Advances in Materials Physics and Chemistry
Vol.4 No.1(2014), Article ID:42009,7 pages DOI:10.4236/ampc.2014.41002
Development and Characterization of Nanovesicles Containing Phenolic Compounds of Microalgae Spirulina Strain LEB-18 and Chlorella pyrenoidosa
1School of Chemistry and Food, Federal University of Rio Grande, Rio Grande, Brazil
2Federal Institute Sul-Rio-Grandense, Campus Pelotas, Visconde da Graça, Pelotas, Brazil
3Institute of Basic Health Sciences, Federal University of Rio Grande do Sul, Porto Alegre, Brazil
Email: leticiamassis@gmail.com, adriana.rodriguesmachado@yahoo.com.br, asmcons@ig.com.br, jorgealbertovc@terra.com.br, leonor.souzasoares@gmail.com
Received December 6, 2013; revised January 3, 2014; accepted January 14, 2014
ABSTRACT
The objective of this study was to elaborate liposomes, through the lipid film hydration methodology, to nanoencapsulate phenolic compounds of Spirulina LEB-18 and Chlorella pyrenoidosa microalgae, and evaluate their physicochemical characteristics and storage stability for 21 days. The total phenolic compounds were evaluated using a calibration curve of gallic acid using methanol and ethanol as extraction solvents. The size and polydispersity index of nanovesicles were determined by light scattering and the percentage encapsulation efficiency was determined by a centrifugation process. The stability of the liposomes at storage time was measured by zeta potential for 21 days. The methanol extracts from Spirulina had a higher content of phenolic compounds (2.62 mg gallic acid∙g−1 of microalgae) compared to the extracts of Chlorella. However, liposomes with ethanolic extracts of the two algae showed higher encapsulation efficiency. The value was higher (96.40%) for Chlorella. All samples obtained nanometric size, with the highest value obtained for the liposome containing ethanol extract of Chlorella (239 nm) differing significantly (p ≤ 0.05) from the others. The liposomes containing extracts of Spirulina were more stable during the 21 days of storage, whereas, those consisting of ethanol extract showed no significant difference (p ≤ 0.05) throughout this period.
Keywords:Microalgae; Phenols; Encapsulation; Liposome
1. Introduction
Liposomes are vesicles composed of one or more phospholipid bilayers encapsulating a volume of aqueous media. The mechanism of liposome formation is based on the unfavorable interactions which occur between amphiphilic compounds (mainly phospholipids) and water molecules, where the polar head groups of phospholipids are subjected to the aqueous phases of the inner and outer media, and the hydrophobic hydrocarbon tails are associated into the bilayer and spherical core shell structures are formed [1,2]. Having a number of benefits, e.g. a possibility of large-scale production using natural ingredients and entrapment and release of water-soluble, lipidsoluble, and amphiphilic materials as well as targetability [3,4], liposomes have been widely used in the food sector both in research and in industry. Liposome manufacture requires input of energy for dispersion of lipid/phospholipid molecules in an aqueous medium. The main objective of such process is to obtain vesicles with the right size, acceptable polydispersity, elasticity, structure and encapsulation efficiency [4,5]. Different methodologies are described in the literature to produce multi-lamellar vesicles (MLVs), large unilamellar vesicles (LUVs) and small unilamellar vesicles (SUVs) [6,7]. Due to its advantages, its use in the food area is being explored and a research is being undertaken [8-13] for the study of its use as a bioactive carrier.
Nowadays, one of the main areas of the research in Food Science and Technology is the extraction as well as the characterization of new natural ingredients with biological activity (e.g. antioxidant) that can contribute to consumer wellbeing as a part of new functional foods [14]. Marine algae has served as an important source of natural bioactive substances. Moreover, many metabolites isolated from marine algae have been shown to possess biological activities and potential health benefits [15]. In fact, some algae lives in complex habitats that are subject to extreme conditions (for example, changes in salinity, temperature, nutrients and UV irradiation); therefore, they must adapt rapidly to new environmental conditions to survive. To do so, they produce a great variety of secondary (biologically active) metabolites that cannot be found in other organisms. Furthermore, considering their great taxonomic diversity, the search for new biologically active compounds in algae is an almost unlimited field [16,17]. Among several alga genera, Spirulina and Chlorella deserve special attention due to their importance to human food and their in vitro and/or in vivo antioxidant potential [18], and are certified by the FDA (Food and Drug Administration) as GRAS (Generally Recognized as Safe), and can be used as a pharmaceutical or nutritive additive with no risk to health [19]. The cyanobacterium Spirulina (Arthrospira) and the fresh water algae Chlorella contain about 50% to 70% protein, vitamins, fiber, minerals and fatty acids at high concentrations[14], and phenolic compounds, such as organic acids (caffeic, chlorogenic, quimic, salicylic, synaptic and trans-cinnamic) which acted individually or synergistically on such antioxidants [20].
Research and the application of polyphenols have recently attracted a great interest in the functional foods, nutraceutical and pharmaceutical industries, due to their potential health benefits to humans. However, the effectiveness of polyphenols depends on preserving the stability, bioavailability and bioactivity of the active ingredients. The unpleasant taste of most phenolic compounds also limits their application. The utilization of encapsulated polyphenolsinstead of free compounds can effectively alleviate these deficiencies. The technologies of encapsulation of polyphenols include liposome entrapment [21]. Therefore, the aim of this work is to elaborate liposomes through the lipid film hydration methodology, to nanoencapsulate phenolic compounds of Spirulina LEB-18 and Chlorella pyrenoidosa microalgae and to assess their physicochemical characteristics and the storage stability for 21 days.
2. Materials and Methods
2.1. Material
The cyanobacterium Spirulina strain LEB-18 isolated from the Mangueira Lagoon was used, and Mangueira Lagoon water supplemented with 20% (v/v) Zarrouk (MLW-S) medium [22] was used for maintenance, inoculum and biomass production. The pilot plant for the production of Spirulina sp. was located near the shore of Mangueira Lagoon (33˚30'13''S; 53˚08'59''W). The microalgae Chlorella pyrenoidosa (Pharma Nostra Co., São Paulo, Brazil) was purchased in powder form. Soy lecithin (Attivos Magisttrais, Barueri, Brazil), was purchased in powder form.
2.2. Extraction and Determination of Total Phenolic Compounds
The extraction of phenolic compounds was based on a previously reported method, with slight modifications [23]. Three grams of Spirulina and Chlorella were homogenised with 75 mL of methanol or ethanol in an orbital shaker at 35˚C for 120 min at 230 rpm. After centrifugation at 3220 g for 15 min, the supernatant was evaporated in a rotary evaporator at 50˚C. The extract was dissolved in 50 mL of distilled water, and the nonphenolics were precipitated with Ba(OH)2 and ZnSO4. The extract was then filtered through 0.45 µmfilter paper, and the volume was adjusted to 100 ml in volumetric flask with distilled water. The phenolic compounds (PC) in the extract were determined via spectrophotometry using the Folin-Ciocalteu reagent and a calibration curve of gallic acid at concentrations of 10 to 100 µg/ml [24]. The PC values in the samples were expressodas mg of gallic acid per gram of microalgae. The extracts were then frozen at −80˚C for 24 h in an ultrafreezer for subsequent lyophilization.
2.3. Liposome Production by Film Hydration
Encapsulation of phenolic extracts in liposomes was carried out by the thin-film hydration method [9], with slight modifications. Briefly, 1 g of soy lecithin was dissolved with 10 mL chloroform in a round-bottom flask and the organic solvent was removed by a rotary evaporator until a thin to filmwas formed on the flask walls. Traces of organic solvents were removed by storage for 18 h in a vacuum desiccator. The resulting dried lipid film was dispersed by the addition of phosphate buffer containing 0.2 g of lyophilized extracts. These mixtures were then mixed exceeding their phase transition temperature (60˚C). Sonication of the preparation, to reduce the size and homogenize liposomes, was carried out in a bath-type ultrasound (40 kHz, Unique USC 700) during 10 cycles for 1 min and kept in cold water for 3 min. A control was performed under the same encapsulation process conditions, but with phenol sample, called control capsule.
2.4. Encapsulation Efficiency Measurement
First, 0.5 mL of the liposomes were placed in a centrifuge tube with 1 mL of acetone since phosphatidylcholine is insoluble in this solvent. The samples were centrifuged at 5000 g for 30 min at 3˚C, separating into two phases. The supernatant containing the non-encapsulated sample was withdrawn and placed in an oven at 60˚C until complete evaporation of the solvent. The remaining dried stuff was resuspended with 5 mL distilled water and the concentration was determined through the phenol method [24]. To determine the total phenol present in the sample, a 0.5 mL aliquot of the initial sample was withdrawn and 1 mL of 0.06% Triton X-100 was added. The equipment was then homogenized in a vortex (Phoenix AP56) until complete solubilization of phosphatidylcholine. The encapsulation efficiency (EE) was calculated as shown in Equation (1):
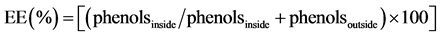 (1)
(1)
Phenol content inside the liposome vesicles quantified compounds after dissolving with Triton, phenols outside the vesicles and quantified compounds that solubilized in acetone were considered.
2.5. Particle Size Distribution and Polydispersity Index
To calculate the average particle size and polydispersity, the dynamic light scattering technique was used (Malvern 4700 MW, Spectra-Physics 127 model) at a wavelength of 632.8 nm, coupled to the BI-200M version 2.0 goniometer and BI-9000AT digital correlator from Brookheaven Instruments [25]. Polydispersity evaluates the size distribution of particles, showing the suspension’s degree of homogeneity. The liposomes were filtered through 0.45 μm filter paper and two drops of the sample dissolved in 8 mL of phosphate buffer pH 7.0 and 0.2 M were used for analyses.
2.6. Capsule Suspension Stability
The suspension stability of the capsules was evaluated through zeta potential using the Zetasizer Nanoseries Nano-Z equipment (Malvern Instruments) at 20˚C and a 90˚ angle, in 4, 11, 14 and 21 days. The suspensions were stored at 4˚C, protected from light and oxygen.
2.7. Statistical Analysis
The results were expressed as mean ± Standard Error (SE) in triplicate. A comparison of the means was ascertained by Tukey’s test at 5% significance level by analysis of variance (ANOVA) using the Statistica 7.0 software.
3. Results and Discussion
3.1. Determination of Total Phenolic Compounds
The total phenolic contents of the methanol and ethanol extracts of the microalgae Spirulina and Chlorella are shown in Table1
The methanol extracts of the two microalgae showed higher content of phenolic compounds (EMS = 2.62 mg/g; EMC = 0.69 mg/g) compared to ethanol extracts (EES = 1.37 mg/g; EEC = 0.41 mg/g), with the values being significantly higher (p ≤ 0.05) for Spirulina. The results showed that the greater polar solvent (methanol) contributed to extract higher concentrations of phenolic compounds for both microalgae studied. It is known that phenolic compounds, particularly phenolic acids, are mostly found in extracts of higher polarity [26], besides the possibility to also find pigments such as phycocyanine and sulfated polysaccharides [27], which may contribute to increase the antioxidant activity [28]. The val ues found for the extracts of Spirulina in this study were higher than those of Souza et al. [23] who obtained 1.15 mg gallic acid.g−1 for methanol extracts of microalgae Spirulina platensis. Cepoi et al. [29] evaluated the influence of ethanol concentration of the solvent for the extraction of phenolic compounds from Spirulina platensis and Nostoc linckia, and concluded that the highest concentration of ethanol (70%) did not provide greater extraction of phenolic compounds from Spirulina, and that a concentration of 55:50 (ethanol:water) did. However, for the microalga Nostoc, the higher content of phenolic compounds was obtained in the highest concentration of ethanolic solvent. Manivannan et al. [30] evaluated the effect of the solvents methanol, diethyl ether and hexane for extraction of phenolic compounds from Chlorella
Table 1 . Total phenolic compounds (mg gallic acid∙g−1 microalgae) of methanolic and ethanolic extracts of Spirulina strain LEB-18 and Chlorella pyrenoidosa.
Mean ± SE. Data in the same column with different letters issignificantly different (p ≤ 0.05). MES = methanolic extracts of Spirulina, MEC = methanolic extracts of Chlorella; EES = ethanolic extracts of Spirulina; EEC = ethanolic extracts of Chlorella.
marina, and found the highest values (0.64 mg gallic acid∙g−1 microalgae) for methanol extracts. This result is very close to that obtained in this study for the methanol extracts of Chlorella pyrenoidosa (0.69 mg gallic acid∙g−1 microalgae). Compared to other plants, Wojdylo et al. [31] evaluated water: methanol (80:20) extracts of 32 plant species and found higher values for Melissa officinalis (0.13 mg gallic acid∙g−1 of the dry plant), Acoruscalamuse and Taraxacum officinale (0.12 mg gallic acid∙g−1 of the dry plant) Polygonum avicularee and Valeriana officinalis (0.11 mg gallic acid∙g−1 of the dry plant).
3.2. Particle Size, Polydispersity Index and Entrapment Efficiency
The average particle size, the polydispersity and the entrapment efficiency of phenolic compounds in liposomes are presented in Table2
The addition of the extracts to liposomes did not affect the average particle diameter, with the exception of the LEC, which showed the largest size (239 nm). The polydispersity index, which provides information about thehomogeneity of the distribution of sizes was low (<0.3) for all samples, indicating the formation of monodisperse systems [32] or narrow range of sizes. The LMC and LEC showed no significant difference (p ≤ 0.05) of the polydispersity index compared to the control (without sample). Some authors [33,34] report that liposomes had a mean size of around 200 nm for the encapsulation of phenolic compounds.
Large unilamellar vesicles (LUV), which are characterized by a particle size greater than 100nm, were obtained for all the samples. In general, LUVs are more homogeneous than MLVs and have a higher encapsulation efficiency than the SUVs, and are often the most useful liposomes [35]. It is widely accepted that large unilamellar vesicles are more suitable for food applica
Table 2. Average size, polydispersity index and encapsulation efficiency of liposomes consisting of phenolic compound of Spirulina LEB-18 and Chlorella pyrenoidosa.
Mean ± SE. Data in the same column with different letters is significantly different (p ≤ 0.05). LMS = liposome with methanol extract of Spirulina; LES = liposome with ethanol extract of Spirulina; LMC = liposome with methanol extract of Chlorella; LEC = liposome with ethanol extract of Chlorella.
tions due to higher encapsulation efficiency (above 45%), increased stability against its melting, and ease of production. However, liposomes smaller than 50 nm in diameter proved to be effective for the simultaneous encapsulation of hydrophilic compounds (inside the vesicle) and hydrophilic antioxidants (α-tocopherol) solubilized in the hydrophobic portion of the lipid bilayer [36]. Ferreira et al. [37] reported that LUVs are very stable physically, when kept at 4˚C showing no change of mean diameter after 5 days, when stored at ambient temperature they show an increase in average diameter of 10 % at the end of same time.
The LEC showed higher encapsulation efficiency, differing significantly (p ≤ 0.05), from the other samples. Priprem et al. [34] prepared liposomes containing quercetin (a flavonoid) from egg phosphatidylcholine/cholesterol (2:1) and obtained encapsulation efficiency of 60% to 80%. Takahashi et al. [33] prepared liposomes using commercial lecithin for encapsulation of curcumin (polyphenolic pigment), in order to increase the bioavailability and functionality of this feed. These authors reported that the results showed greater gastrointestinal absorption and significantly higher antioxidant activity in plasma for curcumin encapsulated in liposomes, and encapsulation efficiency of 68%.
3.3. Stability of the Capsules
Figure 1 shows the results for evaluating the stability of phenolic compounds in liposomes evaluated during 21 days of storage by the zeta potential.
The zeta potential analyses indicated the obtaining of negatively charged particles, due to the presence of lecithin, for all samples. In the first measurements (days 4 and 11), there is no significant difference (p ≤ 0.05) between the samples, compared to the control, except for the LEC, which had the highest value (−34.50) in module in the first evaluation. On the 21st day of evaluation, all samples were significantly different from each other, with the liposomes consisting of extracts of Chlorella showing the highest values in module (Figure 1). The liposomes containing extracts of Spirulina were more stable during the 21 days of storage, and those consisting of ethanol extract showed no significant difference (p ≤ 0.05) throughout this entire period.
The zeta potential reflects the surface potential of the particles, which is influenced by changes to the interface with the dispersing medium, due to the dissociation of functional groups on the particle surface or the adsorption of ionic species present in the aqueous dispersion medium. This study is based on measuring the electrophoretic mobility of phospholipid vesicles, from which it is possible to calculate the zeta-potential. In the module, a relatively high value of the zeta potential is important for good physical and chemical stability of the colloidal
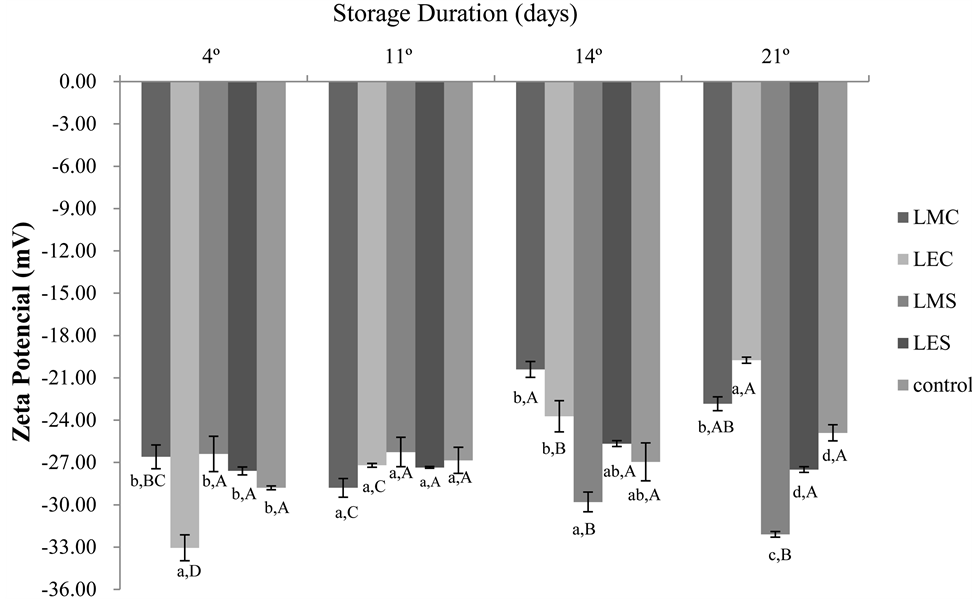
Figure 1. Suspension stability of liposomes comprised of a phenolic compound Spirulina LEB-18 and Chlorella pyrenoidosa in 21 days of storage. Different lowercase lettersof samples differ from each other in the same day and uppercase letters of the sample differs between days, by Tukey test (p ≤ 0.05). LMS = liposome with methanol extract of Spirulina; LES = liposome with ethanolic extract of Spirulina; LMC = liposome with methanol extract of Chlorella; LEC = liposome with ethanol extract of Chlorella.
suspension because large repulsive forces tend to prevent aggregation due to incidental collisions of adjacent nanoparticles [9,38]. The aggregation of liposomes occurs more often for larger structures due to van der Waals interactions that depend on the contact surface. This process is not inevitable for charged structures, so the use of charged lipids in the liposome formulation is an alternative to reducing this phenomenon [39]. Knowledge of the zeta potential of a liposome can help predict the stability and the fate of liposomes in vivo [40]. Phospholipids (lecithins) and the constituent polymers of the nanoparticles are the main components present in the formulations capable of influencing the zeta potential. The liposomes composed of charged polar lipids with higher electric charges can be expected to be more stable than liposomes composed of neutral polar lipids [9]. Caddeo et al. [41] evaluated liposomes prepared with lecithin and the ethanol extract of resveratrol, as an active, and obtained the value of −44.5 mV for the zeta potential.
4. Conclusion
The methanol extracts showed higher levels of total phenolics compared to ethanol extracts. The Spirulina microalgae showed higher phenolic contents than Chlorella. The phenolic compounds of microalgae were satisfactorily adhered to lipid vesicles, especially with regard to nanometric-sized and high encapsulation efficiency for all samples. However, it can be seen that the ethanolic extracts are better able to interact with the liposomes, which can be attributed to the higher lipophilicity of the compounds, compared to the methanol extracts, and are shown to be more stable during 21 days of storage. The results suggest that the use of liposomes can be an interesting alternative and applicable in the encapsulation of bioactive compounds in order to protect them and improve their effectiveness. However, more research is needed with respect to the bioavailability of these compounds encapsulated in vitro and in vivo.
Acknowledgements
The CAPES, Brazil for the financial support that made this study possible.
REFERENCES
- P. Goyal, K. Goyal, S. G. V. Kumar, A. Singh, O. P. Katare and D. N. Mishra, “Liposomal Drug Delivery Systems E Clinical Applications,” Actapharmaceutica, Vol. 55, No. 1, 2005, pp. 1-25.
- A. Jesorka and O. Orwar, “Liposomes: Technologies and Analytical Applications,” AnnualReview of Analytical Chemistry, Vol. 1, 2008, pp. 801-832. http://dx.doi.org/10.1146/annurev.anchem.1.031207.112747
- A. K. Thompson, J. P. Hindmarsh, D. Haisman, T. Rades and H. Singh. “Comparison of the Structure and Properties of Liposomes Prepared from Milk Fat Globule Membrane and Soy Phospholipids,” Journal of Agriculture and Food Chemistry, Vol. 54, No. 10, 2006, pp. 3704- 3711. http://dx.doi.org/10.1021/jf052859b
- M. R. Mozafari, C. Johnson, S. Hatziantoniou and C. Demetzos, “Nanoliposomes and their applications in food nanotechnology,” Journal of liposome research, Vol. 18, No. 4, 2008, pp. 309-327. http://dx.doi.org/10.1080/08982100802465941
- M. R. Mozafari, “Liposomes: An Overview of Manufacturing Techniques,” Cellular & Molecular Biology Letters, Vol. 10, No. 4, 2005, pp. 711-719.
- J. F. Nagle and S. Tristram-Nagle, “Structure of Lipid Bilayers,” Biochimica et Biophysica Acta, Vol. 1469, No. 3, 2000, pp. 159-195. http://dx.doi.org/10.1016/S0304-4157(00)00016-2
- M. Fathi, M. R. Mozafari and M. Mohebbi, “Nanoencapsulation of Food Ingredients Using Lipid Based Delivery Systems,” Trends in Food Science & Technology, Vol. 23, No. 1, 2012, pp. 13-27. http://dx.doi.org/10.1016/j.tifs.2011.08.003
- C. C. Liolios, O. Gortzi,S. Lalas, J. Tsaknis and I. Chinou, “Liposomal Incorporation of Carvacrol and Thymol Isolated from The Essential Oil of Origanumdictamnus L. and in Vitro Antimicrobial Activity,” Food Chemistry, Vol. 112, No. 1, 2009, pp. 77-83. http://dx.doi.org/10.1016/j.foodchem.2008.05.060
- P. S. Malheiros, Y. M. S. Micheletto, N. P. Silveira and A. Brandelli, “Development and Characterization of Phosphatidylcholinenanovesicles Containing the Antimicrobial Peptide Nisin,” Food Research International, Vol. 43, No. 4, 2010, pp. 1198-1203. http://dx.doi.org/10.1016/j.foodres.2010.02.015
- C. H. Peng, C. H. Chang, R. Y. Peng and C. C. Chyau, “Improved Membrane Transport of Astaxanthine by Liposomal Encapsulation,” European Journal of Pharmaceutics and Biopharmaceutics, Vol. 75, No. 2, 2010, pp. 154-61. http://dx.doi.org/10.1016/j.ejpb.2010.03.004
- V. Sant’anna, P. S. Malheiros and A. Brandelli, “Liposome Encapsulation Protects Bacteriocin-Like Substance P34 against Inhibition by Maillard Reaction Products,” Food Research International, Vol. 44, No. 1, 2011, pp. 326-330. http://dx.doi.org/10.1016/j.foodres.2010.10.012
- M. Marsanasco, A. L. Márquez, J. R. Wagner, S. V. Alonso and N. S. Chiaramoni, “Liposome as Vehicles for Vitamins E and C: An Alternative to Fortify Orange Juice and Offer Vitamin C Protection after Heat Treatment,” Food Research International, Vol. 44, No. 9, 2011, pp. 3039-3046. http://dx.doi.org/10.1016/j.foodres.2011.07.025
- L. Wechtersbach, N. P. U. lrih and B. Cigic, “Liposomal Stabilization of Ascorbic Acid in Model Systems and in Food Matrices,” LWT—Food Science and Technology, Vol. 45, No. 1, 2012, pp. 43-49. http://dx.doi.org/10.1016/j.lwt.2011.07.025
- M. Plaza, S. Santoyo, L. Jaime, B. Avalo, A. Cifuentes, G. Reglero, G. G-B. Reina, F. J. Señoráns and E. Ibáñez, “Comprehensive Characterization of the Functional Activities of Pressurized Liquid and Ultrasound-Assisted Extracts from Chlorella vulgaris,” LWT—Food Science and Technology, Vol. 46, No. 1, 2012, pp. 245-253. http://dx.doi.org/10.1016/j.lwt.2011.09.024
- R. Pangestuti and S. K. Kim, “Biological Activities and Health Benefit Effects of Natural Pigments Derived from Marine Algae,” Journal of functional foods, Vol. 3, No. 4, 2011, pp. 255-266. http://dx.doi.org/10.1016/j.lwt.2011.09.024
- H. B. Li, K. W. Cheng, C. C. Wong, K. W. Fan, F. Chen and Y. Jiang, “Evaluation of Antioxidant Capacity and Total Phenolic Content of Different Fractions of Selected Microalgae,” Food Chemistry, Vol. 102, No. 3, 2007, pp. 771-776. http://dx.doi.org/10.1016/j.foodchem.2006.06.022
- M. Plaza, A. Cifuentes and E. Ibáñez, “In the Search of New Functional Food Ingredients from Algae,” Trends in Food Science & Technology, Vol. 19, No. 1, 2008, pp. 31-39. http://dx.doi.org/10.1016/j.tifs.2007.07.012
- M. S. Mirand, R. G. Cintra, S. B. Barros and J. ManciniFilho, “Antioxidant Activity of the Microalga Spirulina maxima,” Brazilian Journal of Medical and Biological Research, Vol. 31, No. 8, 1998, pp. 1075-1079. http://dx.doi.org/10.1590/S0100-879X1998000800007
- M. G. Morais, E. M. Radmann, M. R. Andrade, G. G, Teixeira, L. R. F. Brusch and J. A. V. Costa, “Pilot Scale Semicontinuous Production of Spirulina Biomass in Southern Brazil,” Aquaculture, Vol. 294, No. 1-2, 2009, pp. 60-64. http://dx.doi.org/10.1016/j.aquaculture.2009.05.009
- L. M. Colla, E. B. Furlong and J. A. V. Costa, “Antioxidant Properties of Spirulina (Arthospira) platensis Cultivated under Different Temperatures and Nitrogen Regimes,” Brazilian Archives of Biology and Technology, Vol. 50, No. 1, 2007, pp. 161-167. http://dx.doi.org/10.1590/S1516-89132007000100020
- Z. Fang and B. Bhand ari, “Encapsulation of Polyphenols—A Review,” Trends in Food Science & Technology, Vol. 21, No. 10, 2010, pp. 510-523. http://dx.doi.org/10.1016/j.tifs.2010.08.003
- J. A. V. Costa, L. M. Colla, P. D. Filho, K. Kabke and A. Weber, “Modelling of Spirulinaplatensis Growth in Fresh Water Using Response Surface Methodology,” World Journal of Microbiology and Biotechnology, Vol. 18, No. 7, 2002, pp. 603-607.
- M. M. Souza, L. Prietto, A. C. Ribeiro, T. D. Souza and E. Badiale-Furlong, “Assessment of the Antifungal Activity of spirulina platensis Phenolic Extract against aspergillus flavus,” Ciência e Agrotecnologia, Vol. 35, No. 6, 2011, pp. 1050-1058.
- M. M. Souza, V. M. Recart, M. Rocha, E. P. Cipolatti and E. Badiale-Furlong, “Study on the Extracting Conditions of Phenolic Compounds from Onion (Allium cepa L.),” Revista do Instituto Adolfo Lutz, Vol. 68, No. 2, 2009, pp. 192-200.
- M. L. Teixeira, J. Santos, N. P. Silveira and A. Brandelli, “Phospholipid Nanovesicles Containing a Bacteriocin-Like Substance for Control of Listeria monocytogenes,” Innovative Food Science and Emerging Technologies, Vol. 9, No. 1, 2008, pp. 49-53. http://dx.doi.org/10.1016/j.ifset.2007.05.001
- S. E. Soares, “Ácidos Fenólicos Como Antioxidantes,” Revista da Nutrição, Vol. 15, No. 1, 2002, pp. 71-81. http://dx.doi.org/10.1590/S1415-52732002000100008
- E. A. Shalaby, S. M. M. Shanab and V. Singh, “Salt Stress Enhancement of Antioxidant and Antiviral Efficiency of Spirulina platensis,” Journal of Medicinal Plants Research, Vol. 4, No. 24, 2010, pp. 2622-2632.
- N. Siriwardhana, K. W. Lee, S. H. Kim, J. W. Ha and Y. J. Jeon, “Antioxidant Activity of Hizikia fusiformis on Reactive Oxygen Species Scavenging and Lipid Peroxidation Inhibition,” Food Science and Technology International, Vol. 9, No. 6, 2003, pp. 339-346. http://dx.doi.org/10.1177/1082013203039014
- L. Cepoi, L. Rudi, V. Miscu, A. Cojocari, T. Chiriac and D. Sadovnic, “Antioxidative Activity of Ethanol Extracts from Spirulina platensis and Nostoc Linckia Measured by Various Methods,” Fascicula Biologie, Vol. 16, No. 2, 2009, pp. 43-48.
- K. Manivannan, P. Anantharaman and T. Balasubramanian, “Evaluation of Antioxidant Properties of Marine Microalgae chlorella marina (Butcher, 1952),” Asian Pacific Journal of Tropical Biomedicine, Vol. 2, No. 1, 2012, pp. 342-346. http://dx.doi.org/10.1016/S2221-1691(12)60185-3
- A. Wojdylo, J. Oszmianski and R. Czemerys, “Antioxidant Activity and Phenolic Compounds in 32 Selected Herbs,” Food Chemistry, Vol. 105, No. 3, 2007, pp. 940- 949. http://dx.doi.org/10.1016/j.foodchem.2007.04.038
- D. Nemen and E. Lemos-Senna, “Preparação e Caracterização de Suspensões Coloidais de Nanocarreadores Lipídicos Contendo Resveratrol Destinados à Administra- ção Cutânea,” Química Nova, Vol. 34, No. 3, 2011, pp. 408-413. http://dx.doi.org/10.1590/S0100-40422011000300008
- M. Takahashi, S. Uechi, K. Takara, Y. Asikin and K. Wada, “Evaluation of an Oral Carrier System in Rats: Bioavailability and Antioxidant Properties of LiposomeCapsulated Curcumin,” Journal of Agricultural and Food Chemistry, Vol. 57, No. 19, 2009, pp. 9141-9146. http://dx.doi.org/10.1021/jf9013923
- A. Priprem, J. Watanatorn, S. Sutthiparinyanont, W. Phachonpai, S. Muchimapura, “Anxiety and Cognitive Effects of Quercetin Liposomes in Rats,” Nanomedicine: Nanotechnology, Biology and Medicine, Vol. 4, No. 1, 2008, pp. 70-78. http://dx.doi.org/10.1016/j.nano.2007.12.001
- R. B. Pegg and F. Shahidi, “Encapsulation, Stabilization, and Controlled Release of Food Ingredients and Bioactives,” 2nd Edition, Hand Book of Food Preservation, CRC Press, Boca Raton, 2007.
- S. E. Acosta, “Regulatory Aspects of Nutrient Delivery. Part IV: Regulatory Issues and Future Trends,” University of Toronto, Toronto, 2008.
- H. Ferreira, M. Lúcio, C. Siquet and S. Reis, “Utilização de Modelos Membranares na Avaliação da Actividade de Fármacos” Boletim da Sociedade Portuguesa de Química, No. 99, 2005, pp. 39-51.
- S. R. Schaffazick, S. S. Guterres, L. L. Freitas and A. R. Pohlmann, “Caracterização e Estabilidade Físico-Química de Sistemas poliméricos Nanoparticulados Para Administração de Fármacos,” Química Nova, Vol. 26, No. 5, 2003, pp. 726-737. http://dx.doi.org/10.1590/S0100-40422003000500017
- D. C. Drummond, O. Meyer, K. Hong, D. B. Kirpotin, D. Papahadjopoulos, “Optimizing Liposomes for Delivery of Chemotherapeutic Agents to Solid Tumors,” Pharmacological reviews, Vol. 51, No. 4, 1999, pp. 691-744.
- B. Ruozi, G. Tosi, F. Forni, M. Fresta and M. A. Vandelli, “Atomic Force Microscopy and Photon Correlation Spectroscopy: Two Techniques for Rapid Characterization of Liposomes,” European Journal of Pharmaceutical Sciences, Vol. 25, No. 1, 2005, pp. 81-89. http://dx.doi.org/10.1016/j.ejps.2005.01.020
- C. Caddeo, K. Teskac, C. Sinico and J. Kristl, “Effect of Resveratrol Incorporated in Liposomes on Proliferation and UV-B Protection of Cells,” International Journal of Pharmaceutics, Vol. 363, No. 1-2, 2008, pp. 183-191. http://dx.doi.org/10.1016/j.ijpharm.2008.07.024
Abbreviations
MES: methanolic extracts of Spirulina; MEC: methanolic extracts of Chlorella; EES: ethanolic extracts of Spirulina; EEC: ethanolic extracts of Chlorella; LMS: liposome with methanol extract of Spirulina; LES: liposome with ethanolic extract of Spirulina; LMC: liposome with methanol extract of Chlorella; LEC: liposome with ethanol extract of Chlorella.


