Open Journal of Ecology
Vol.3 No.2(2013), Article ID:30965,8 pages DOI:10.4236/oje.2013.32011
Seasonal comparison of aquatic macroinvertebrate assemblages in a flooded coastal freshwater marsh
![]()
1School of Renewable Natural Resources, Louisiana State University Agricultural Center, Baton Rouge, USA; *Corresponding Author: skang1@tigers.lsu.edu
2U.S. Geological Survey, Louisiana Cooperative Fish and Wildlife Research Unit, School of Renewable Natural Resources, Louisiana State University Agricultural Center, Baton Rouge, USA
Copyright © 2013 Sung-Ryong Kang, Sammy L. King. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 18 January 2013; revised 20 February 2013; accepted 22 March 2013
Keywords: Freshwater Emergent Marsh; Freshwater Pond; Aquatic Macroinvertebrate Assemblage; Hydrologic Connection
ABSTRACT
Marsh flooding and drying may be important factors affecting aquatic macroinvertebrate density and distribution in coastal freshwater marshes. Limited availability of water as a result of drying in emergent marsh may decrease density, taxonomic diversity, and taxa richness. The principal objectives of this study are to characterize the seasonal aquatic macroinvertebrate assemblage in a freshwater emergent marsh and compare aquatic macroinvertebrate species composition, density, and taxonomic diversity to that of freshwater marsh ponds. We hypothesize that 1) freshwater emergent marsh has lower seasonal density and taxonomic diversity compared to that of freshwater marsh ponds; and 2) freshwater emergent marsh has lower taxa richness than freshwater marsh ponds. Seasonal aquatic macroinvertebrate density in freshwater emergent marsh ranged from 0 organisms/m2 (summer 2009) to 91.1 ± 20.53 organisms/m2 (mean ± SE; spring 2009). Density in spring was higher than in all other seasons. Taxonomic diversity did not differ and there were no unique species in the freshwater emergent marsh. Our data only partially support our first hypothesis as aquatic macroinvertebrate density and taxonomic diversity between freshwater emergent marsh and ponds did not differ in spring, fall, and winter but ponds supported higher macroinvertebrate densities than freshwater emergent marsh during summer. However, our data did not support our second hypothesis as taxa richness between freshwater emergent marsh and ponds did not statistically differ.
1. INTRODUCTION
Spatial and temporal variation in habitat conditions affects movements of aquatic macroinvertebrates [1,2] and contributes to the regulation of assemblages [3]. In riverine ecosystems, flow regime plays a major role in structuring patterns of biotic composition and diversity [1,2,4,5]. Similarly, marsh flooding and drying in freshwater marshes may be important factors affecting seasonal aquatic macroinvertebrate density and distribution. Lateral hydrologic connectivity between ponds and freshwater emergent marsh during flooded marsh conditions may decrease aquatic macroinvertebrate density in ponds while aquatic macroinvertebrate density in the freshwater emergent marsh may increase due to aquatic macroinvertebrate movement from ponds to the freshwater emergent marsh. Several studies suggest that ponds (or other habitats) that have a low degree of connection with adjacent waterways support relatively few organisms due to limited recruitment [6] and severe environmental conditions [7-9]. The effects of marsh flooding and drying between ponds and emergent marsh on aquatic macroinvertebrate assemblages in coastal marshes are relatively unknown and poorly studied.
Besides hydrologic connectivity, several studies suggest that hydroperiod affects the assemblages of wetlands macroinvertebrates [10-12]. [13] noted that desiccation stress and the physical environment (e.g., extreme temperatures, low dissolved oxygen) are expected to exert a dominant influence on aquatic macroinvertebrate assemblages in wetlands with short hydroperiods. [14] indicated that temporary ponds support relatively few aquatic macroinvertebrates when compared to more permanent sites. Also, [15] noted that ephemeral and temporary lakes tended to have fewer taxa than semi-permanent channel or terminal lake habitats in a central Australian arid-zone river. In this sense, the relatively long hydroperiod of ponds may allow for higher macroinvertebrate density than in the freshwater emergent marsh due to limited availability of macroinvertebrates as a result of drying in freshwater emergent marsh.
Coleopterans are known to possess physiological and behavioral mechanisms to survive desiccation during dry periods (e.g., Dytiscidae; [16]) and these traits may allow them to avoid the deep water habitat that commonly support relatively large and strong predators (e.g., fish, odonates; [17]).Thus, coleopterans in freshwater emergent marsh may be more abundant than in ponds. In contrast, odonates require a relatively long hydroperiod for the full development of nymphs even though they occur in shallow water [18,19]. Therefore, ondonates may avoid freshwater emergent marsh because of the risk of drying. In addition, macrophyte (e.g., SAV: submerged aquatic vegetation) cover appears to affect macroinvertebrate distribution by providing refuge from predators [20], and increasing the availability of food resources [21]. [17] characterized aquatic macroinvertebrate assemblages in freshwater marsh ponds, however, a paucity of information [22] exists on the aquatic macroinvertebrate assemblages in freshwater marshes and their similarity to assemblages in freshwater marsh ponds.
A clear understanding of the similarity and differences between freshwater emergent marsh and marsh ponds would enhance our understanding of aquatic macroinvertebrate habitat requirements in freshwater marshes because freshwater marsh and pond habitats represent very different physical and chemical conditions. The principal objectives of this study are to characterize the seasonal aquatic macroinvertebrate assemblage in a freshwater emergent marsh and compare aquatic macroinvertebrate species composition, density, and taxonomic diversity to that of freshwater marsh ponds. We hypothesize that 1) freshwater emergent marsh has lower seasonal density and taxonomic diversity compared to that of freshwater marsh ponds; and 2) freshwater emergent marsh has lower taxa richness than freshwater marsh ponds.
2. STUDY AREA AND METHODS
2.1. Study Area
This study was conducted in White Lake Wetlands Conservation Area (WLWCA, 29˚52'N, 92˚31'W) in the Chenier Plain of southwestern Louisiana. WLWCA is bounded on the south by White Lake (28.2 km north of the Gulf of Mexico) and is a 28,719 ha freshwater marsh. Dominant vegetation is maidencane (Panicum hemitomon Schultes) and bulltongue arrowhead (Sagittaria lancifolia Linnaeus).
2.2. Data Collection
We randomly selected three sites for more intensive study in the edge of each emergent marsh site (i.e., 100 m from channel or pond margin). To assess variation in environmental variables, monthly we measured salinity, dissolved oxygen (DO, mg/l), and water temperature (˚C) with a YSI Model 85 Water Quality Meter. These variables were measured 2 - 3 cm above the sediment at each sampling point between 08:00 AM and 17:00 PM (one time per month). Percent cover of SAV in a 1 × 1-m frame was also randomly determined at three points around each sampling point (i.e., sampling point + 2 random points) and the mean coverage of SAV in the 1 m2 was calculated. We used a meter stick to check sampling point water depth (SPWD, water depth at sampling points).
To determine aquatic macroinvertebrate assemblage structure, we sampled each emergent marsh monthly from April 2009 to February 2010. For purposes of this study, seasons were defined as: 1) Spring 2009 (AprilMay); 2) Summer 2009 (June-August); 3) Fall 2009 (September-November); 4) Winter 2009 (DecemberFebruary). We sampled water-column macroinvertebrates using a D-shaped sweep net with a 30-cm opening and 1-mm mesh size. Many previous studies [23-28] have found that a D-shaped sweep net is an effective sampling method for water-column macroinvertebrates in ponds. We conducted a total of 10 continuous sweeps of 2-m long each (surface covered 6 m2; [23]) at a randomly selected point.
[17] provides detailed results of environmental variables that include salinity, DO, temperature, SAV, and SPWD and of aquatic macroinvertebrate species composition, density, and taxonomic diversity in two pond types (i.e., permanently connected pond [PCP: permanently connected channel during all seasons]; temporarily connected pond [TCP: temporarily connected by surface water to the surrounding marsh but not permanently connected to a channel]).
2.3. Statistical Analysis
Data are reported as mean ± SE, and significance level was set at α = 0.05. Analyses of variance (ANOVA) and T-test (Proc Mixed, Version 9.3, SAS Institute, Cary, North Carolina) were used to test for statistical differences in environmental variables and aquatic macroinvertebrate density by season. We used a one-way ANOVA for each response variable. Models included the environmental variable, as well as density and taxonomic diversity. We conducted a one-way ANOVA with one fixed effect. Significant one-way ANOVA effects were tested using post-hoc comparisons of Tukey adjusted least squares means. For one-way ANOVA analyses, data were tested for normality with the Shapiro-Wilks test. In the event that the residuals were not normally distributed, the data were natural log-transformed.
3. RESULTS
Seasonal salinity was higher in spring and fall than in summer (F3,8 = 12.0, p = 0.003). Comparison of DO and water depth indicated their highest values were in winter but temperature values were lowest in winter (DO: F3,8 = 38.6, p < 0.001; temperature: F3,8 = 132.1, p < 0.001; water depth: F3,8 = 512.6, p < 0.001). Seasonal SAV cover did not differ. In freshwater ponds, salinity, temperature, and SAV were the lowest in winter but DO was the highest. The highest SPWD was in fall. The full results of water chemistry (i.e., salinity, DO, temperature), SAV cover, and SPWD in freshwater marsh ponds (i.e., PCPs and TCPs) are presented in Table 1 and [17].
We collected 5,114 aquatic macroinvertebrates of 35 taxa from 33 samples. The dominant taxon during all flooded seasons was Chironomidae (spring 2009: 38.3%; fall 2009: 14.8%; winter 2009: 12.2%; Table 2). Seasonal aquatic macroinvertebrate density in freshwater emergent marsh ranged from 91.1 ± 20.53 organisms/m2 (mean ± SE; spring 2009) to 0 organisms/m2 (summer 2009; Table 3). Density in spring 2009 was higher than in all other seasons (F3,7 = 31.3, p < 0.001). Taxonomic diversity did not differ. Table 4 provides a detailed comparison of aquatic macroinvertebrate densities among freshwater emergent marsh and ponds. Aquatic macroinvertebrate density between freshwater emergent marsh and ponds did not differ in spring, fall, and winter but ponds supported higher macroinvertebrate densities than freshwater emergent marsh (F2,6 = 13.2, p = 0.006) during summer. Taxa richness between freshwater emergent marsh and ponds did not statistically differ.
4. DISCUSSION
The present study considered the hypothesis that freshwater emergent marsh had lower aquatic macroinvertebrate density and taxonomic diversity than freshwater marsh ponds. When emergent marsh is flooded (i.e., lateral connectivity with pond or channel), aquatic macroinvertebrates can move from ponds into the freshwater emergent marsh, resulting in increased aquatic macroinvertebrate density in the freshwater emergent marsh. Our results indicated that aquatic macroinvertebrate density and taxonomic diversity among freshwater emergent marsh and ponds did not statistically differ in spring, fall, and winter (i.e., flooded marsh condition).
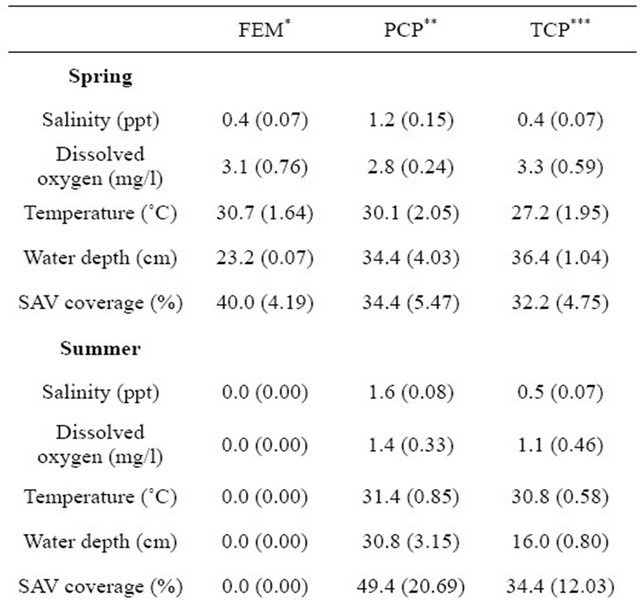
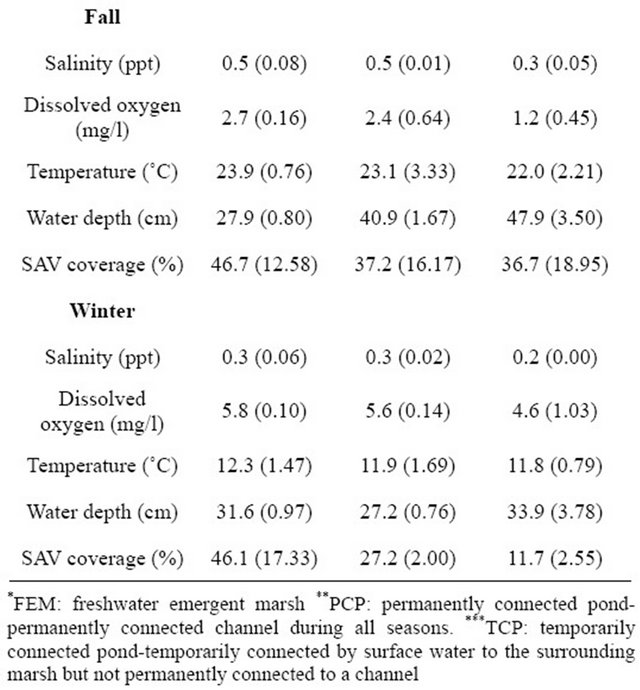
Table 1. Comparison of seasonal means (±SE) of water chemistry (n = 108), water depth (n = 108), and SAV cover (n = 108) in freshwater ponds and emergent marsh.
High variability in macroinvertebrate density within the emergent marsh and ponds suggests that macroinvertebrates in freshwater emergent marsh are patchily distributed. Furthermore, the lack of macroinvertebrates during the summer when the marsh dried also suggests that this resource is also temporally limited. In spite of the high variability and limited temporal availability, the freshwater emergent marsh is still an important and widely distributed habitat for aquatic macroinvertebrates along the Louisiana coast.

Table 2. Comparison of mean density (ind·m−2 (SE)) of aquatic macroinvertebrates in freshwater emergent marsh by season. No sample in summer (i.e., June, July, August 2009) because of drying.

Table 3. Seasonal aquatic macroinvertebrate density (organisms/m2 (±SE)) and taxonomic diversity (Index H’) in freshwater emergent marsh from April 2009 to February 2010.
We hypothesized freshwater emergent marsh may have fewer aquatic macroinvertebrate taxa than freshwater marsh ponds due to the seasonal drying of the marsh. The dry condition in freshwater emergent marsh may decrease the taxa richness because of limited marsh accessibility for macroinvertebrates. However, our data did not support the hypothesis as taxa richness between freshwater emergent marsh and ponds did not statistically differ and common pond inhabitants were also common in the freshwater emergent marsh (Table 4). Similarly, [29] found that macroinvertebrate species in temporary communities are a nested subset of those in permanent communities.
Previous studies [14,30] emphasized the role of water permanence in determining the macroinvertebrate occur-
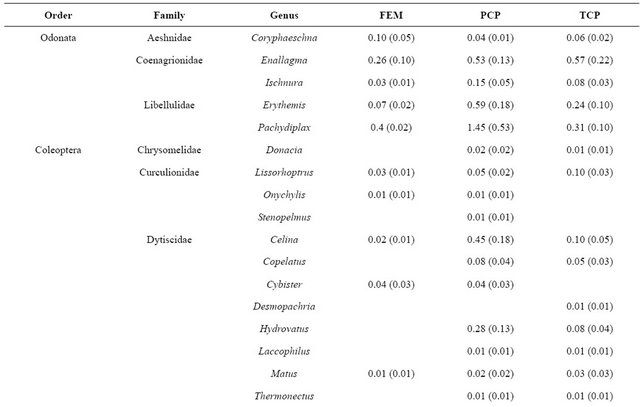
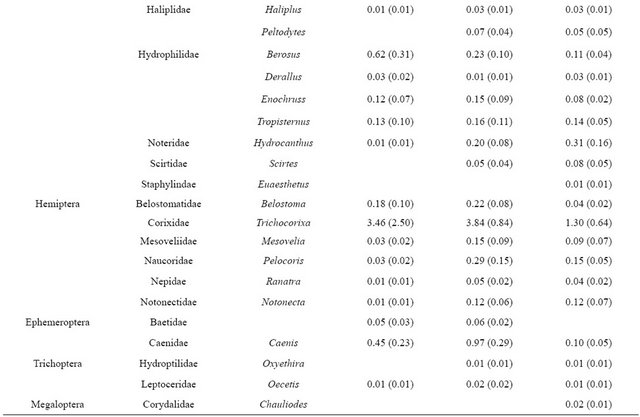
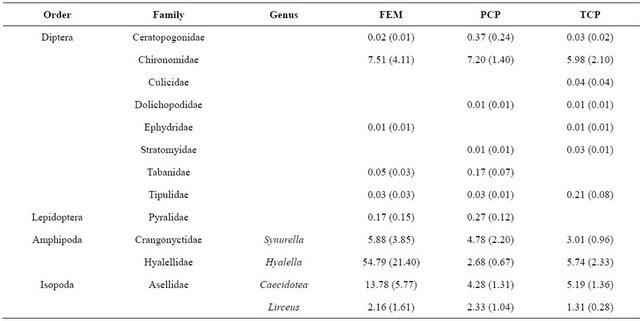
Table 4. Comparison of mean density (ind·m−2 (SE)) of aquatic macroinvertebrates in freshwater emergent marsh and ponds from April 2009-February 2010.
rence in freshwater wetlands. [26] noted that the hydroperiod gradient (i.e., long, short) influenced the dominant macroinvertebrate genera. Our results, however, were not consistent with a hydroperiod effect on assemblage structure as we did not detect a difference in dominant species between ponds and freshwater emergent marsh. Based on [17], most aquatic macroinvertebrates in these coastal marshes appear to be poorly adapted to dry conditions. In freshwater emergent marsh, midges (Chironomidae) were relatively abundant species (i.e., >10 %) during all flooded seasons. The family Chironomidae is frequently the most abundant group in freshwater communities [31] and their larvae are known to have a certain degree of resistance to desiccation although they live in water [32]. In this sense, Chironomidae in our study sites may be well adapted to desiccation stress, resulting in relatively high density in freshwater marsh ponds and freshwater emergent marsh.
Variation in life history traits of macroinvertebrates seems to be correlated with hydrologic condition (i.e., flooding duration, water depth). The low fall densities in emergent marshes appear to be a life history strategy to avoid harsh conditions and maximize reproductive success by synchronizing egg hatch with more favorable winter conditions. It is also possible that low fall densities represent a residual effect of summer drying. In addition, individual taxa have different habitat requirements. According to previous studies [16,18,19], odonates require a relatively long hydroperiod for the full development of nymphs; thus, they may avoid the marshes due to the risk of dry conditions. However, coleopterans have relatively high desiccation tolerance;
therefore, they may avoid the ponds because they support an abundance of relatively large and strong predators (e.g., fish, dragonfly; [17]). Despite differences in physical and chemical conditions between marshes and ponds, our results indicated that the density of odonates and coleopterans between marshes and ponds did not statistically differ. These results could have been affected by our relatively small sample sizes. Additional research could provide important insights into aquatic macroinvertebrate use patterns in these habitats.
Flooding and drying conditions are common in wetlands and are an important part of the hydrological cycle. The relatively long inundation promotes higher densities and taxonomic diversity of aquatic macroinvertebrates. In our study, the results suggest that anthropogenic activities such as marsh management that increase or decrease the duration of lateral hydrologic connection between emergent marsh and adjacent waterbodies may potentially affect aquatic macroinvertebrate habitat value in freshwater marshes.
5. ACKNOWLEDGEMENTS
This project was supported by a Louisiana Department of Wildlife and Fisheries and U.S. Fish and Wildlife Service State Wildlife Grant with support also from the International Crane Foundation. We thank M. La Peyre, J. A. Nyman, R. Keim, A. Rutherford, and M. Ferro for their critical insights. The authors would like to acknowledge the field and laboratory contributions of J. Linscombe, R. Cormier, M. Huber, and A. Williamson. In addition, we extend gratitude to M. Kaller for helping with the aquatic macroinvertebrate identification and statistical analysis. Collections were made under Louisiana State University AgCenter Animal Care and Use protocol (#AE2008-012). Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
![]()
![]()
REFERENCES
- Paillex, A., Castella, E. and Carron, G. (2007) Aquatic macroinvertebrate response along a gradient of lateral connectivity in river floodplain channels. Journal of the North American Benthological Society, 26, 779-796. doi:10.1899/06-12.1
- Zilli, F.L. and Marchese, M.R. (2011) Patterns in macroinvertebrate assemblages at different spatial scales. Implications of hydrological connectivity in a large floodplain river. Hydrobiologia, 663, 245-257. doi:10.1007/s10750-010-0576-1
- Leigh, C. and Sheldon, F. (2009) Hydrological connectivity drives patterns of macroinvertebrate biodiversity in floodplain rivers of the Australian wet/dry tropics. Freshwater Biology, 54, 549-571. doi:10.1111/j.1365-2427.2008.02130.x
- Poff, N.L., Allan, J.D., Bain, M.B., Karr, J.R., Prestegaard, K.L., Richter, B.D., Sparks, R.E. and Stromberg, J.C. (1997) The natural flow regime. A paradigm for river conservation and restoration. BioScience, 47, 769-784. doi:10.2307/1313099
- Puckridge, J.T., Sheldon, F., Walker, K.F. and Boulton, A.J. (1998) Flow variability and the ecology of large rivers. Marine and Freshwater Research, 49, 55-72. doi:10.1071/MF94161
- Rozas, L.P. and Minello, T.J. (1999) Effects of structural marsh management on fishery species and other nekton before and during a spring drawdown. Wetlands Ecology and Management, 7, 121-139. doi:10.1023/A:1008434727703
- Dunson, W.A., Friacano, P. and Sadinski, W.J. (1993) Variation in tolerance to abiotic stresses among sympatric salt marsh fish. Wetlands, 13, 16-24. doi:10.1007/BF03160861
- Rowe, C.L. and Dunson, W.A. (1995) Individual and interactive effects of salinity and initial fish density on a salt marsh assemblage. Marine Ecology Progress Series, 128, 271-278. doi:10.3354/meps128271
- Gascon, S., Boix, D., Sala, J. and Quintana, X.D. (2008) Relation between macroinvertebrate life strategies and habitat traits in Mediterranean salt marsh ponds (Emporda wetlands, NE Iberian Peninsula). Hydrobiologia, 597, 71-83. doi:10.1007/s10750-007-9215-x
- Wiggins, G.B., Mackay, R.J. and Smith, I.M. (1980) Evolutionary and ecological strategies of animals in annual temporary pools. Archiv für Hydrobiologie, 58, 97-206.
- Spencer, M., Blaustein, L., Schwartz, S.S. and Cohen, J.E. (1999) Species richness and the proportion of predatory animal species in temporary freshwater pools; relationships with habitat size and permanence. Ecology Letters, 2, 157-166. doi:10.1046/j.1461-0248.1999.00062.x
- Zimmer, K.D., Hanson, M.A. and Butler, M.G. (2000) Factors influencing invertebrate communities in prairie wetlands: a multivariate approach. Canadian Journal of Fisheries and Aquatic Sciences, 57, 76-85. doi:10.1139/f99-180
- Welborn, G.A., Skelly, D.K. and Werner, E.E. (1996) Mechanisms creating community structure across a freshwater habitat gradient. Annual Review of Ecology and Systematics, 27, 337-363. doi:10.1146/annurev.ecolsys.27.1.337
- Collinson, N.H., Biggs, J., Corfield, A., Hodson, M.J., Walker, D., Whitefield, M. and Williams, P.J. (1995) Temporary and permanent ponds: an assessment of the effects of drying out on the conservation value of aquatic macroinvertebrate communities. Biological Conservation, 74, 125-133. doi:10.1016/0006-3207(95)00021-U
- Sheldon, F., Boulton, A.J. and Puckridge, J.T. (2002) Conservation value of variable connectivity: Aquatic invertebrate assemblages of channel and floodplain habitats of a central Australian arid-zone river, Cooper Creek. Biological Conservation, 103, 13-31. doi:10.1016/S0006-3207(01)00111-2
- Nilsson, A.N. (1986) Life cycles and habitats of the northern European Agabini (Coleoptera, Dytiscidae). Entomologica Basiliensia, 11, 391-417.
- Kang, S.R. and King, S.L. (2013) Effects of hydrologic connectivity on aquatic macroinvertebrate assemblages in different marsh types. Aquatic Biology, 18,149-160. doi:10.3354/ab00499
- Wissinger, S.A. (1988) Spatial distribution, life history, and estimates of survivorship in a fourteen-species assemblage of larval dragonflies (Odonata: Anisoptera). Freshwater Biology, 20, 329-340. doi:10.1111/j.1365-2427.1988.tb00458.x
- Zimmer, K.D., Hanson, M.A., Butler, M.G. and Duffy, W.G. (2001) Size distribution of aquatic invertebrates in two prairie wetlands, with and without fish, with implications for community production. Freshwater Biology, 46, 1373-1386. doi:10.1046/j.1365-2427.2001.00759.x
- Mittlebach, G.G. (1988) Competition among refuging sunfishes and effects of fish density on littoral zone invertebrates. Ecology, 69, 614-623. doi:10.2307/1941010
- Campeau, S., Murkin, H.R. and Titman, R.D. (1994) Relative importance of algae and emergent plant litter to freshwater marsh invertebrates. Canadian Journal of Fisheries and Aquatic Sciences, 51, 681-692. doi:10.1139/f94-068
- Batzer, D.P., Pusateri, C.R. and Vetter R. (2000) Impacts of fish predation on marsh invertebrates: Direct and indirect effects. Wetlands, 20, 307-312. doi:10.1672/0277-5212(2000)020[0307:IOFPOM]2.0.CO;2
- Bolduc, F. and Afton, A.D. (2003) Effects of structural marsh management and salinity on invertebrate prey of waterbirds in marsh ponds during winter on the Gulf Coast Chenier Plain. Wetlands, 23, 897-910. doi:10.1672/0277-5212(2003)023[0897:EOSMMA]2.0.CO;2
- Batzer, D.P., Palik, B.J. and Buech, R. (2004) Relationships between environmental characteristics and macroinvertebrate communities in seasonal woodland ponds of Minnesota. Journal of the North American Benthological Society, 23, 50-68. doi:10.1899/0887-3593(2004)023<0050:RBECAM>2.0.CO;2
- Nicolet, P., Biggs, J., Fox, G., Hodson, M.J., Reynolds, C., Whitfield, M. and Williams, P. (2004) The wetland plant and macroinvertebrate assemblages to temporary ponds in England and Wales. Biological Conservation, 120, 261- 278. doi:10.1016/j.biocon.2004.03.010
- Tarr, T.L., Baber, M.J. and Babbitt, K.J. (2005) Macroinvertebrate community structure across a wetland hydroperiod gradient in southern New Hampshire, USA. Wetlands Ecology and Management, 13, 321-334. doi:10.1007/s11273-004-7525-6
- Hornung, J.P. and Foote, A.L. (2006) Aquatic invertebrate responses to fish presence and vegetation complexity in western boreal wetlands, with implications for waterbird productivity. Wetlands, 26, 1-12. doi:10.1672/0277-5212(2006)26[1:AIRTFP]2.0.CO;2
- Kratzer, E.B. and Batzer, D.P. (2007) Spatial and temporal variation in aquatic macroinvertebrates in the Okefenokee swamp, Georgia, USA. Wetlands, 27, 127-140. doi:10.1672/0277-5212(2007)27[127:SATVIA]2.0.CO;2
- Wissinger, S.A., Greig, H. and McIntosh, A. (2009) Absence of species replacements between permanent and temporary lentic communities in New Zealand. Journal of the North American Benthological Society, 28, 12-23. doi:10.1899/08-007.1
- Sanderson, R.A., Eyre, M.D. and Rushton, S.P. (2005) Distribution of selected macroinvertebrates in a mosaic of temporary and permanent freshwater ponds as explained by autologistic models. Ecography, 28, 355-362. doi:10.1111/j.0906-7590.2005.04093.x
- Pinder, L.C.V. (1986) Biology of freshwater Chironomidae. Annual Review of Entomology, 31, 1-23. doi:10.1146/annurev.en.31.010186.000245
- Suemoto, T., Kawai, K. and Imabayashi, H. (2004) A comparison of desiccation tolerance among 12 species of chironomid larvae. Hydrobiologia, 515, 107-114. doi:10.1023/B:HYDR.0000027322.11005.20

