campine region of Belgium. The Universal Transeverse Mercator coordinates of the sampled plot is 31 656535 E and 56 75647 N. The soil samples were collected from a site which had been used as a smelter from the 19th century to the mid 1970’s [14]. Soil samples were collected from a depth of 0 - 30 cm, stored in plastic bags and transported to the laboratory. The soil samples were air-dried for four weeks and passed through a 2 mm sieve mesh. Field capacity was estimated by adding an excess of water to 400 g dry soil. The soil was assumed to be at field capacity when formation of further droplets at the base of the pot after free percolation had completely stopped. Based on the increase in weight, the field capacity (Table 1) was calculated.
The total carbonate (CaCO3) (Table 1) content of the soil was determined by adding a known excess quantity of sulphuric acid and back titrating the excess with sodium hydroxide. The pH of the soil was determined by allowing 5 g of air-dried soil to equilibrate in 25 mL of deionized water for 24 h and subsequently measuring the

Table 1. Some selected physicochemical properties of the soil used for the experiment.
pH of the supernatant with a pH glass electrode (Model 520 A, Orion, Boston, MA, USA). The determination of electrical conductivity (EC) was made with a conductivity cell by measuring the electrical resistance of a 1:5 soil: water suspension. Soil organic matter content was determined by the Walkley-Black method [15] while the cation exchange capacity (CEC) was determined using the method explained by Reeuwijk [16].
2.2. Experimental Setup and Sampling
Pots were filled with 450 g of dry soil, together with the different treatments (Table 2) and brought to 60% field capacity. Immediately after the addition of the treatments, sample of soil (10 g) was taken and analysed for the levels of solubilized cadmium (Cd) and zinc (Zn). Each treatment was performed in triplicate. All the treatments were transported to a green house and frequently watered. Once a week, soil samples of 10 g was taken for CaCl2 extraction for the determination of Cd and Zn as well as soil pH.
2.3. Extraction and Determination of Cd and Zn in the Soil Samples
Extraction of Cd and Zn was carried out by adding 25 mL of 0.01 M CaCl2 to 5 g of air dried soil. The mixture was shaken for 2 h on a mechanical shaker and filtered through a white ribbon filter (Macherey-Nagel, Germany, 640 m, Ø 125 mm, Cat No. 203210). Heavy metal analysis was performed on the filtrate using inductively coupled plasma-optical emission spectrometry (ICP-OES: Varian vista MPC, Varian, Palo Alto, California, USA).
2.4. Statistical Analysis
Statistical analyses were performed with S-Plus 8.0 for windows. Normality and equality of variance of the samples was tested using a Kolmorogov-Smirnov-test and a Modified Levene test (equality of variance). For each sampling time and each group of treatment, analysis of variance (ANOVA) and a Tukey test was performed to
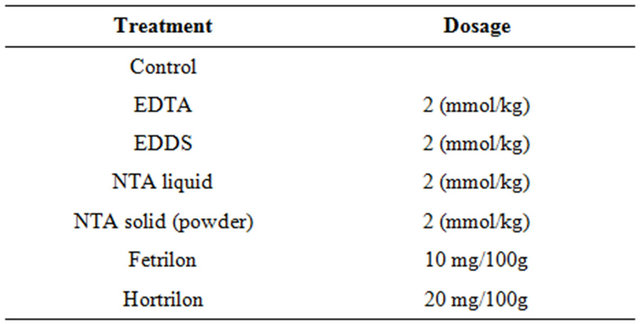
Table 2. Overview of the different treatments, together with the dosage of each amendment.
investigate differences between the different treatments. Also for each treatment ANOVA was performed to investigate significant differences of treatments with time. The results were evaluated on the basis of homogeneous groups at a significant level of p < 0.05.
3. Results and Discussion
3.1. Quality Control
In order to estimate the efficiency, precision and accuracy of the extraction and analytical methods, certified reference material CRM 141R (Cd 14.0 ± 0.4, and Zn 270 ± 8) No 347 trace element in Calcareous Loam soil from Community Bureau of Reference (BCR) was extracted and analyzed with the ICP-OES: Varian Vista MPX, Varian. The observed concentrations were 13.3 ± 0.1 for Cd and 264 ± 3 mg/kg for Zn. The CRM 141R No 347 reference material gave mean recoveries of 87.4% for Cd and 94.9% for Zn. This is an indication that the methodology was good.
3.2. Solubilizing Effect of EDTA and NTA on Cd and Zn
The potential use of some APCAs in phytoextraction was studied by applying these APCAs to contaminated soils and measuring the solubilized levels of Cd and Zn over a period of eight weeks. Figure 1 shows the changes in the levels of Cd and Zn in the soil samples without chelating agents. There was no significant change in the levels of Cd over the duration of the experiment while the levels of Zn increased ten times (from 1.35 to 13.5 mg/kg) within seven days. The levels of Zn continued to increase and reached levels of 56 mg/kg after 14 days. From the 14th day, the levels of Zn did not change significantly till the end of the experiment.
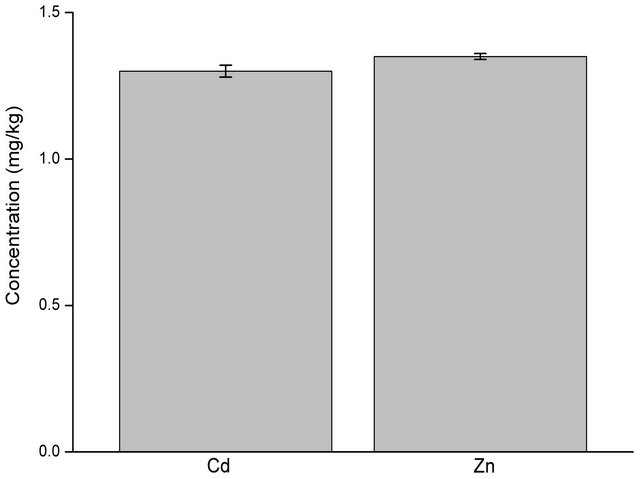
Figure 1. Levels of Cd and Zn in the soil solution used for the experiment determined by CaCl2 extraction protocol before the introduction of the different amendments.
The effect of the different amendments on the solubility of the heavy metals was juxtaposed with the control. Therefore the levels of Cd, Zn and pH were also determined in the control (untreated soil) throughout the experimental period (Figure 2).
The ability of EDTA and NTA to solubilize Cd is shown in Figures 3 and 4 respectively.
In addition to the application of NTA in the form of a solution, we were also interested in determining the solubilizing potential of NTA applied in the form of a powder (Figure 5). The application of EDTA to the soil samples immediately increased the amount of solubilized Cd by a factor of 1.6 while both NTA liquid and the powdered form did not alter the levels of Cd in the soils samples. The highest amount of solubilized Cd in the soil samples after the application of EDTA, NTA solution and powder (2.50, 1.92 and 1.50 mg/kg respectively) were observed after 7 days from the start of the experiment. From seventh day, the levels of solubilized Cd in all three treatments (EDTA, NTA solution and NTA powder) decreased gradually to levels of 1.5, 1.3 and 1.15 mg/kg respectively.
The addition of EDTA, NTA solution and NTA powder to the soil caused a significant increase in the levels of solubilized Zn. More than a 100 fold increase in the levels of solubilized Zn was observed immediately upon the addition of EDTA (Figure 3) while NTA solution (Figure 4) and NTA powder (Figure 5) increased the levels of solubilized Zn by a factor of 70 and 73 respectively. After the initial increase, the levels of solubilized Zn continued to increase significantly to levels of 480 mg/kg in the EDTA treated samples after 7 days. After the seventh day, the levels of solubilized Zn began to decrease and reached levels similar to that observed immediately after the application of EDTA (Figure 3). In both NTA treated soils (NTA solution and powder), the highest levels of solubilized Zn (121.5 and 124 mg/kg respectively) was observed after 14 days (Figures 4 and 5), even though these started to decrease and reached levels of 80 and 53 mg/kg respectively after 56 days.
The application of EDTA to the contaminated soil increased the levels of solubilized Zn as has been reported in previous studies [17,18]. According to Chen et al., the addition of EDTA to contaminted soils increases the soluble or exchangeable fraction of heavy metals in the soil solution making them available for uptake by plants [19]. In our study, significant amounts of the the soluble fraction of Cd and Zn could be oberved in the soil solution several weeks following the application of EDTA. This shows that the solubilizing effect of EDTA did not decrease with time which could be due to its high environmental persistence of EDTA [20].
The application of both types of NTA did not signifi-
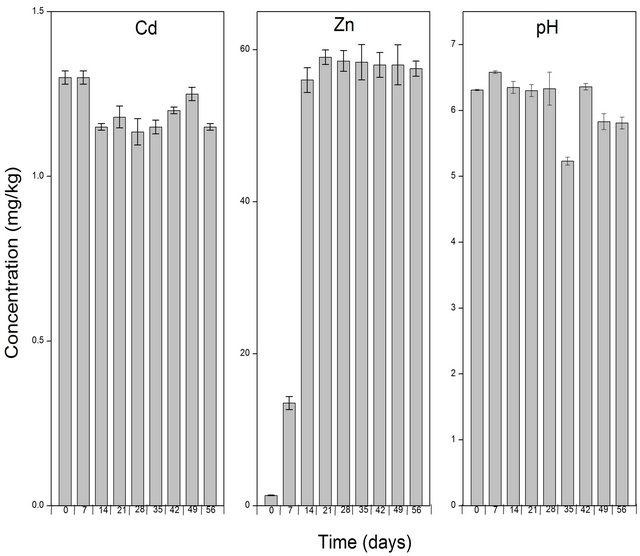
Figure 2. Levels of Cd and Zn in the untreated soil solution (control) determined after extraction with CaCl2. The bar columns shows the mean ± standard deviation of three (3) independent replicates.
cantly increase the solubility of Cd in the contaminated soils even though both NTA types solubilized large fractions of Zn immediately after its application. The ability of NTA to solubilize Zn but not Cd could mean that the application of NTA to a heavy metal contaminated soil could be heavy metal specific. Compared to EDTA, NTA is known to be a weak chelant [21,22], which might explain its low solubilizing potential on Cd. However, in studies using Nicotiana tabacum, repeated NTA application increased the levels of Zn, Cu and Cd [23].
3.3. Effect of EDDS on the Solubility of Cd and Zn
The application of EDDS caused an increase in the levels of Cd and Zn (Figure 6). Cd increased by a factor of 1.3, whereas Zn levels increased by a factor 66. Cd levels further increased to levels of 2 mg/kg after the first week. Subsequetly the levels of Cd started to decrease and reached levels significanlty lower than the starting value from the 2nd to the 8th week. With respect to solubilised Zn, the levels continued to increase, reaching a apeak of 128 mg/kg after 21 days. From the 21st day, the levels of Zn decreased and reached levels of about 90 mg/kg towards the end of the experiment. The increase in the levels of solubilised metals following the application of EDDS with a subsequent decrease in the soluble fractions with time shows that the the solubilizing effect of EDDS dissipated with time. Similar patterns of the dissipating effect of EDDS on the solubility of heavy metals have been observed following the application of EDDS to a heavy metal contaminated soil [11].
3.4. Solubilizing Potential of Hortrilon® and Fetrilon® on Cd and Zn
Following the application of Hortrilon® and Fetrilon®, the concentration of Zn increased about 9 and 5 folds respectively. Comparatively, Hortrilon® solubilised the highest concentration of Zn, and this can probably be ascribed to the various ETDA complexes (Cu-EDTA complex, Fe-EDTA complex, Mn-EDTA complex and Zn-EDTA complex) incalcated in the amendment. The potential use Hortrilon® and Fetrilon® in phytoextraction is advantageous because they can be used as fertilizers. However, the ability to solubilize high levels of Cd and Zn is offset by the fact that both Hortrilon® and Fetrilon® persisted highly in the soil environment even after 8 weeks of the experimental period. This implies a potential risk of leaching of mobilised heavy metals to contaminate the groundwater following.
3.5. Effect of the Chelating Agents on Soil pH
Soil pH is an important factor in determining the effectiveness of metal chelating agents. The pH of many soils
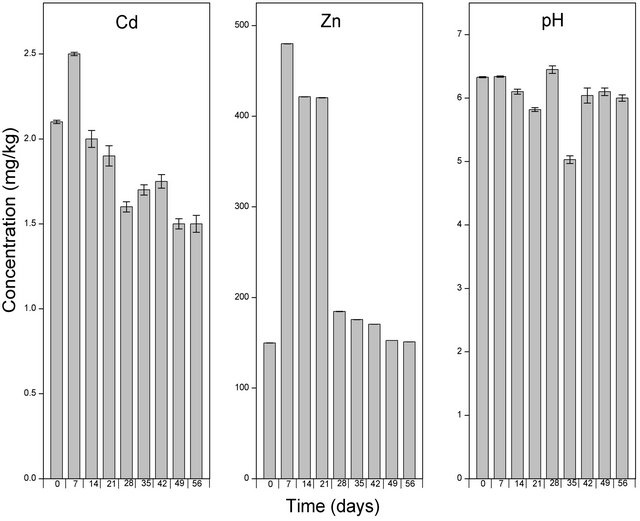
Figure 3. Levels of Cd, Zn and pH of the soil solution following amendment with EDTA and extraction using CaCl2. Samples at time zero were taken immediately after the application of EDTA. The bar columns shows the mean ± standard deviation of three (3) independent replicates.
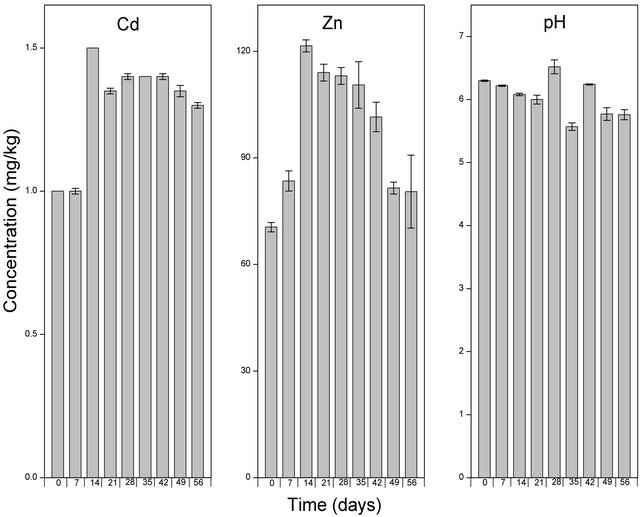
Figure 4. Levels of Cd, Zn and pH of the soil solution following amendment with EDDS and extraction using CaCl2. Samples at time zero were taken immediately after the application of EDDS. The bar columns shows the mean ± standard deviation of three (3) independent replicates.
is often near neutral, and an acidic or alkaline soil environment might cause dramatic ecological impacts. The lowest pH (5.03) observed upon the application of the different solubilizing agents was seen after 5 weeks of EDTA treatment (Figure 3) while the highest pH (8.51) was observed following the application of EDDS for 6
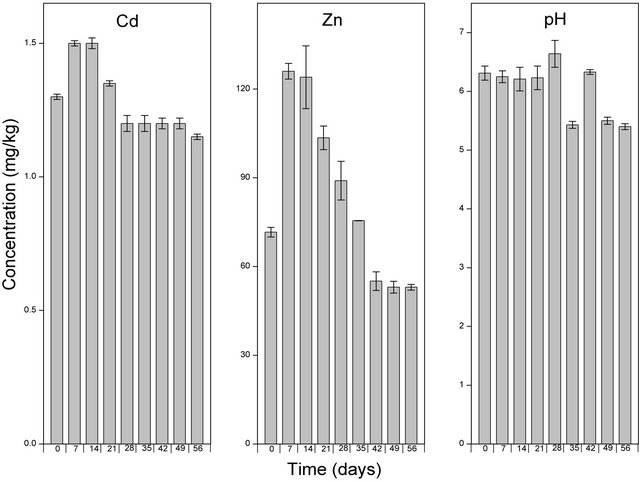
Figure 5. Levels Cd, Zn and pH of the soil solution following amendment with NTA Liquid and extraction using CaCl2. Samples at time zero were taken immediately after the application of NTA Liquid. The bar columns shows the mean ± standard deviation of three (3) independent replicates.

Figure 6. Levels of Cd, Zn and pH of the soil solution following amendment with NTA Solid and extraction using CaCl2. Samples at time zero were taken immediately after the application of NTA Solid. The bar columns shows the mean ± standard deviation of three (3) independent replicates.
weeks (Figure 6). The application of Hortilon® (pH range: 6.19 - 6.71; Figure 7) and Fertrilon® (pH range: 6.1 - 6.72; Figure 8) did not significantly alter the pH of the soil. Even though the application of EDTA and NTA (both solid and liquid) was characterised by a gradual decrease in pH (Figures 3-5), the change in pH after 7 weeks was less than 1.5 units. These changes in pH is in conformity with the observations made by Wu et al. [24] and Lai et al. [25] that the use of EDTA in phytoextraction does not significantly alter soil pH. The observed change in soil pH following the use of NTA is in agreement with the findings of Wenger et al. [23] who ob-
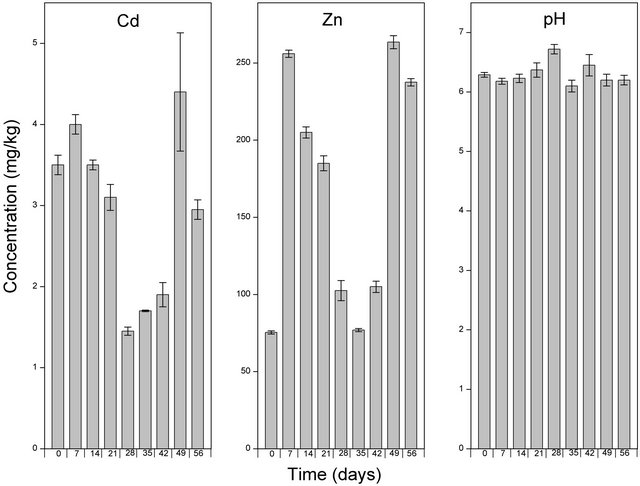
Figure 7. Levels of Cd, Zn and pH of the soil solution following amendment with Fetrilon and extraction using CaCl2. Samples at time zero were taken immediately after the application of Fetrilon. The bar columns shows the mean ± standard deviation of three (3) independent replicates.
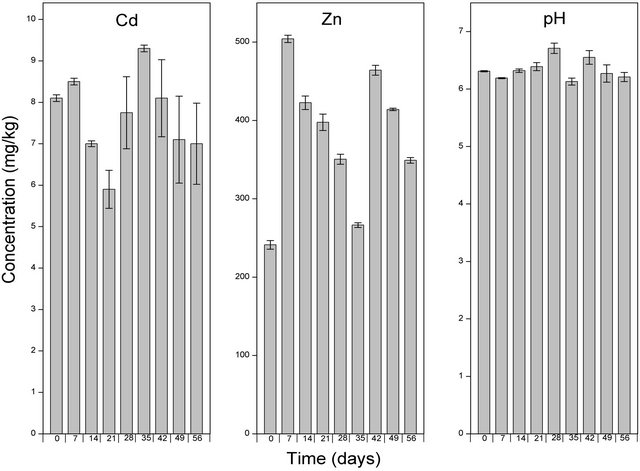
Figure 8. Levels of Cd, Zn and pH of the soil solution following amendment with Hortrilon and extraction using CaCl2. Samples at time zero were taken immediately after the application of Hortrilon. The bar columns shows the mean ± standard deviation of three (3) independent replicates.
served no significant decrease in soil pH following the application of NTA.
4. Conclusion
The solubilizing potential of different chelating agents for use in phytoextraction was assesed based on their ability to solubilize Cd and Zn in contaminated soils as well as their effect on soil pH. Hortrilon® solubilised the highest concentration of Zn, followed by EDTA. The solubilizing potential of NTA, EDDS and Fetrilon® on Zn were comparable, even though these were all signifantly higher than the non-treated soil samples. With respect to Cd, Hortrilon® solubilized the highest amount followed by Fetrilon®. The application of both EDTA and EDDS increased the levels of Cd in the soil, even though NTA applied in the form of a solution did not alter Cd levels in the soil. Even though the solubilizing effect of EDDS on Cd and Zn decreased with time showing that EDDS had a short life-span, it caused an increase in the levels of the soil pH. Based on the results obtained, the two commercial fertilizers (Hortrilon® and Fetrilon®), have a potential to be used in soil phytoextraction based on their ability to solubilize Cd and Zn without significanlty altering the pH of the soil.
REFERENCES
- R. A. Wuana, F. E. Okieimen and J. A. Imborvungu, “Removal of Heavy Metals from a Contaminated Soil Using Organic Chelating Acids,” International Journal of Environment Science and Technology, Vol. 7, No. 3, 2010, pp. 485-496.
- M. Ghosh and S. P. Singh, “A Review on Phytoremediation of Heavy Metals and Utilization of Its Byproducts,” Applied Ecology and Environmental Research, Vol. 3, No. 1, 2005, pp. 1-18.
- N. von Wiren, H. Marschner and V. Romheld, “Roots of Iron-Efficient Maize Also Absorb Phytosiderophore-Chelated Zinc,” Plant Physiology, Vol. 111, No. 4, 1996, pp. 1119-1125.
- D. E. Crowley, Y. C. Wang, C. P. P. Reid and P. J. Szanislo, “Mechanism of Iron Acquisition from Siderophores by Microorganism and Plants,” Plant and Soil, Vol. 130, No. 1-2, 1991, pp. 179-198. doi:10.1007/BF00011873
- M. N. V. Prasad and H. M. O. Freitas, “Metal Hyperaccumulation in Plants—Biodiversity Prospecting for Phytoremediation Technology,” Electronic Journal of Biotechnology, Vol. 6, No. 3, 2003, p. 3. doi:10.2225/vol6-issue3-fulltext-6
- J. W. Huang, J. Chen, W. R. Berti and S. D. Cunningham, Phytoremediation of Lead-Contaminated Soils: Role of Synthetic Chelates in Lead Phytoextraction,” Environmental Science and Technology, Vol. 31, No. 3, 1997, pp. 800-805. doi:10.1021/es9604828
- R. R. Brooks, “Plants That Hyperaccumulate Heavy Metals,” CAB International, New York, 1998.
- T. Egli, “Biodegradation of Metal-Complexing Aminopolycarboxylic Acids,” Journal of Bioscience and Bioengineering, Vol. 92, No. 2, 2001, pp. 89-97.
- S. D. Cunningham and D. W. Ow, “Promises and Prospects of Phytoremediation,” Plant Physiology, Vol. 110, No. 3, 1996, pp. 715-719.
- V. Sykora, P. Pitter, I. Bittnerova and T. Lederer, “Biodegradability of Ethylenediamine-Based Complexing Agents,” Water Researcher, Vol. 35, No. 8, 2001, pp. 2010-2016. doi:10.1016/S0043-1354(00)00455-3
- D. V. Greman, S. Velikonja-Bolta and D. Lestan, “Ethylenediaminedissucinate as a New Chelate for Environmentally Safe Enhanced Phytoextraction,” Journal of Environmental Quality, Vol. 32, No. 2, 2003, pp. 500-506.
- L. H. Wu, Y. M. Luo, X. R. Xing and P. Christie, “EDTAEnhanced Phytoremediation of Heavy Metal Contaminated Soil with Indian Mustard and Associated Potential Leaching Risk,” Agriculture, Ecosystem and Environment, Vol. 102, No. 3, 2004, pp. 307-318. doi:10.1016/j.agee.2003.09.002
- D. Lestan and H. Greman, “Chelate Enhanced Pb Phytoextraction in Plant Uptake, Leaching and Toxicity,” 17th WCSS World Congress of Soil Science, Bangkok, 14-21 August 2002, Symposium 42, Paper 1701.
- S. Van Slycken, E. Meers, L. Meiresonne, N. Witters, K. Adriaensen, A. Peene, W. Dejonghe, T. Thewys, J. Vangronsveld and F. M. G. Tack, “The Use of Bio-Energy Crops for Phytoremediation of Metal Enriched Soils in the Campine Region,” Communications in Agricultural and Applied Biological Sciences, Vol. 73, No. 1, 2008, pp. 19-22.
- A. Walkley and I. A. Black, “An Examination of the Method for Determining Soil Oganic Matter and Proposed Modification of the Chromic Acid Titration Method,” Soil Science, Vol. 37, No. 1, 1934, pp. 29-28. doi:10.1097/00010694-193401000-00003
- L. P. Reeuwijk, “Procedures for Soil Analysis,” 6th Edition, International Soil and Reference Information Centre, Food and Agriculture Organisation, Wageningen, 2002.
- H. Y. Lai and Z. S. Chen, “Effects of EDTA on Solubility of Cadmium, Zinc and Lead and Their Uptake by Rainbow Pink and Vertivers Grass,” Chemosphere, Vol. 55, No. 3, 2004, pp. 421-430. doi:10.1016/j.chemosphere.2003.11.009
- C. Luo, Z. Shem and X. Li, “Enhanced Phytoextraction of Cu, Pb, Zn and Cd with EDTA and EDDS,” Chemosphere, Vol. 59, No. 1, 2004, pp. 1-11. doi:10.1016/j.chemosphere.2004.09.100
- H. Chen and T. Cutright, “EDTA and HEDTA Effects on Cd, Cr and Ni Uptake by Helianthus annus,” Chemosphere, Vol. 45, No. 1, 2001, pp. 21-28. doi:10.1016/S0045-6535(01)00031-5
- E. Lombi, F. J. Zhao, S. J. Dunham and S. P. McGrath, “Phytoremediation of Heavy Metal Contaminated Soils: Natural Hyper Accumulation versus Chemically Enhanced Phytoextraction,” Journal of Environmental Quality, Vol. 30, No. 6, 2001, pp. 1919-1926. doi:10.2134/jeq2001.1919
- E. Meers, M. Hopgood, E. Lesage, F. M. G. Tack and M. G. Verloo, “Enhanced Phytoextraction: In Search of EDTA Alternatives,” International Journal of Phytoremediation, Vol. 6, No. 2, 2004, pp. 95-109. doi:10.1080/16226510490454777
- A. T. Ruley, N. C. Sharna, S. V Sahi, S. R. Singh and K. S. Sajuran, “Effects of Lead and Chelators on Growth, Photosynthetic Activity and Pb Uptake in Sesbania drummondii Grown in Soil,” Environmental Pollution, Vol. 144, No. 1, 2006, pp. 11-18. doi:10.1016/j.envpol.2006.01.016
- K. Wenger, A. Kayser, S. K. Gupta, G. Furvts and R. Schulin, “Comparison of NTA and Elemental Sulphur as Potential Soil Amendments in Phytoremediation,” Soil and Sediment Contamination, Vol. 11, No. 5, 2002, pp. 655-672. doi:10.1080/20025891107023
- L. H. Wu, Y. H. Luo, P. Christie and M. H. Wong, “Effects of EDTA and Low Molecular Weight Organic Acids on Soil Solution Properties of a Heavy Metal Polluted Soil,” Chemosphere, Vol. 50, No. 6, 2003, pp. 819-822. doi:10.1016/S0045-6535(02)00225-4
- H. Y. Lai and Z. S. Chen, “The EDTA Effect on Phytoextraction of Single and Combined Metals-Contaminated Soils Using Rainbow Pink (Dianthus chinensis),” Chemosphere, Vol. 60, No. 8, 2005, pp. 1062-1071. doi:10.1016/j.chemosphere.2005.01.020
NOTES
*Corresponding author.

