Open Journal of Modern Hydrology
Vol.07 No.02(2017), Article ID:74912,15 pages
10.4236/ojmh.2017.72005
Search for Environmental Causation of the Cladoceran Dynamics in Lake Kinneret, Israel
Gophen Moshe
Migal Scientific Research Institute, Kiryat Shmona, Israel

Copyright © 2017 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: February 2, 2017; Accepted: March 24, 2017; Published: March 27, 2017
ABSTRACT
Ten years of monthly dataset of cladoceran (Diaphanosoma sp., Bosmina spp., Ceriodaphnia spp.) density in Lake Kinneret (spatially scattered lake sampling stations), Israel, was studied with the aim of searching impact factors controlling their dynamics. Statistical regressions indicated that out of several environmental factors (among others, non-pyrrhophyte algal biomass, invertebrate predation) only temperature factor was relevant. Additional speculative assumptions of zooplanktivore fish predation suggest this factor as a significant impact on cladoceran dynamics.
Keywords:
Cladocera, Monthly, Dynamics, Temperature, Fish, Predation

1. Introduction
The spato-temporal and bathymetrical distributions, as well as the eco-physio- logical characteristics of Cladocera in Lake Kinneret, were widely studied by the author. Metabolic activities during 1972-1977 comprised of two cycles: 1972- 1974 and 1975-1977 suggested changes of nutritional values of potential food resources [1] . The role of cladoceran organisms within the Carbon flow pattern of the Kinneret ecosystem was analyzed [2] . The impact of food availability, fish predation and temperature on their distribution was documented as well [3] . The potential impact of fish and invertebrate predation on cladoceran densities was documented [4] . The temporal changes of cladoceran metabolic traits in relation to changes in nutrient availability in Lake Kinneret were documented [5] . The impact of temperatures on the densities of cladocerans assemblages was documented [6] [7] [8] . The relation of cladoceran population dynamics to fish predation and consumed algal preferences based on annual means were documented [9] . The temporal indications in those studies were annual or several months (seasonal) timing intervals. In the present paper, the monthly time units were considered. It makes it possible to implicate a precise insight into the temporal trend of changes of the cladoceran population.
Recent environmental changes within and around Lake Kinneret eco-system, such as, climate, hydrology, phytoplankton, and nutrients, did not have a direct impact on the cladoceran densities fluctuations. Nevertheless, an indirect impact on the cladoceran community was documented. Although climate changes of temperature increase, precipitation, river discharges and Lake-water-level decline, did not directly influenced long-term Cladoceran densities although seasonal fluctuations of temperature passively affected their dynamics. Climate changes also affected nutrient regimes in the lake. The Kinneret eco-system was shifted from P to N limited system. Phytoplankton composition was changed; hydrochemical cycles were modified as well; the metabolic trait of cladocerans were responsively changed but long-term change of density fluctuations of as a result of nutrient ava
2. Material and Methods
The density of Cladocera in Lake Kinneret was monitored since 1969 and onwards. A decade (1975-1985) of the dataset was chosen for the analysis of monthly changes of the numerical density values (No/L) averaged for the whole lake. A program of spatial, temporal and bathymetrical sampling was previously described [2] [5] [10] . The cladoceran organisms in Lake Kinneret were grouped into three categories: Diaphanosoma (D. brachiurum: Lieven) [11] ; Bosmina (B. longirostris, O.F. Muller; B. longirostris Var. Cornuta, Jurine [10] ; and Ceriodaphnia (C. reticulate Jurine)) [10] . The cladocerans were separated into two age groups: 1 - 3 neonates as “Small” and 4-adults as “Large” [8] .
Statistical analyses included: Fractional Polynomial (FP) Regression models which are based on functions of a continuous covariate [12] . This type of regression provides flexible parameterization for continuous variables which provide a wide range of shapes that include other shapes provided by ordinary polynomials. Data of phytoplankton and Epilimnetic temperatures were maintained from the Kinneret Limnological Data base [13] . The data presented here re- present huge bulk of collected sample analyses, with spatial, temporal and bathymetrical justified cover, and therefore standard error and standard deviation values are statistically sufficient for the evaluation processes.
The estimation of population growth rate was done using the standard exponential population growth model:

And: 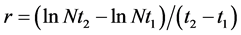 (in: 30 for da
(in: 30 for da
where,
Nt2―the population size at time t.
Nt1―the initial population size.
r―the intrinsic rate of growth.
3. Results
The study of the relations between cladoceran densities and limnological parameters was initiated by computing Simple Correlation (Dependence Coefficient) between monthly averages of zooplankton groups and epilimnetic temperatures and Chlorophyll concentrations; results are given in Table 1.
Results in Table 1 indicate high dependence relations between zooplankton and temperature and lower dependence relations with Chlorophyl concentration. Nevertheless, Small and Large Bosmina and small Ceriodaphnia declined with Temperature increase; Small and Large Diaphanosoma and Small Ceriodaphnia decreased when Chlorophyll increased.
The next step of the statistical analysis was Polynomial Regressions between the Zooplankters Densities (monthly means of No/l) and Ep
Table 1. Correlation/Dependence Coefficients between Mean Monthly Densities (No/l) of Small (S) and Large (L) Diaphanosoma, Bosmina and Ceriodaphnia and Epilimnetic Temperature (˚C) and Chlorophyll concentrations (µg/l) (Positive = Increase; Negative = Decline).
Table 2. Polynomial Regressions between Monthly means of Zooplankton (Diaphanosoma, Bosmina, Ceriodaphnia) density (No/l) (S = Small, L = Large) and Ep
All cladoceran parameters (S&L, Diaphanosoma, and Ceriodaphnia and large Bosmina) were declining with increase of Chlorophyll concentration: r2 for Diaphanosoma, and small Bosmina and Ceriodaphnia (S&L) Vs Chlorophyll indicated enhancement densities with chlorophyll concentration increase. The third step was grouping monthly values of the values of Small/Large Zooplankter body Ratios. The two groups were seasonal periods: Winter: January-June and Summer: July-December. The results indicated only one significant difference between summer and winter mean ratios for Ceriodaphnia: Winter = 0.65, and 1.13 in summer. An insignificant difference of higher in summer for Bosmina and lower in summer for Diaphanosoma were found as well.
Several other types of statistical relations between Zooplankton parameters and algal groups or limnological features (Water Level, water inflows, Nutrient concentrations Secchi Depth, etc.) were insignificant. Polynomial Regressions between the Zooplankton body size ratios (S/L) and algal groups (Cyanophyta, Chlorophyta, Diatoms), ep
3.1. The S/L Ratio (Figure 1 and Figure 2)
High value of the ratio between Small (S) and Large (L) (S/L) body cladocerans might be an implication of three environmental parameter impacts: 1) Intensive reproduction; 2) Preferential predation of large body cladocerans by Fish; 3) Both. Results presented in Figure 1 represent high S/L monthly ratios of Ceriodaphnia during July-September. Sim
3.2. Intrinsic Rate of Population Growth (r) (Figures 3-6)
The actual size and dynamic fluctuations of the entire population are shown in Figures 3-6. Results in Figure 1 and Figure 2 are not reflected or even corre-
Figure 1. Monthly Lake Means of Small/Large (S/L) density (No./L) ratios of Diaphanosoma, Bosmina and Ceriodaphnina.
Figure 2. Polynomial Regressions (r2 and p values are given) between lake averages of “Small” and “Large” organism densities (No./L): 201 S/202 L = Diaphanosoma; 203 S/204 L = Bosmina; 207 S/208 L = Ceriodaphnia.
Figure 3. Monthly lake averages of the intrinsic rate of population growth (r, see text) of Diaphanosoma, Bosmina and Ceriodaphnia.
lated with results given in Figure 3. The population’s intrinsic growth rate (r) is not dependent on S/L ratios or vice versa. Seasonal (monthly) fluctuation trait of the intrinsic rate of population growth and S/l ratios are dissim
Figure 4. Fractional Polynomial Regressions between Monthly lake averages of Intrinsic Rate of Population growth (r, see text) and Years of Diaphanosoma, Bosmina and Ceriodaphnia.
Figure 5. Fractional Polynomial Regressions between Monthly lake averages of Intrinsic Rate of Population growth (r, see text) and Months of Diaphanosoma, Bosmina and Ceriodaphnia.
similar annual fluctuations of r values (Figure 4): high in the late 1970’s and declining during the early 1980’s. It conclusively points to a sim
Figure 6. Fractional Polynomial Regressions between Monthly lake averages of Intrinsic Rate of Population growth (r, see text) and Monthly means of ep
3.3. Epilimnetic Temperature (Figures 6-8)
Because of causative relations between most of the biological parameters and cladoceran fluctuations (monthly, annually) of densities, the Temperature parameter was tested. The impact of temperature increase on the cladocerans population rate of growth (r) is given in Figure 6: none of the Cladocera genera represents a unidirectional trend of relation. The optimal r values (highest rate of population growth) for Diaphanosoma and Bosmina are similar: 15˚C - 23˚C (corresponding to the Winter-Spring season; whilst for Ceriodaphnia two ranges: 13 - 17 and 26˚C - 29˚C (corresponding to Winter and late Summer) (Figure 6). Figure 7 and Figure 8 represent annual (Figure 7) and seasonal (Figure 8) fluctuations of epilimnetic temperatures smoothed (FP). From the early 1980’s the temperature declines towards 1985; Winter 15 - 20; Summer 20 - 27.
4. Discussion
Relations between zooplankton densities and environmental conditions in lakes, either physical or biological factors, were widely studied. Lampert [14] documented zooplankton biomass build-up due to both favorable food conditions and low predation pressure followed by the development of “clear-water- phase”. Manipulation of planktonic communities might be beneficial for lake restoration as water quality improvements [14] [15] [16] [17] [18] ). The case study of cladoceran communities in Lake Kinneret presented in this paper is an attempt aimed at searching causative environmental parameters which control the cladoceran dynamics. It is done here, the first time, by place reliance on multiannual monthly resolution of the assemblage densities. Periodical (2 year
Figure 7. Fractional Polynomial Regressions between Monthly lake averages of Epilimnetic Temperature (˚C) and Years.
Figure 8. Fractional Polynomial Regressions between Monthly lake averages of Epilimnetic Temperature (˚C) and months.
groups: 1972-1974 & 1975-1977) changes of the food nutritional value comparatively analyzed and the impact on cladoceran metabolic activity was concluded [1] . Temperature effect on the metabolism of Ceriodaphnia was previously documented [6] . It was found that under a temperature of 15˚C (Kinneret winter time) metabolic activities of Ceriodaphnia reticulata was lower by 61%, 58% and 62% in comparison with 27˚C as measured by food intake, Ammonia excretion, and respiration respectively. The production enhancement of Ceriodapohnia in summer is therefore concluded as presented here (Figure 6) by the increase of r values. The opposite trend of lower production (decline of r values) of Bosmina and Diaphanosoma in summer (Figure 6) was indicated. It is suggested that the temperature elevation effect on the metabolic activity of Diaphanosoma and Bosmina is sim
Here, an attempt was tentatively made at the implementation of experimental data to the long-term monthly record of lake population densities. Results of Polynomial Regression analysis (r2 and p, values) (Figure 2) between young (“small”) and matured life cycle stages (“Large”) indicates significant relations where the highest is due to Ceriodaphnia (r2 = 0.614) and the lowest to Bosmina (r2 = 0.162). Consequently, it is suggested that environmental factors are similarly affecting the “small” and the “large”. For example, if food condition parameter is improved and production rate enhanced, as well as intensification of visual particulate attacker fish predation pressure on “Large”, the increase of S/L ration is predicted. Several other options are also relevant: cladoceran food sources deficiency followed by a lower production rate (relative decline of “small”) or stock increase of f
Figure 9. Polynomial Regression (shadow = 95% Confidence Interval) between monthly lake averages of biomass (g(ww)/m2) of predator (Copepodite 5 and adult cyclopoid stages) and herbivore (young cyclopoid, cladocerans and small rotifers) in Lake Kinneret.
riodaphnia and three months periodical fluctuations of Bosmina. Fractional Polynomial Regression Model (FP) predictions of r (intrinsic rate of population growth) (in No/l density values) in relation to years, months and temperature are presented in Figures 4-6. The advantage of FP is to increase the flexibility afforded by conventional polynomial models (StataCorp 2005) [20] . The fact that Coefficient of Determination (r2) between two variables was found to be high is not necessarily a clear indication of a circumstantial relationship. The existence of a third variable as a link between the two is possible. Conclusive attri- bute to the r2 level is required.
The concept of isolating related parameters of cladoceran densities and biological and/or chemical environmental parameters did not indicate strict causative variables. Even those variables which were partly validated cannot be confirmed as sole causatively correlated. Temperature impact partly confirms causation impact on a monthly basis. As known from numerous studies about Plankton-Fish relations in the Kinneret ecosystem, it is reasonable that fish predation factor plays a major role in the complicated Kinneret ecosystem aimed at cladoceran dynamics. Since deta
Usually, if from statistical evaluations a perfect answer to the search for causative parameters of the cladoceran dynamics is insufficient, a Model construction is the common solution. The modularity of complex interactions is very problematic or complicated. In a comprehensive model aimed at eco-limnological trait, there is a common option of an unwanted discord between noisy data when simulated with observed findings. A comprehensive eco-limnological (coupled physical and biological parameters) approach is commonly caused by the usage of many parameters and state variables whose validity is questionable. Several different parameter sets can produce sim
Figure 10. Monthly averages of Small (left 3 panels) and Large (right 3 panels) organisms of Diaphanosoma (upper 2 panels), Bosmina (middle 2 panels) and Ceriodaphnia (2 lower panels) densities (No./l) in Lake Kinneret (1975-1985).
Figure 11. Monthly averages of densities (No./l) of total number of cladocerans in Lake Kinneret (1975-1985).
Ceriodaphnia, Small and Large, represent sim
The fish removal by fishery management was documented by Sondergaard et al. [22] . This kind of management, namely, Biomanipulation, has been used in many shallow lakes aimed at water quality improvement. They summarized two such operations with an interval of 20 years between and concluded that repeated managements of fish removal might be a relevant strategy to improve the water quality of highly external nutrient loaded lakes. It is important to account the incomplete implementation of a model constructed for temperate ecosystems to the subtropical lake Kinneret. The advantage of a model is due to its simplicity and generality. Nevertheless, wrong implications are quite common: Peridinium was a bloom forming phytoplankter comprised >50% of the annual carbon fixation in Kinneret. But this alga is not edible to zooplankton and some of the fish species in Kinneret. The ichthyofauna of Kinneret comprised mostly of planktivorous species but only one of them, the most common Bleak, consume zooplankton solely. The Kinneret Bleaks prey on Cladocera more efficiently than predator life stages of Cyclopoida. Fishing removal of Bleaks could potentially have a significant impact on the Kinneret ecosystem (Bendorf et al. 1984). The food resources of Bleak is significantly different from other fish species, resulting in a degree of freedom for Bleaks removal. The grazer zooplankters preferential food are chlorophytes and diatoms; therefore, fishing removal of bleaks might improve water quality by cladoceran-mediated algal suppression. Nevertheless, as documented by Bendorf et al. [17] , reduction of algal biomass by removal of un-wanted fish (Bleaks in Kinneret) requires Phosphorus control; otherwise, failure of Biomanipulation is predicted. During the 1969-1993, zopplankton biomass declined in Lake Kinneret and removal of about 5000 tons of Bleaks during 1994-2001 was subsidized and the biomass of zooplankton significantly increased (Figure 12). As an immediate response, zooplankton biomass was significantly enhanced but algal biomass was enhanced due to an increase of Phosphorus in the lake ep
Figure 12. Annual means of total Cladoceran biomass (g(ww)/m2) In Lake Kinneret (1969- 2001). Periodical decline (1969-1993) and increase (1993-2001) are arrowed.
many state variables and parameter values for the production of an adequate model. A simplified model using a low number of state variable and parameter values is more constructive and therefore recommended.
Conclusively, the cladoceran density dynamics is mostly affected by fish predation and periodical temperature changes.
5. Summary
A 10-year period of monthly record of cladoceran density (No./l) dynamic was statistically evaluated. Several indications were tested of which no direct significant relationship was identified as a major component affecting animal fluctuations. It is not impossible that food conditions and invertebrate predations are optimal. Two relevant major impacts were indicated: Ep
Cite this paper
Moshe, G. (2017) Search for Environmental Causation of the Cladoceran Dynamics in Lake Kinneret, Is- rael. Open Journal of Modern Hydrology, 7, 90-104. https://doi.org/10.4236/ojmh.2017.72005
References
- 1. Gophen, M. (1981) Metabolic Activity of Herbivorous Zooplankton in Lake Kinneret (Israel) during 1972-1977. Journal of Plankton Research, 3, 15-24.
https://doi.org/10.1093/plankt/3.1.15 - 2. Serruya, C., Gophen, M. and Pollingher, U. (1980) Lake Kinneret: Carbon Flow Pattern and Ecosystems Management. Archiv fur Hydrobiologie, 88, 265-302.
- 3. Gophen, M., Serruya, S. and Spataru, P. (1990) Zooplankton Community Changes in Lake Kinneret (Israel) during 1969-1985. In: Biro, P. and and Talling, J.F., Eds., Trophic Relationships in Inland Waters. Developments in Hydrobiology, Vol. 53, Springer, Netherlands, 39-46.
https://doi.org/10.1007/978-94-009-0467-5_6 - 4. Gophen, M. (2004) Ecohydrological Management of Lake Kinneret: A Case Study. Ecohydrology and Hydrobiology, 4, 237-408.
- 5. Gophen, M. (2011) The Cladoceran Trophic Status in the Nitrogen Limited Ecosystem of Lake Kinneret (Israel). Journal of Environmental Biology, 32, 455-462.
- 6. Gophen, M. (1976) Temperature Dependence of Food Intake, Ammonia Excretion and Respiration in Ceriodaphnia reticulate (Jurine) (Lake Kinnere, Israel). Freshwater Biology, 6, 451-455.
https://doi.org/10.1111/j.1365-2427.1976.tb01634.x - 7. Gophen, M. (2016) Innovated Management Design of Lake Kinneret (Israel) and Its Drainage Basin. Research in Business and Management, 3, 1-15.
https://doi.org/10.5296/rbm.v3i1.9041 - 8. Gophen, M. and Azoulay, B. (2002) The Trophic Status of Zooplankton Communities in Lake Kinneret (Israel). Verhandlungen des Internationalen Verein Limnologie, 28, 836-839.
- 9. Gophen, M. (2003) Water Quality Management in Lake Kinneret (Israel): Hydrological and Food Web Perspectives. Journal of Limnology, 62, 91-101.
https://doi.org/10.4081/jlimnol.2003.s1.91 - 10. Gophen, M. (1978) Zooplankton. In: Serruya, C., Ed., Lake Kinneret, Monographoiae Biologicae, Vol. 32, Springer, Netherlands, 299-311.
https://doi.org/10.1007/978-94-009-9954-1_11 - 11. Korovchinski, N.M. (1987) A Study of Diaphanosoma Species (Crustacea; Cladocera) of the “Mongolianum” Group. International Review of Hydrobiology, 72, 727-758.
https://doi.org/10.1002/iroh.19870720609 - 12. Royston, P. and Altman, D.G. (1994) Regressions Using Fractional Polynomials of Continuous Covariates: Parsimonious Parametric Modeling. Journal of the Royal Statistical Society. Series C, 43, 429-467.
https://doi.org/10.2307/2986270 - 13. KLL-LKDB (1975-1985) Kinneret Limnological Laboratory—Lake Kinneret Data Base. Directed by M. Schleichter.
- 14. Lampert, W. (1988) The Relationship between Zooplankton Biomass and Grazing: A Review. Limnologica (Berlin), 19, 11-20.
- 15. Shapiro, J. (1980) The Importance of Trophic-Level Interactions to the Abundance and Species Composition of Algae in Lakes. In: Barica, J. and Mur, L.R., Eds., Hypertrophic Ecosystems. Developments in Hydrobiology, Vol. 2, Springer, Netherlands, 105-116.
https://doi.org/10.1007/978-94-009-9203-0_12 - 16. Shapiro, J. and Wright, D.I. (1984) Lake Restoration by Biomanipulation: Round Lake, Minnesota, the First Two Years. Freshwater Biology, 14, 371-383.
https://doi.org/10.1111/j.1365-2427.1984.tb00161.x - 17. Bendorrf, J., Kneschke, H., Kossatz, K. and Penz, E. (1984) Manipulation of the Pelagic Food Web by Stocking with Predacious Fishes. International Review of Hydrobiology, 69, 407-428.
https://doi.org/10.1002/iroh.19840690308 - 18. Carpenter, S., Kitchell, J.F. and Hodgson, F.R. (1985) Cascading Trophic Interactions and Lake Productivity. BioScience, 35, 634-639.
https://doi.org/10.2307/1309989 - 19. Gophen, M. (2016) Population Dynamics of Cyclopoid Copepods in Lake Kinneret (Israel). Open Journal of Modern Hydrology, 6, 212-221.
https://doi.org/10.4236/ojmh.2016.64017 - 20. StataCorp (2005) Stata Statistical Software: Release 9. Chapter Volume 1: Fracpoly: Fractional Polynomial Regression. StataCorp LP, College Station, 357-370.
- 21. Vanni, M.J., Layne, C.D. and Arnott, S.E. (1997) “Top-Down” Trophic Interactions in Lakes: Effects of Fish on Nutrient Dynamics. Ecology, 78, 1-20.
- 22. Sondegaard, M., Lauridsen, T.L., Johansson, L.S. and Jeppessen, E. (2017) Repeated Fish Removal to Restore Lakes: Case Study of Lake Vaeng, Denmark—Two Biomanipulations during 30 Years of Monitoring. Water, 9, 43.
https://doi.org/10.3390/w9010043 - 23. Walline W.P., Pizanty, S., Gophen, M. and Berman, T. (1993) The Ecosystem of Lake Kinneret, Israel. In: Christensen, V. and Pauly, D., Eds., Trophic Models of Aquatic Ecosystems, ICLARM Conf. Proc. 26, 103-109.














