Open Journal of Immunology
Vol.2 No.4(2012), Article ID:26245,5 pages DOI:10.4236/oji.2012.24018
The epidemiology and prevalence of Ulcerative colitis in the South of India
![]()
1Centre for Liver Research and Diagnostics, Deccan College of Medical Sciences, Hyderabad, India
2Society for Translational and Regenerative Medicine, Secunderabad, India; *Corresponding Author: aleemakhan.clrd@gmail.com
3Department of Immunology and Infectious Diseases, AR Zellen GmbH, Hannover, Germany
4Department of Genetics, Osmania University, Hyderabad, India
Received 1 August 2012; revised 1 September 2012; accepted 22 October 2012
Keywords: Ulcerative Colitis; Epidemiology; Prevalence; South India
ABSTRACT
Ulcerative colitis is considered frequent in majority of European and North American population and exceptional in most of the developing Asian countries. There is a dearth of reported data from South India on the incidence of the disease and its prevalence. Hence the present study was designed to estimate the prevalence of Ulcerative colitis in a tertiary care hospital of Hyderabad, South India. The study population consisted of 157 Ulcerative colitis and 204 healthy subjects. All subjects were interviewed by means of a questionnaire for general demographical details and socioeconomic conditions, health related quality of life and history of UC. Patients were categorized based on disease severity; moderate: 95, and severe: 62 and disease manifestation: 73 (46.5%) pancolitis, 60 (38.2%) left-sided colitis and 24 (15.3%) had proctosigmoiditits. Disease prevalence was high in patients of <35 years age. Junk/outside food consumers were significantly high in Ulcerative colitis than controls 68 (43.3%) vs 67 (32.8%) p = 0.048. There was no significant difference of disease prevalence with diet, drinking habits and alcoholic consumption. Whereas UC prevalence was high in nonsmokers than smokers (p = 0.025). This report establishes the importance of various factors in ulcerative colitis. This is the first population based study from South India that reports the prevalence of ulcerative colitis. Ulcerative colitis is more predominant in young age. Further, junk food consumers and Nonsmokers/ex-smokers were found to be high in terms of UC prevalence.
1. INTRODUCTION
Ulcerative colitis (UC) is a chronic idiopathic inflamematory condition of gastrointestinal tract, caused by inappropriate and continuing inflammatory response to gut microbiota on a background of genetic susceptibility [1]. UC is precipitated by a complex interaction of environmental, genetic, and immunoregulatory factors. Family history is a major predisposing factor for UC, although sporadic cases do occur at large [2]. The pathophysiology of UC has been actively investigated recently. The common end pathway is inflammation of the mucosal lining of the intestinal tract, causing ulceration, edema, bleeding, diarrhea and fluid and electrolyte loss. Many inflammatory mediators have been identified in UC, and considerable evidence suggests that these mediators play an important role in the pathologic and clinical characteristic of these disorders. Treatment of Ulcerative colitis depends on the extent and severity of the condition. A mild relapse may be treated with oral corticosteroids or steroidal enemas or suppositories which are directly ad- - ministered to the site of inflammation. Mesalazine and olsalazine has made the management much easier than ever before and are most preferred regimens than sulphasalazine [3]. The global prevalence of UC has seen a discernible shift in the past decade. UC once considered to be common in the Western population has witnessed a relative stable or decreasing trend in Western European region [4]. However, its prevalence has seen an upsurge in previously low incidence areas, such as Asia, Eastern Europe [5-7] and North Indians [8]. More reliable information is available from Asian migrants in Western countries than from Asians in Asia [9]. Only scanty information is available from South Asia, including India. Except for a couple of studies from North India in 1965 and 2003 [8,10] there is a dearth of reports on the present prevalence pattern of UC in India. It is therefore a definite need for descriptive population based epidemiological studies to reflect the true magnitude of the disease, which will define the public health burden of the disease in Indian population. Hence the present study was designed to evaluate the prevalence rate of ulcerative colitis in a population attending a tertiary care hospital in Hyderabad, South India.
2. MATERIALS AND METHODS
A total of 2847 patients attending the Department of Gastroenterology and Hepatology between March 2008 to July 2012 with complaints of irregular bowel movements, bloody diarrhea, and mucous in the stools were retrospectively screened for the present study. Of these, 404 patients agreed to take part in the study and abide by the protocol which included completing health related quality of life (HR-QoL) questionnaire. Among 404 patients, 43 lost to follow-up and only 361 patients completed the study. The major reason for patients lost to follow-up was found to be symptomatic improvement in the disease status. However, a small subset of them withdrew their consent due to personal reasons.
Study subjects with 18 to 60 years of age, histologically and endoscopically confirmed diagnosis of established UC with a Mayo Score of 6 to 12 were enrolled in the present study. Subjects with indeterminate colitis, Crohn’s disease, colonic malignancy, seropositive of human immunodeficiency virus and hepatitis B virus, symptoms of active tuberculosis were excluded from the study.
The study population consisted of 361 patients of which 157 had confirmed with moderate-to-severe UC and 204 were healthy subjects. The demographic and disease characteristics of the enrolled study participants are presented in Table 1. The study population had an essentially equal male/female ratio (88 males to 69 females). All UC patients had a past history of more than 5 years of UC whereas healthy controls were those without any prior history of GI presentations/autoimmune diseases. Diagnosis of UC was done as per conventional clinical, endoscopic and histological criteria [11,12].
The activity of disease was calculated using MAYO score index which consists of four parameters such as stool frequency, rectal bleeding, mucosal appearance and physician’s rating of disease activity. The mean MAYO score of patients with UC at the time of participation was 8.40 ± 1.78 indicating moderate to severe disease. The study protocol was approved by the Institutional Ethics Committee, Deccan College of Medical Sciences, Hyderabad, India. All the study-related procedures were performed after obtaining written and signed informed consent from all the participants.
All subjects were interviewed by means of a questionnaire for general demographical details and socioeconomic conditions, and history of UC. Information about their food habits (intake at home or outside), drinking habits (like consumption of municipal tap water, boiled or filtered water) were asked. Patients were also inquired about smoking and alcohol intake.
Patients with UC were categorized broadly based on the type of disease phenotype (i.e. localization of the disease in the large intestine), histological score and also on the basis of total MAYO for disease activity. However for the present study, only patients with moderate to severe disease activity index were included.
3. RESULTS
The age at which UC was diagnosed ranged between 18 - 75 years. Males and females were equally affected in all age groups (Table 1). Of the total 157 patient with UC, 88 (56%) were males and the rest were females 69 (44%). The proportion of patients with moderate UC was: 95 (males: 58% and females: 63.8%), and severe UC was: 62 (42.0%) males and 25 (36.2%) females had severe UC. Based on the extent of disease manifestation, 73 (46.5%) had pancolitis, 60 (38.2%) had left-sided colitis and 24 (15.3%) had proctosigmoiditits. The mean MAYO score was found to be 8.3 ± 1.7 in males and 8.3 ± 1.7 in female patients according to their disease activity. The prevalence of UC was high in the age group of 18 - 35 years (Table 2).
Of the total 157 UC patients, 89 (57.7%) and 68 (43.3%) preferred home-cooked food and outside food respectively. Among the 204 healthy controls, 67 (32.8%) preferred home-cooked food whereas the remaining and 137 (67.2%) had outside food (UC vs healthy controls p = 0.048) (Table 2). 146 (93%) of the 157 UC patients were non-vegetarian and the rest of the 11 (7%) were vegetarians, On the contrary, in the controls 19 (9.3%) and 185 (90.7%) were vegetarian and non-vegetarian respectively. Evaluation of drinking habits showed 135 (86%) patients used municipal water, and 22 (14%) patients used boiled or filtered water in UC sub-population. Among healthy controls, 177 (86.8%) used municipal water and 27 (13.2%) used boiled or filtered water.
Of the 157 UC patients, only 8 (5.1%) were smokers (significantly low than controls p = 0.025) and among the controls 25 (12.3%) were smokers. Forty-six (29.3%) UC patients consumed alcohol on a regular basis whereas 111 (71.7%) were non-alcoholic. Among the 204 controls, 51 (25%) consumed alcohol and 153 (75%) were non-alcoholic.
Sensitivity to gluten-rich diet was found in 35 (22.2%) UC patients and 29 (14.2%) healthy controls.
4. DISCUSSION
Since there is a real dearth of epidemiological studies in India except for few single centre studies reported from north India [8], there is a desperate need to evaluate the exact magnitude of ulcerative colitis prevalence in Indian sub-population. This is the first study which evaluated the prevalence of UC in typical South Indian population at a tertiary care hospital. The results of the present study demonstrated the effect of parameter such as age, gender, eating habits, gluten rich food, drinking habits,
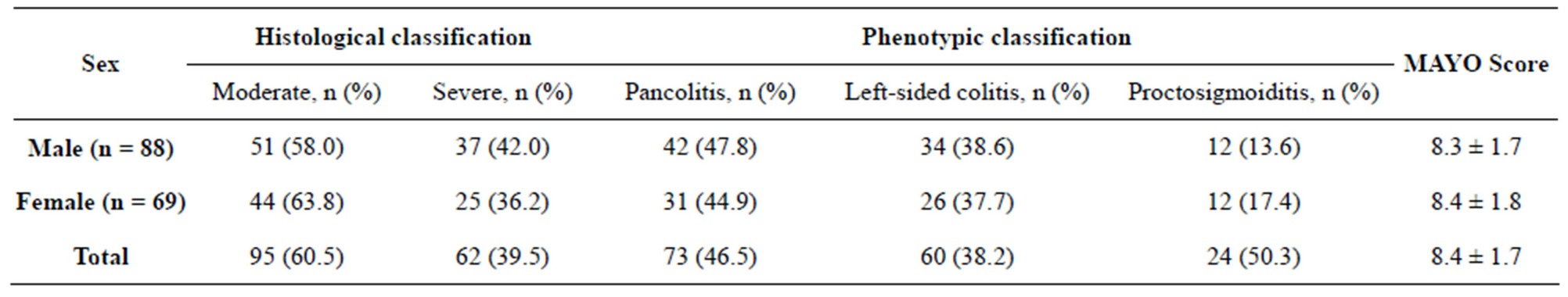
Table 1. UC categorization by disease type and severity.
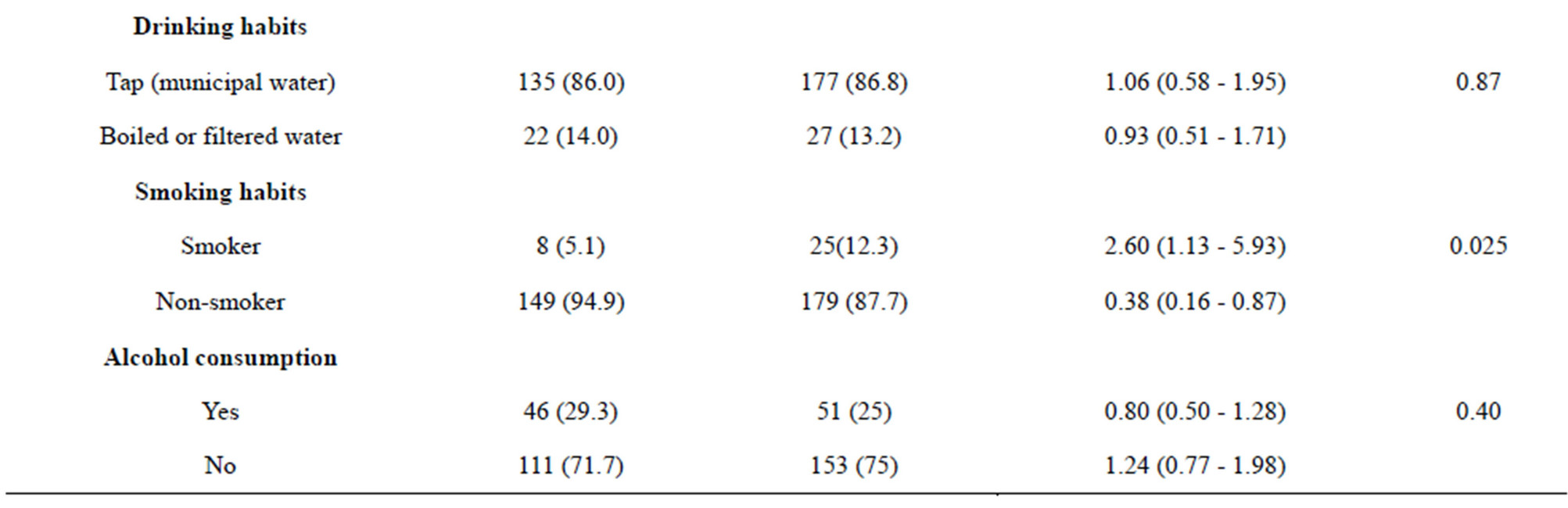
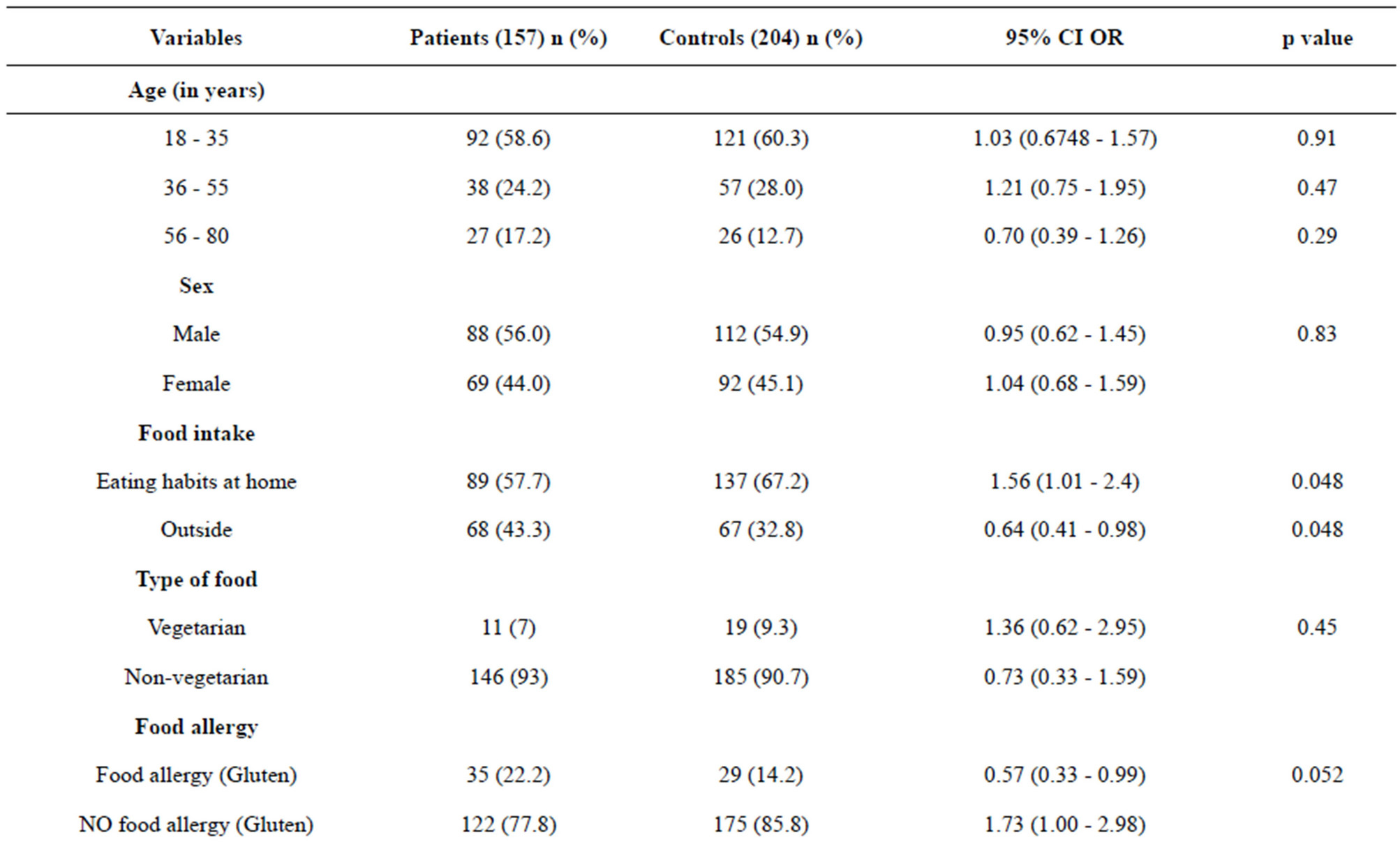
Table 2. Multivariate analysis of different variables and its association with UC.
smoking and consumption of alcohol etc., which may contribute in the development of UC.
The tertiary care hospital where the study was carried out is one of the important hospitals especially known for its Gastroenterology specialty. Due to its strategic location, patients of diverse background and religion seek medical facilities for an array of diseases. For this study, data regarding various parameters such as age, gender, drinking and smoking habits, alcohol consumption etc. were collected from individual patients with an history of ulcerative colitis and naïve patients who had attended the department of Gastroenterology for their lower abdominal discomfort.
Disease prevalence was high (58.6%) in patients between 18 to 35 years of age. There was no much difference in the prevalence observed among the patients with ages between 36 - 55 years and 56 - 80 years (24.4% vs 17.2%). In our study, males and females were equally affected which is in line with those reported by Langan et al., (2007) [13] which reported ulcerative colitis to be most common in 15 and 40 years of age, with a second peak in incidence between 50 and 80 years.
Eating habit contributed significantly to the UC prevalence as in our study, patients taking food outside had high risk to develop UC than the controls (43.3% vs 32.84%; p = 0.045) This is very important index as many factors such as unhygienic food, reuse of oils, spicy food and other fast food (junk food), has been reported to cause various intestinal health problems [14]. However, type of food taken (i.e. vegetarian vs non-vegetarian) by the study population was not found to be a predisposing factor for UC.
Sensitivity to gluten-rich food is one of the important factors that triggers inflammation [15]. Many prospective studies have already demonstrated that patients who avoid gluten-rich food products have less number of relapses per year [16]. In our study a similar trend was observed wherein 22.2% were found to be allergic to gluten-rich diet compared to healthy controls (14.2%), and this was found to be statistically significant (p = 0.05). These observations are supported by Gillberg et al., (1982) [16] that demonstrated exposure to gluten significantly increased the number of UC relapse.
Evaluation of source of drinking water had no impact in the study population as subjects who resorted to municipal tap water and those who utilized boiled or filtered water had almost similar prevalence (p = 0.87). This is in contrast to Aamodt et al., (2008) [17] that reported positive association of the content and quality of drinking water and the incidence of inflammatory bowel disease.
Active tobacco smoking has been recognized as one of the most prominent protective factors against ulcerative colitis, and ex-smokers have an increased risk of developing UC reported by Bastida et al., (2011) [18]. The present study found UC to be most prevalent among nonsmokers than the smokers for the development of UC (94.9% vs 5.1%; p = 0.025). These findings are well in line with the study done by Samuelsson et al., (1991) [19]. One of the reasons attributed to this may be nicotine which increases mucin synthesis in colon and rectum in UC patients and hence may impart protection to the epithelium [20]. However, the increased prevalence of UC among non-smokers/ex-smokers in our study population deems further research to be undertaken.
Alcohol consumption is also a potential trigger for flares in UC because of pro-oxidant effects exerts deleterious effects on gut barrier function [21]. The data obtained from our study however showed lack of association between alcohol intake and UC (p = 0.40).
5. CONCLUSION
In conclusion, results of the present study suggest prevalence of ulcerative colitis to be predominant at the young age (<35 years). Further, gluten-rich diet and outside food were found to be important factors responsible for increased prevalence in our study population. Nonsmokers/ex-smokers were found to be high in terms of UC prevalence; this however warrants series of studies to be designed to evaluate the epicentre of trigger especially among the non-smoking population.
6. ACKNOWLEDGEMENTS
We would also like to thank Ms. Anuradha, Department. of Genetics, Osmania University her help in performing statistical analysis of data and interpretation. The study was not supported by any intraor extra mural funds.
REFERENCES
- Danese, S. and Fiocchi, C. (2006) Etiopathogenesis of inflammatory bowel diseases. World Journal of Gastroenterology, 12, 4807-4812.
- Peeters, M., Cortot, A., Vermeire, C., et al. (2000) Familial and sporadic inflammatory bowel disease: Different entities. Inflammatory Bowel Diseases, 6, 314-320.
- Kefalides, P.T. and Hanauer, S.B. (2002) Ulcerative colitis: Diagnosis and management. Journal of Clinical Outcomes Management, 8, 40-48.
- Molinie, F., Gower-Rousseau, C., Yzet, T., et al. (2004) Opposite evolution in incidence of Crohn’s disease and ulcerative colitis in Northern France (1988-1999). Gut, 53, 843-848. doi:10.1136/gut.2003.025346
- Sincic, B.M., Vucelic, B., Persic, M., et al. (2006) Incidence of inflammatory bowel disease in Primorsko-goranska County, Croatia, 2000-2004: A prospective population-based study. Scandinavian Journal of Gastroenterology, 41, 437-444. doi:10.1080/00365520500320094
- Lakatos, P.L., Fischer, S. and Lakatos, L. (2006) Is the epidemiology of inflammatory bowel disease changing in Eastern Europe? Scandinavian Journal of Gastroenterology, 41, 870-871. doi:10.1080/00365520600636167
- Thia, K.T., Loftus Jr., E.V., Sandborn, W.J., et al. (2008) An update on the epidemiology of inflammatory bowel disease in Asia. American Journal of Gastroenterology, 103, 3167-3182. doi:10.1111/j.1572-0241.2008.02158.x
- Sood, A., Midha, V., Sood, N., et al. (2003) Incidence and prevalence of ulcerative colitis in Punjab, North India. Gut, 52, 1587-1590. doi:10.1136/gut.52.11.1587
- Siew, C.N.(2010) Changing epidemiology and future challenges of inflammatory bowel disease in Asia. Intestinal Research, 8, 1-8. doi:10.5217/ir.2010.8.1.1
- Tandon, B.N., Mathus, A.K., Mohapatra, L.N., et al. (1965) A study of the prevalence and clinical pattern of non-specific ulcerative colitis in northern India. Gut, 6, 448-453. doi:10.1136/gut.6.5.448
- Podolsky, D.K. (1991) Inflammatory bowel disease. The New England Journal of Medicine, 325, 928-937. doi:10.1056/NEJM199109263251306
- Satsangi, J., Silverberg, M.S., Vermaire, S., et al. (2006) The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut, 55, 749-753. doi:10.1136/gut.2005.082909
- Langan, R.C., Gotsch, P.B., Krafczyk, M.A., et al. (2007) Ulcerative colitis: Diagnosis and treatment. American Family Physician, 76, 1323-1330.
- Cencic, A. and Chingwaru, W. (2010) The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients, 2, 611-625. doi:10.3390/nu2060611
- Marsh, M.N. and Crowe, P.T. (1995) Morphology of the mucosal lesion in gluten sensitivity. Bailliere’s Clinical Gastroenterology, 9, 273-293. doi:10.1016/0950-3528(95)90032-2
- Gillberg, R., Dotevall, G. and Ahren, C. (1982) Chronic inflammatory bowel disease in patients with coeliac disease. Scandinavian Journal of Gastroenterology, 17, 491-496. doi:10.3109/00365528209182237
- Aamodt, G., Bukholm, G., Jahnsen, J., et al. (2008) The association between water supply and inflammatory bowel disease based on a 1990-1993 cohort study in Southeastern Norway. American Journal of Epidemiology, 168, 1065-1072. doi:10.1093/aje/kwn218
- Bastida, G. and Beltran, B. (2011) Ulcerative colitis in smokers, non-smokers and ex-smokers. World Journal of Gastroenterology, 17, 2740-2747.
- Samuelsson, S.M., Ekbom, A., Zack, M., et al. (1991) Risk factors for extensive ulcerative colitis and ulcerative proctitis: A population based case-control study. Gut, 32, 1526-1530. doi:10.1136/gut.32.12.1526
- Finnie, I.A., Campbell, B.J., Taylor, B.A., et al. (1996) Stimulation of colonic mucin synthesis by corticosteroids and nicotine. Clinical Science, 91, 359-364.
- Swansona, G.R., Sedghib, S., Farhadia, A., et al. (2010) Pattern of alcohol consumption and its effect on gastrointestinal symptoms in inflammatory bowel disease. Alcohol, 44, 223-228. doi:10.1016/j.alcohol.2009.10.019

