Open Journal of Marine Science
Vol.3 No.2A(2013), Article ID:33657,6 pages DOI:10.4236/ojms.2013.32A002
Feeding and Growth Characteristics of a Diatom-Feeding Flagellate Isolated from the Bottom Sediment of Onagawa Bay, Northeastern Japan
Graduate School of Agricultural Science, Tohoku University, Sendai, Japan
Email: hohno@bios.tohoku.ac.jp
Copyright © 2013 Hiromasa Ohno et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 9, 2013; revised May 14, 2013; accepted May 27, 2013
Keywords: Diatom-Feeding; Heterotrophic Nanoflagellate; Growth; Grazing Impact; Pelagic-Benthic Coupling
ABSTRACT
In recent years, several marine heterotrophic nanoflagellates have been reported to feed on diatoms much larger than themselves. However, very little is known about the grazer-prey relationships between heterotrophic nanoflagellates and diatoms in aquatic ecosystems. A diatom-feeding flagellate was discovered in the bottom sediment of Onagawa Bay. It fed and proliferated on diatoms, especially Skeletonema costatum sensu lato. This study determined growth and ingestion rates of the flagellate on Skeletonema costatum s.l. under laboratory conditons. Its grazing pressure on the sinking flux of S. costatum s.l. was calculated by combining field data. Specific growth rate of the flagellate increased with increasing prey concentration. The maximum specific growth rate was 0.79 d–1 and maximum ingestion rate of the flagellate was 0.66 ng·C·grazer–1·d–1. Calculated grazing pressure of the flagellate on S. costatum s.l. flux was up to 25.6%. The results imply that the flagellate has a considerable grazing impact on S. costatum s.l. flux at least in a restricted season of the year. Therefore, the present study suggests that this benthic flagellate plays an important role in pelagic-benthic coupling in Onagawa Bay.
1. Introduction
Heterotrophic nanoflagellates have generally been considered to be grazers of bacteria [1]. Thus, they were not thought to be herbivorous protists like ciliates and dinoflagellates [2,3]. In recent years several marine heterotrophic nanoflagellates have been reported to feed on diatoms much larger than themselves [4-6]. Therefore, some nanoflgellates may play an important but underestimated role in the control of phytoplankton assemblages. Onagawa Bay is the southernmost embayment on the Pacific coast of the Sanriku District in northeastern Japan. It is known that three major currents are present off Onagawa Bay: the cold-water Oyashio Current, the warm-water Kuroshio Current and the Tsugaru Warm Current ([7] and reference therein). Water in the bay is affected directly and indirectly by these currents seasonally. Planktonic diatoms are major components in coastal waters where environmental conditions fluctuate drastically. In particular, the diatom Skeletonema costatum s.l. is thought to comprise a cosmopolitan species group and is also one of the dominant diatom species in Onagawa Bay [8]. Recently, Masuda [9] found a diatom-feeding flagellate in the bottom sediment. It could feed and grow on S. costatum s.l. Unfortunately, light microscopy observation alone was insufficient to identify it at the species level, and its identification in detail is currently in preparation. Masuda [9] also suggested that the abundance of the flagellate is positively correlated with the sinking flux of diatoms in Onagawa Bay. Therefore, it is postulated that the flagellate feeds on sinking diatoms and increases its population. However, the magnitude of the growth rate of the flagellate and the grazing impact on sinking flux of diatoms are not known. Hence the aim of this study is to investigate the biological characteristics of this diatom-feeding flagellate and its ecological role in the marine food web of Onagawa Bay.
2. Materials and Methods
2.1. Sample Collection
Mud samples were collected from St. 1 (38˚26'14''N, 141˚27'83''E, ca. 20 m deep) in Onagawa Bay, Miyagi Prefecture on 22 May, 2007. Masuda [9] isolated one cell of the flagellate from the mud sample (Figure 1) and it was cultured using ESNW medium [10] in seawater with a 12:12 light:dark cycle at 15˚C with the diatom Skeletonema costatum s. l. as a food source.
2.2. Growth and Ingestion Rates
This experiment was designed to measure growth and ingestion rates of the flagellate as a function of the prey S. costatum s.l. concentration. The flagellates in stock culture growing on S. costatum s.l. under a 12:12 h light: dark cycle in ESNW medium were transferred to a 30 ml bottle. Before conducting experiments, the flagellate culture was left unfed for 24 hours to reduce the residual prey concentration in the culture. Three 1 - ml subsamples were taken from the bottle and the cell concentration of the flagellate was determined using a microscope, and the culture was then used for experiments, which were conducted in triplicate.The initial concentrations of the flagellate and the diatom were established as follows. Experimental bottles contained a mixture of grazer (the flagellate, 30 cells·ml–1) and prey (S. costatum s.l., 1000, 5000, 10,000, and 20,000 cells·ml–1). Control bottles contained the same densities of prey but no flagellates. After dispensing 1 ml from each bottle to each well, the culture plate was incubated for 3 days at 15˚C in the dark, mimicking conditions at the seabed in autumn, when the flagellate is most abundant [9]. At the end of the experiment, each well was fixed with 10% glutaraldehyde to a final concentration of 2% and S. costatum s.l. and the flagellate abundances were counted using an inverted mi-

Figure 1. Scanning electron micrograph of the diatomfeeding flagellate. The specimen is smaller than average probably because of shrinkage during preparation. Scale bar 9 µm.
croscope (Olympus IX70). The flagellate was observed to dwell on the bottom of culture plates, so it is thought to utilize only diatoms settled on the bottom.
The specific growth rate of the flagellate, µ(d–1), was calculated as follows:,
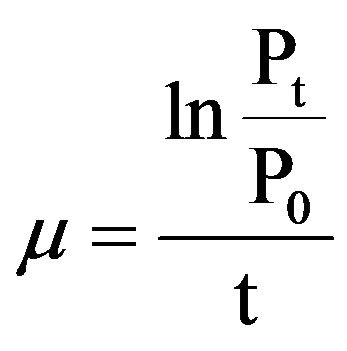
where P0 and Pt denote the flagellate densities at the beginning and end, respectively, of the incubation. The relationship between the specific growth rate of the flagellate and the initial prey concentration was fitted to a Michaelis-Menten equation:
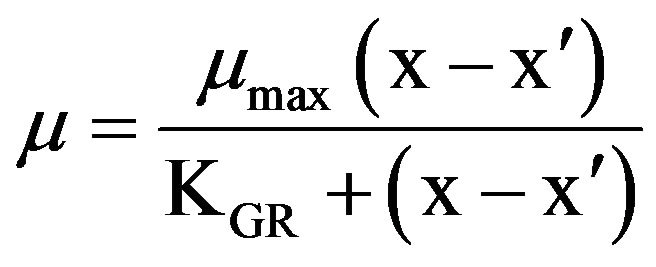
where µmax is the maximum growth rate (d–1), x is the prey concentration on the bottom surface of the well (cells·cm–2), x’ is the threshold prey concentration (the prey concentration where µ = 0), and KGR is the prey concentration sustaining 1/2 µmax.
Ingestion rates were calculated from cell counts of the experimental and control wells [11]. The growth constant, k, for prey was calculated from
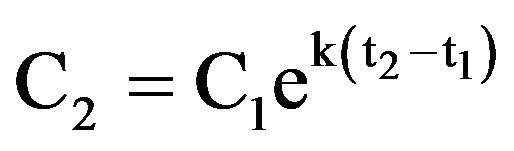
where C1 and C2 are prey concentrations (cells cm–2) in the control wells at times t1 and t2, respectively. For each well with grazers, the grazing coefficient, g, was calculated from
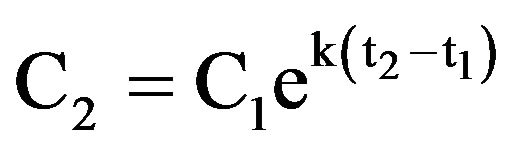
where  and
and  are prey concentrations in a well with grazers at times t1 and t2, respectively. Using values of k and g, the average cell concentration,
are prey concentrations in a well with grazers at times t1 and t2, respectively. Using values of k and g, the average cell concentration,
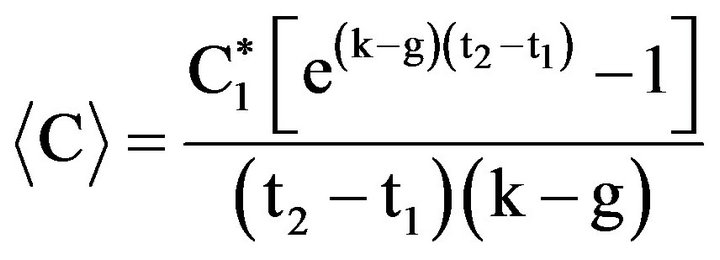
The ingestion rate, IR, is then

where V is the volume (ml) of the medium and N is the mean number of flagellates in the well from t1 to t2. The incubation time for calculating the ingestion rate was the same as for estimating the growth rate. Ingestion rate data were fitted to a Michaelis-Menten equation:
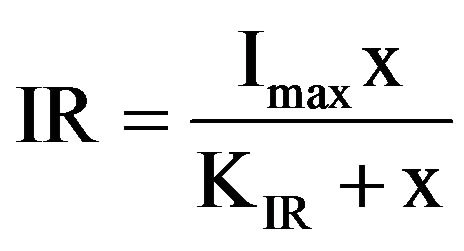
where Imax is the maximum ingestion rate (d–1), x is the prey concentration (cells·cm–2), and KIR is the prey concentration sustaining 1/2 Imax.
Cell length and width of S. costatum s.l. were measured using an inverted microscope (OLYMPUS IX70), and the cell volume was calculated according to Miyai [12]. The carbon content of S. costatum s.l. was estimated from cell volume according to Strathmann [13]
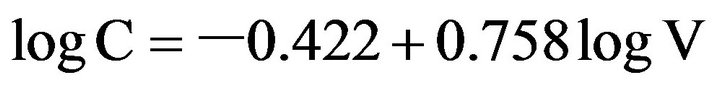
where C is the cell carbon (pg C),V is the cell volume (µm3).
The cell volume of the flagellate (V, µm3) was calculated as follows:
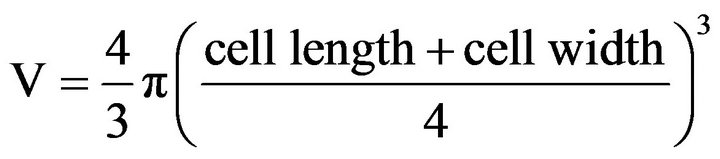
The carbon content of the flagellate (C, pg C) was estimated from cell volume (V, µm3) according to MendenDeuer [14].

Gross growth efficiency (GGE), defined as grazer biomass produced per prey biomass ingested, was calculated from estimates of carbon content per cell based on cell volume for each mean prey concentration.
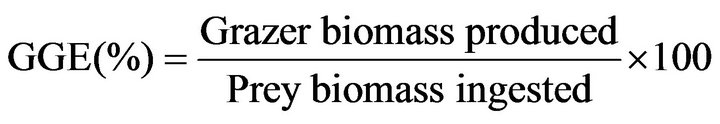
The grazing impact of the flagellate was estimated using field data on the abundance of the flagellate and S. costatum s.l. and the ingestion rates of the flagellate obtained in this study.
3. Results
The isolated flagellate was broadly oval in shape with a longitudinal groove, and had a diameter of approximately 17 µm (Figure 1).
The sspecific growth rate of the heterotrophic flagellate increased with increasing prey concentration before saturating at a S. costatum s.l. concentration of 15,000 cells cm–2 (220 ng·C·cm–2, Figure 2). The maximum specific growth rate (µmax) and half-saturation constant (KGR) of the flagellate were 0.79 d–1 and 113.7 ng·C·cm–2, respectively. The threshold prey concentration (where net growth = 0) for the flagellate was 11.3 ng·C·cm–2. The Michaelis-Menten equation for the relationship between prey concentration (x) and growth rate (µ) was
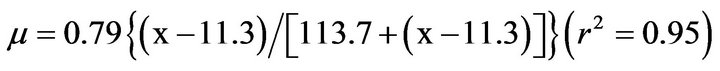 .
.
The ingestion rate of the flagellate on S. costatum s.l. increased with increasing mean prey concentration (Figure 3). The maximum ingestion rate (IRmax) and halfsaturation constant (KIR) of the flagellate were 0.66
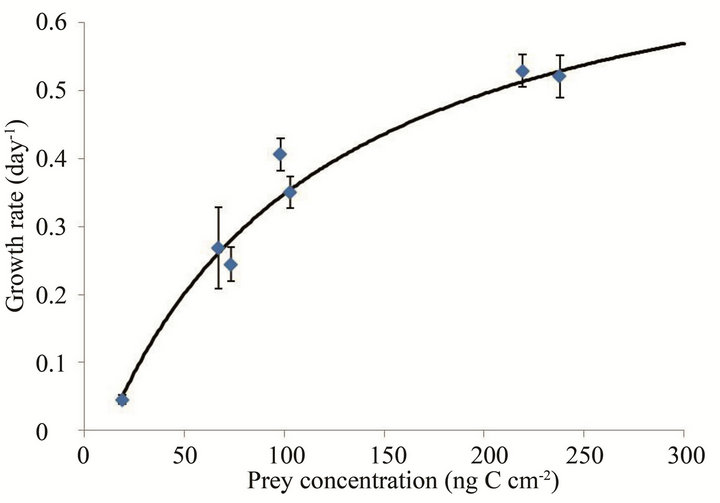
Figure 2. Growth rates (µ, day–1) of the flagellate on S. costatum s.l. as a function of mean prey concentration (x, ng·C·cm–2). Symbols represent treatment means ± 1 SE. The fitted equation was µ = 0.79 {(x – 11.3)/[113.7 + (x – 11.3)]} (r2 = 0.95).
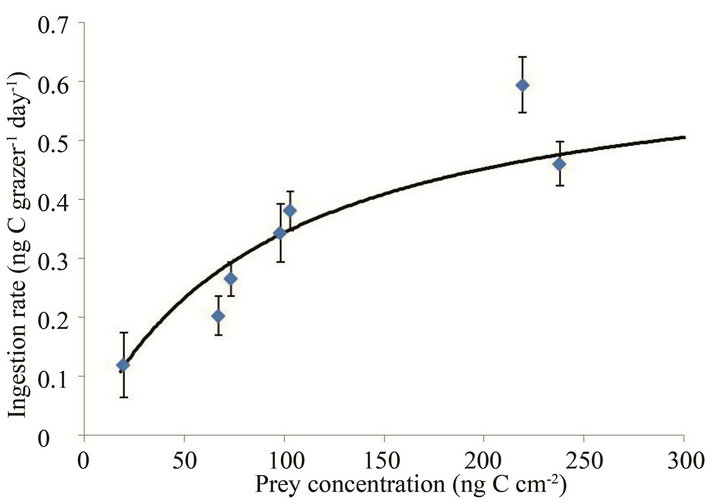
Figure 3. Ingestion rates (IR, ng·C·grazer–1·day–1) of the flagellate on S. costatum s.l. as a function of mean prey concentration (x, ng·C·cm–2). Symbols represent treatment means ± 1 SE. The fitted equation was IR = 0.66 x/(92.0 + x) (r2 = 0.81).
ng·C·grazer–1·d–1 (45 cells grazer–1·d–1) and 92.0 ng·C·cm–2, respectively. The Michaelis-Menten equation for the relationship between prey concentration (x) and ingestion rate (IR) was
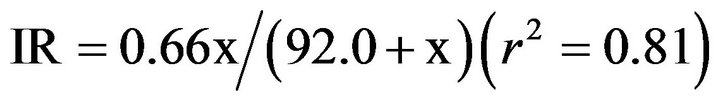 .
.
The GGE of the flagellate on S. costatum s.l. ranged from 13.9% to 45.9% (Figure 4). The maximum efficiency was observed at 67 ng·C·cm–2.
4. Discussion
The specific growth rates observed in this study were compared with those reported for dinoflagellate and benthic ciliates ([15-17] and reference therein), since very little is known about the growth rates of flagellates
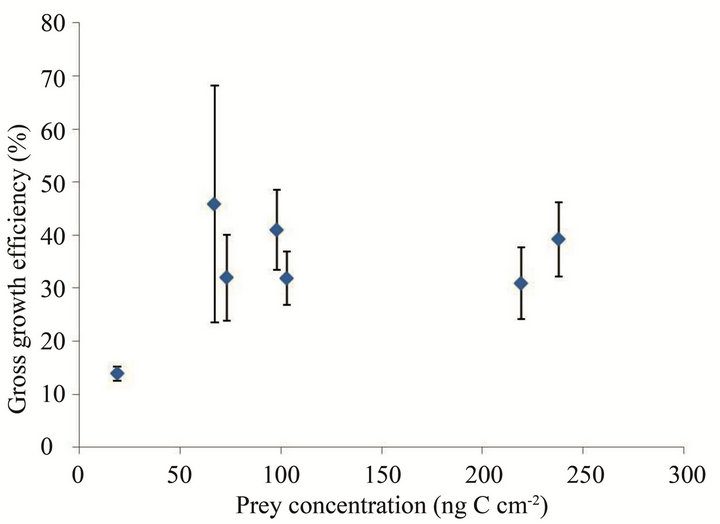
Figure 4. Gross growth efficiency (GGE), defined as the flagellate biomass produced per S. costatum s.l. biomass ingested, as a function of mean prey concentration (x, ng·C·cm–2). Symbols represent treatment means ± 1 SE.
that feed on diatoms [18]. Previous investigators have generally reported that growth rates could be described as a Michaelis-Menten function of food concentration [16,19,20]. The results obtained in this study are generally consistent with this growth model in that growth rates increase with increasing food concentrations but then tend to level off above a moderate level of food.
The maximum specific growth rate of the flagellate was higher than that of any other benthic heterotrophic nanoflagellate or ciliates so far reported with the exception of the soil flagellate Heteromita globosa (Table 1). However, the cell volume of H. globosa is 41 µm3, which is more than 60 times smaller than that of the flagellate (2590 µm3).
The ingestion rates obtained in this study were compared with some estimates made previously for protozoans. The functional response (i.e. the ingestion rates as a function of food concentration) of the flagellate is quite similar to that of other grazers studied previously in that ingestion rate increases with increasing food concentrations until a plateau is reached. Jeong et al. [16] and Yoo et al. [19] measured the ingestion rates of the dinoflagellates Protoperidinium bipes, Prorocentrum micans and Gonyaulax polygramma as a function of prey S. costatum s.l. The maximum specific ingestion rates of these grazers on S. costatum s.l. were 2.9, 0.35, and 0.34 ng C grazer–1·d–1, respectively (Table 1). The maximum specific ingestion rate of the flagellate was higher than that of the above mentioned grazers on S. costatum s.l., with the exception of P. bipes, even though the volume of the flagellate (ca. 2,590 µm3) is greater than that of P. bipes (ca. 1000 µm3). The feeding mechanism of the flagellate may be less efficient than that of P. bipes. It is suggested also that the lower swimming speed of grazers might cause lower encounter rates between the grazers and prey
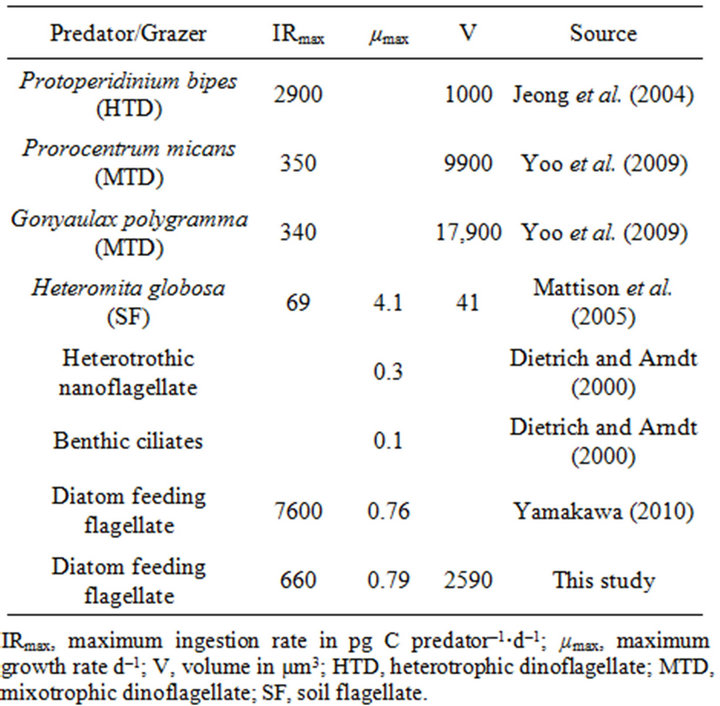
Table 1. Comparison of the ingestion and growth rates of some flagellates, dinoflagellates and ciliates.
[19].
Published growth efficiency estimates for oligotrichous ciliates range from approximately 10% to 80% [21]. In addition, the highest GGE of heterotrophic dinoflagellate Protoperidinium huberi feeding on the diatom Ditylum brightwellii was 59% [22]. The GGE of the flagellate, 14% - 46%, was lower than that of the other protists. It is believed that the GGE of flagellates is highly dependent on prey condition. Food quality can have a considerable effect on oligotrichous ciliate growth [21], and the GGE of heterotrophic nanoflagellates can vary depending on prey condition (density, type, and chemical composition) [23].
The relationship shown in Figure 4 must be considered carefully, because the conversion factor from cell volume to carbon content is available only for bacterivorous flagellates and the cell volume of flagellates may change according to feeding condition. In fact, ciliate cell volumes are reported to be highly responsive to prey concentration ([24] and references therein).
The grazing pressure on S. costatum s.l. was calculated by combining field data and ingestion rate obtained in the present study. Data on the abundance of the flagellate were obtained from Masuda [9], and those on the sinking flux of S. costatum s.l. from Nakamura [8] from February to June 2006 (Table 2). The grazing pressure of the flagellate on S. costatum s.l. averaged 15.5%. Therefore, from these results it can be concluded that the flagellate may have a considerable grazing impact on S. costatum s.l. flux.
The benthic flagellate feeding on the pelagic diatom S. costatum s.l. is an interesting ecological phenomenon.
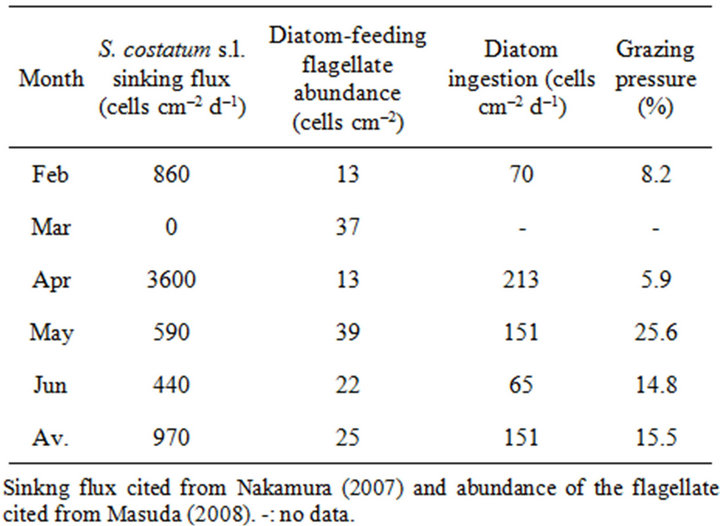
Table 2. Sinking flux of S. costatum s.l., abundance of the diatom-feeding flagellate, consumption of S. costatum s.l. by the flagellates, and grazing pressure from February to June 2006.
Masuda [9] reported that the abundance of the flagellate has a positive correlation with the sinking flux of diatoms in Onagawa Bay. The feeding by the flagellate on the ubiquitous diatom S. costatum s.l. may be of broad importance in marine communities. Prior to the present study, no particularly close relationship was suspected between S. costatum s.l. and benthic flagellates, since their habitats are separated vertically. Accordingly, the results of the present study suggest that the flagellate has an important role in pelagic-benthic coupling.
However, there are some uncertainties arising from this study. Firstly, all the experiments were conducted in the dark, which differs from the natural light-dark cycle even if light is dim on the seabed in autumn, and the prey organism S.costatum s.l. forms resting cells under dark conditions [25]. In this study, vegetative cells were used as prey, since it is presumed that the biomass of the flagellate increases after grazing on sinking diatoms. In nature, the abundance of S. costatum s.l. resting cells is high in the mud in Onagawa Bay [8], so it is important to clarify whether or not the flagellate can graze on resting cells. Secondly, the number of experiments was limited, and therefore the estimate of threshold food concentration is only approximate. Thirdly, the grazing pressure in this study may be overestimated because the calculation was made based only on abundance of the flagellate and the sinking flux of S. costatum s.l. In the wild, diatoms other than S. costatum s.l. will sink, so the flagellate may graze on these other diatoms. Further investigations are needed to address these uncertainties. Finally, the flagellate may be widespread, not restricted to Onagawa Bay, since S. costatum s.l. is a cosmopolitan species group in coastal regions around the world, so its grazers may show a similarly wide distribution.
5. Acknowledgements
We thank Dr. Yoshio Masuda (Miyagi Prefecture Fisheries Technology Institute) for contributing to this study through supplying the diatom-feeding flagellate and Yuta Yamakawa for technical support. We are grateful to Dr. Ian G. Gleadall for his critical comments on and polishing up English in our manuscript. The authors would like to thank the anonymous reviewers for their valuable comments and suggestions to improve the quality of the paper. We also owe a debt of gratitude to Captain Toyokazu Hiratsuka and the staff of the Field Science Center of the Graduate School of Agricultural Science, Tohoku University, for their kind cooperation in helping us to collect samples in Onagawa Bay.
REFERENCES
- E. B. Sherr, B. F. Sherr and J. McDaniel, “Clearance Rates of <6 µm Fluorescently Labeled Algae (FLA) by Estuarine Protozoa: Potential Grazing Impact of Flagellates and Ciliates,” Marine Ecology Progress Series, Vol. 69, 1991, pp. 81-92. doi:10.3354/meps069081
- E. B. Sherr and B. F. Sherr, “Capacity of Herbivorous Protists to Control Initiation and Development of Mass Phytoplankton Blooms,” Aquatic Microbial Ecology, Vol. 57, No. 3, 2009, pp. 253-262. doi:10.3354/ame01358
- E. B. Sherr and B. F. Sherr, “Heterotrophic Dinoflagellates: A Significant Component of Microzooplankton Biomass and Major Grazers of Diatoms in the Sea,” Marine Ecology Progress Series, Vol. 352, 2007, pp. 187- 197. doi:10.3354/meps07161
- G. Drebes, S. F. Kühn, A. Gmelch and E. Schnepf, “Cryothecomonas aestivalis sp. nov., a Colourless Nanoflagellate Feeding on the Marine centric diatom Guinardia delicatula (Cleve) Hasle,” Helgoländer Meeresunters, Vol. 50, No. 4, 1996, pp. 497-515. doi:10.1007/BF02367163
- S. Kühn, L. Medlin and G. Eller, “Phylogenetic Position of the Parasitoid Nanoflagellate Pirsonia Inferred from Nuclear-Encoded Small Subunit Ribosomal DNA and Description of Pseudopirsonia n. gen. and Pseudopirsonia mucosa (Drebes) comb. nov.,” Protist, Vol. 155, No. 2, 2004, pp. 143-156. doi:10.1078/143446104774199556
- E. Schnepf and S. F. Kühn, “Food Uptake and Fine Structure of Cryothecomonas longipes spec. nov., a Marine Nanoflagellate Incertae Sedis Feeding Phagotrophically on Large Diatoms,” Helgoland Marine Reserach, Vol. 54, No. 1, 2000, pp. 18-32. doi:10.1007/s101520050032
- A. Ishikawa and A. Taniguchi, “Vegetative Cell and Cyst Assemblages of Armored Dinoflagellates in Onagawa Bay, Northeast Japan,” Plankton Biology and Ecology, Vol. 47, No. 1, 2000, pp. 12-22.
- S. Nakamura, “Seasonal Change and Sinking of Diatom Community in Onagawa Bay,” Master Thesis, Tohoku University, Sendai, 2007.
- Y. Masuda, “Pelagic and Benthic Microbial Food Webs in Onagawa Bay,” Ph. D. Thesis, Tohoku University, Sendai, 2008.
- P. J. Harrison, R. E. Waters and F. J. R. Taylor, “A Broad Spectrum Artificial Seawater Medium for Coastal and Open Ocean Phytoplankton,” Journal of Phycology, Vol. 16, No. 1, 1980, pp. 28-35.
- B. W. Frost, “Effects of Size and Concentration of Food Particles on the Feeding Behavior of the Marine Planktonic Copepod Calanus pacificus,” Limnology and Oceanography, Vol. 17, No. 6, 1972, pp. 805-815. doi:10.4319/lo.1972.17.6.0805
- H. Miyai, K. Matsuzaki, K. Ogawa and T. Sugihara, “A Simple Method for the Estimation of Phytoplankton Biomass Based on Cell Morphology,” Bulletin of Plankton Society of Japan, Vol. 35, 1988, pp. 121-126.
- R. R. Strathmann, “Estimating the Organic Carbon Content of Phytoplankton from Cell Volume or Plasma Volume,” Limnology and Oceanorgraphy, Vol. 12, No. 3, 1967, pp. 411-418. doi:10.4319/lo.1967.12.3.0411
- S. Menden-Deuer and E. J. Lessard, “Carbon to Volume Relationships for Dinoflagellates, Diatoms, and other Protist Plankton,” Limnology and Oceanography, Vol. 45, No. 3, 2000, pp. 569-579. doi:10.4319/lo.2000.45.3.0569
- D. Dietrich and H. Arndt, “Biomass Partitioning of Benthic Microbes in a Baltic Inlet: Relationships between Bacteria, Algae, Heterotrophic Flagellates and Ciliates,” Marine Ecology, Vol. 136, No. 2, 2000, pp. 309-322. doi:10.1007/s002270050689
- H. J. Jeong, Y. D. Yoo, S. T. Kim and N. S. Kang, “Feeding by the Heterotrophic Dinoflagellate Protoperidinium bipes on the Diatom Skeletonema costatum,” Aquatic Microbial Ecology, Vol. 36, 2004, pp. 171-179. doi:10.3354/ame036171
- R. G. Mattison, H. Taki and S. Harayama, “The Soil Flagellate Heteromita globosa Accelerates Bacterial Degradation of Alkylbenzenes through Grazing and Acetate Excretion in Batch Culture,” Microbial Ecology, Vol. 49, No. 1, 2005, pp. 142-150. doi:10.1007/s00248-003-0226-5
- Y. Yamakawa, “Morphology and Feeding Characteristics of a Diatom-Feeding Flagellate Isolated from the Water Column of Onagawa Bay,” Ph. D. Thesis, Tohoku University, Sendai, 2010.
- Y. D. Yoo, H. J. Jeong, M. S. Kim, N. S. Kang, J. Y. Song, W. Shin, K. Y. Kim and K. Lee, “Feeding by Phototrophic Red-Tide Dinoflagellates on the Ubiquitous Marine Diatom Skeletonema costatum,” Journal of Eukaryotic Microbiology, Vol. 56, No. 5, 2009, pp. 413-420. doi:10.1111/j.1550-7408.2009.00421.x
- S. Menden-Deuer, E. J. Lessard, J. Satterberg and D. Grünbaum, “Growth Rates and Starvation Survival of Three Species of the Pallium-Feeding, Thecate Dinoflagellate Genus Protoperidinium,” Aquatic Microbial Ecology, Vol. 41, No. 2, 2005, pp. 145-152. doi:10.3354/ame041145
- B. Chen, H. Liu and M. T. S. Lau, “Grazing and Growth Responses of a Marine Oligotrichous Ciliate Fed with Two Nanoplankton: Does Food Quality Matter for Micrograzers?” Aquatic Ecology, Vol. 44, No. 1, 2010, pp. 113-119. doi:10.1007/s10452-009-9264-5
- E. J. Burskey, C. J. Coulter and S. L. Brown, “Feeding, Growth and Bioluminescence of the Heterotrophic Dinoflagellate Protoperidinium huberi,” Marine Biology, Vol. 121, 1994, pp. 373-380. doi:10.1007/BF00346747
- J. W. Choi and F. Peters, “Effects of Temperature on Two Psychrophilic Ecotypes of a Heterotrophic Nanoflagellate, Paraphysomonas imperforata,” Applied and Environmental Microbiology, Vol. 58, No. 2, 1992, pp. 593-599.
- J. Laybourn-Parry, “A Functional Biology of Free-Living Protozoa,” University of California Press, California, 1984.
- S. Itakura, I. Imai and K. Itoh, “Morphology and Rejuvenation of Skeletonema costatum (Bacillariophyceae) Resting Cells from the Bottom Sediments of Hiroshima Bay, the Seto Inland Sea, Japan,” Bulletin of Plankton Society of Japan, Vol. 38, No. 2, 1992, pp. 135-145.

