Open Journal of Molecular and Integrative Physiology
Vol.3 No.1(2013), Article ID:27859,9 pages DOI:10.4236/ojmip.2013.31002
The role of histamine H4 receptors as a potential targets in allergic rhinitis and asthma
![]()
Department of Pathophysiolohy, Jessenius Faculty of Medicine, Comenius University, Martin, Slovakia
Email: *eva.hanuskova@gmail.com, ehanuskova@jfmed.uniba.sk
Received 9 November 2012; revised 13 December 2012; accepted 28 December 2012
Keywords: Allergic Rhinits; Asthma; Histamine; H4R; Novel Drug Target
ABSTRACT
Histamine—the main product of mast cells plays critical role in the pathogenetic pathways of both allergic rhinitis and asthma. The novel concept of the unique airway diseases its only supported by the similarities within pathogenetic process. Antagonists of H1 and H2 receptors are quite effective in allergic rhinitis, but not effective enough in asthma. In an era of corticosteroids, leucotriene antagonists and AntiIgE treatment, there is still a challenge to search for more effective, more acurate and more safe treatment option. Antagonists (inversive agonists) of histamine receptors H4 seems to be one of the promising targets in the allergic rhinitis and asthma treatment. The first H4 antagonist entered to clinics and the results from a proof-of-concept Phase II clinical study is expected to be disclosed soon. This review article summarizes current knowledge on H4R that have been collected in various studies sharing evidences about efficacy of H4R as a reasonable target for diseases with histamine involved pathogenetic pathways.
1. INTRODUCTION TO THE TOPIC
The increasing recognition over the last 50 years that allergic rhinitis and allergic asthma frequently co-exist, has led to the concept that these seemingly separate disorders are possibly the same disease, with symptoms occurring to a greater or lesser extent in the upper airways (rhinitis) or lower airways (asthma). When patients with either allergic rhinitis or allergic asthma are thoroughly investigated, it is frequently found that they have allergic inflammation and airway sensitivity throughout all of the airways [1].
One of the important biological signals involved in the pathogenesis of both of them is histamine, which is released after relevant antigenic stimulation of sensitized subjects, initiating the early phase of allergic reaction [2]. Many patients with allergic rhinitis and asthma could be managed quite easily, mainly if they respond to the treatment in an expected pattern. But still, there are challenges to find better treatment targeting the pathogenetic pathways more acuratelly, more effectivelly and more safely. In an era of local or systemic corticosteroids, antihistamines and antileukotrienes and new class of anti-allergy drugs, anti-IgE antibodies, there is still a need and provocation to search novel, possibly effective treatment options. This review article analyzes the role of H4 receptors in pathogenesis of allergic rhinitis and asthma and possible clinical applications of H4 antagonists.
2. HISTAMINE AND ITS MAIN BIOLOGICAL ACTIVITY
Histamine, is biogenic amine, that was isolated from the mould ergot in 1910 by Sir Henry Dale and his colleagues at the Wellcome Laboratories. Very early, in 1924, Lewis described the classic “triple response” to histamine consisting of a red spot due to vasodilatation, a wheal which was the consequence of increased permeability and flare due to an axon reflex [3]. Since that time a lot of knowledge had been collected about histamine and its action in various system of human body concerning gastrointestinal, cardiovascular and respiratory systems [4,5]. Later this amine was found to be a natural constituent of the body, hence, the name histamine was given after the Greek word for tissue, histos.
Nowadays we know, that histamine belongs to the biogenic amines and is synthesized by the pyridoxal phosphate (vitamin B-6)–containing l-histidine decarboxylase (HDC) from the amino acid histidine [6]. Gastric enterochromaffin-like cells, histaminergic neurons as well as mast cells and basophils are classical cellular sources of histamine, where it is stored intracellularly in vesicles and released on stimulation [7]. De novo histamine synthesis has also been shown in other cell types, such as platelets, monocytes/macrophages, dendritic cells, neutrophils and lymphocytes [8-10].
Histamine is a potent mediator of numerous biologic reactions. Besides the well-known triggering of degranulation of mast cells by crosslinking of the FcεRI receptor by specific allergens, several other nonimmunologic stimuli, such as neuropeptides, substance P, complement factors (i.e., C3a and C5a), cytokines (IL-1, IL-3, IL-8, GM-CSF), platelet-activating factor (PAF), hyperosmolarity, lipoproteins, adenosine, superoxidases [11]. Hypoxia, chemical and physical factors (e.g., extreme temperatures, traumas, vibration) [12] or alcohol and certain food and drugs, may activate mast cells thus releasing histamine [7,13]. The pleiotropic effects of histamine are triggered by activating through one or several histamine membrane receptors on different cells. Four subtypes of receptors (histamine 1 receptor (H1R), histamine 2 receptor (H2R), histamine 3 receptor (H3R), and histamine 4 receptor (H4R)) have been described. All these receptors belong to the G-protein-coupled receptor family. They are heptahelical transmembrane molecules that transduce the extracellular signal by using G-proteins and intracellular second messenger systems [14]. Differences in the affinities of these receptors are highly decisive on the biological effects of histamine and agents that target. The active and inactive states of HRs exist in equilibrium. However, it has been shown in recombinant systems that HRs can trigger downstream events in the absence of receptor occupancy by an agonist, which accounts for constitutive spontaneous receptor activity [15]. HR agonists stimulate the active state in the receptor and inverse agonists stimulate the inactive one. An agonist with a preferential affinity for the active state of the receptor stabilizes the receptor in its active conformation leading to a continuous activation signal. An inverse agonist with a preferential affinity for the inactive state stabilizes the receptor in this conformation and consequently induces an inactive state, which is characterized by blocked signal transduction via the HR [16]. HRs form dimers and even oligomers, which allow cooperation between HRs and other G-protein-coupled receptors. The affinity of histamine binding to different HRs varies significantly, with Ki values ranging from 5 - 10 nM for the H3 and H4 receptors to 2 - 10 mM for the H1 and H2 receptors [6,17]. Specific activation or blockade of HRs showed that they differ in expression, signal transduction or function and improved the understanding of the role of histamine in physiology and disease mechanisms [18]. Histamine can act not only on cell surface receptors (H1, H2, H3 and H4 receptors), but may also bind to some intracellular receptors such as cytochrome p450 and cytochrome c [19,20] and highaffinity lipocalins isolated from the saliva of ticks [21].
Histamine causes smooth muscle cell contraction, vasodilatation, increased vascular permeability and mucus secretion, tachycardia, alterations of blood pressure, and arrhythmias, and it stimulates gastric acid secretion and nociceptive nerve fibers. In addition, histamine has been known to play various roles in neurotransmission, immunomodulation, hematopoiesis, wound healing, day-night rhythm, and the regulation of histamineand polyamineinduced cell proliferation and angiogenesis in tumor models [22,23] and intestinal ischemia [24]. The important roles of histamine in body physiology and various pathologic events have been well established, whereas new and exciting findings are still being uncovered.
The major routes of histamine inactivation in mammals are methylation of the imidazole ring, catalyzed by histamine N-methyltransferase (HNMT), and oxidative deamination of the primary amino group, catalyzed by diamine oxidase (DAO) also known as histaminase [25]. The DAO protein is stored in plasma membraneassociated vesicular structures in epithelial cells and is secreted into the circulation on stimulation [26].
3. H4 RECEPTOR—BIOLOGICAL AND CLINICAL RELEVANCE FOR THE H4 ANTAGONISTS USE
Histamine has long been known to mediate inflammatory, and allergic responses acting predominately through H1 receptors and H1 receptor antagonists have been used to treat allergies for many years [27]. Accumulating evidence derived from diverse in vivo and in vitro studies using animal models of disease and human biological samples substantiates the fundamental role of the H4 receptor in histamine-induced chemotaxis of mast cells, eosinophils and other immune cells [17,28,29]. In addition, the presence of H4 receptors mostly in immune system organs and their immunomodulatory role in cytokine production [30-32] argue for the pathophysiological significance of H4 receptors in inflammatory conditions that are characterized by increases in immune cell numbers, such as asthma, allergic disorders and autoimmune diseases and imply their contribution not only in the histamine-mediated initial inflammatory signal but also in the maintenance of inflammation [17,33- 35]. At the end of 2000, both Oda et al. (2000) [36] and Nakamura et al. (2000) [14] reported the cloning of the human H4 receptor cDNA from fetus and leukocyte cDNA, respectively. Subsequently, six other laboratories reported the same finding [37-42]. The gene encoding the H4 receptor is on chromosome 18q11.2, spans > 20.6 kb and has a similar intron-exon arrangement as the gene encoding the H3 receptor [42]. The human H4 receptor is related most closely to the human H3 receptor, which shares 31% sequence identity at the protein level. In the transmembrane domains, the sequence identity with the H3 receptor increases to 54%, whereas, taking into account the similarities in physico-chemical properties of the different amino acids, the similarity in the transmembrane region is even higher (68%). Sequence identity with the H1 receptor and H2 receptor is 23% and 22%, respectively [36,37]. Following the cloning of the human H4 receptor, cDNAs that encode the H4 receptor were cloned from mouse, rat, guinea pig and pig [43,44].
The H4R couples to members of the PTX-sensitive Gi/o proteins (Figure 1). Thus, activation of the H4R reduces cAMP formation and further downstream events like CREB-mediated gene transcription [45]. In addition, the H4R can activate the MAPK pathway via PTX-sensitive mechanisms [39]. Furthermore, activation of H4R in mast cells and eosinophils leads to a mobilization of intracellular [Ca2+]i [46,47]. An increase in [Ca2+]i is sensitive to PTX and PLC inhibitors, indicating that PLC is activated by the dissociated G βγ subunit after H4R activation [48]. Recently, signaling of the H4R, presumably via a G protein-independent β-arrestin pathway, resulting in a phosphorylation of ERK 1/2 in U2OS cells has been reported [49,50]. The H4R exerts high levels of constitutive activity [39,51].
H4R expresses on the surface of various hematopoietic cells including T cells [31,53,54], B cells [37], mast cells [17,41,47,55,56], eosinophils [36,46,57], dendritic cells [37,58], monocytes [36,37], neutrophils [36,39] and natural killer (NK) cells [58] and also on the surface of non-hematopoietic cells such as fibroblasts [59] and endocrine cells in gastrointestinal tract [60]. There are controversial reports about H4R expression in neutrophils, spleen, thymus, lymph node, kidney, testis, brain, lung, liver, placenta, heart, skeletal muscle and pancreas [14,61].
The H4 receptor shows little homology to the classical proinflammatory H1 receptor or the H2 receptor. H3 and H4 receptors are most closely related to each other, and they have a closer phylogenetic relationship with peptide ligand GPCRs, while they are remotely related to other biogenic amine receptors, including H1 and H2 receptors [62]. The H4 receptor appears to have higher affinity for
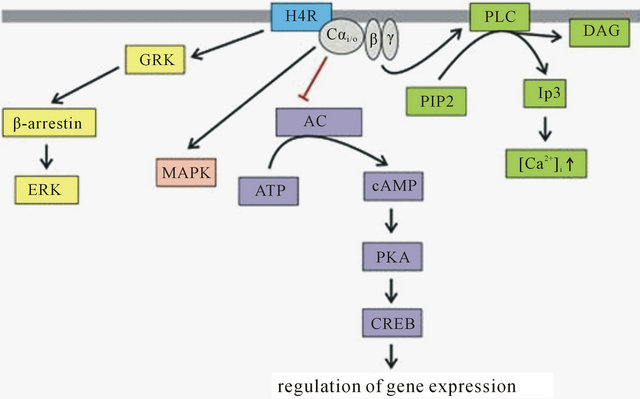
Figure 1. Signal transduction pathways activated by the H4R stimulation [52].
histamine compared with the H1 receptor, activation leading to leukocyte chemotaxis to sites of inflammation via G ai/o proteins and increases in intracellular Ca2+ concentration [47,48]. In addition to histamine, liverexpressed chemokine LEC/CCL16 has been reported to be a non-histamine endogenous H4 receptor agonist, demonstrating additive effects with histamine and involved in eosinophil trafficking [62].
H4 receptors control leukocyte trafficking and proinflammatory responses. It is caused by H4 receptorediated histamine-induced activation of eosinophils, ineased expression of adhesion molecules like CD11b/ D18(Mac1) and CD54(ICAM-1) and rearrangement of the actin cytoskeleton leading to eosinophil migration from the bloodstream into the sites of inflammation [41,46,57,63].
Human mast cells constitutively express H4 receptors that govern autocrine and paracrine histamine-induced processes [56]. H4 receptor activation mediates chemotaxis and intracellular Ca2+ mobilization in murine mast cells, without affecting degranulation, thus providing a mechanism for the selective recruitment of these effector cells into the tissues and the amplification of the histamine-mediated reaction eventually leading to chronic allergic inflammation [47]. Supportive evidence for an autoregulatory function of the mast cell-expressed H4 receptor comes from its critical role in zymosan-induced recruitment of neutrophils in vivo, possibly via regulation of leukotriene B4 release from mast cells [17,64].
The H4 receptor expressed on dendritic cells, CD4+ and CD8+ T cells appears to control cytokine and chemokine production. In general, histamine can enhance TH1 responses through H1 receptor activation and negatively regulate both TH1 and TH2 responses by acting on H2 receptors. H4 receptor, which alongside H1 and H2 receptors, modulates cytokine secretion during the integration of TH1/TH2 differentiation [65]. Cytokines mediate their effects via the signal transduction and activators of transcription (STAT), with STAT6 activation causing a shift towards the TH2 response implicated in allergic state development and STAT1 and STAT4 playing a role in the pathogenesis of asthma with distinct responses existing in non-atopic and atopic states. Histamine acting on the H4 receptor has been reported to suppress ex vivo the mitogen-induced STAT1 phosphorylation and its specific interaction with DNA in peripheral blood mononuclear cells derived from non-atopic individuals [53], while the H4 receptor antagonist JNJ7777120 inhibited STAT6 DNA binding in cells derived from atopic subjects [54]. Additional evidence for the immunomodulatory function of the H4 receptor is provided by its involvement in the release of the CD4+ cell chemoattractant interleukin (IL)-16 from human CD8+ T-lymphocytes in vitro [31], the influence on mouse CD4 + T cell activation possibly via signalling in dendritic cells [29], as well as by its up-regulation during monocyte differentiation, suppression of IL-12p70 production and chemoattraction of human monocyte-derived dendritic cells [32].
A reciprocal crosstalk between histamine and cytokines or chemokines involving the H4 receptor seems to be in operation. Interferon (IFN)-γ up-regulates H4 receptor expression in human peripheral blood monocytes/CD14 + [66] and in inflammatory dendritic cells from atopic dermatitis skin [67]. The H4 receptormediated induction of Ca2+ mobilization and down-regulation of synthesis and release of the TH2-linked chemokine CCL2 from monocytes is indicative of a negative feedback mechanism that would avoid the TH2 environment in case of high histamine levels in allergic inflammation and contribute to the shift to TH1 that is observed in the transition from acute to chronic allergic inflammation [66]. Comparably, H4 receptor stimulation downregulates the production of the TH1 cytokine IL-12 and that of CCL2 in human monocyte-derived inflammatory dendritic epidermal cells, the latter leading to decreased monocyte migration [67].
4. EVIDENCES FOR H4 RECEPTORS INVOLVEMENT IN ALLERGIC AIRWAY INFLAMMATION
Asthma
Histamine has been closely associated with pathophysiology of asthma. Histamine is a known airway constrictor and increased levels have been found in airways and plasma of asthma patients following antigen challenge [68]. Many cell types associated with asthma express histamine receptors, most notably the H1R, H2R and H4R. Eosinophils, T cells, mast cells and smooth muscle cells have all been shown to express both H1R and H2R and these receptors can mediate cytokine and chemokine secretion [69].
H1antagonists were shown to be effective only when given during sensitization, but not during the antigen challenge phase. Despite the clinical data, currently H1R antagonists are not a front—line treatment for asthma and indeed a meta—analysis of clinical trial data indicates that H1R antagonists are not effective in treating asthma [70]. H2R antagonists have largely had no efficacy in asthma [71].
The identification of H4R has offered new inslights into the effect of histamine and histamine receptors in asthma. The H4R is expressed in many important immune cells involved in pathophysiology of asthma. It is present in low amounts in the lung, where its expression in bronchial epithelial and smooth muscle cells and microvascular endothelial cells [31] may contribute to the airway disease phenotypes in various ways. The general tenor of H4R reviews suggests that H4R has a pro-inflammatory role in asthma [72].
For example, mast cells are main source of histamine in the lung and it has been shown, that histamine enhances mast cells chemotaxis via the H4R [47]. The H4 receptor mediates redistribution and recruitment of mast cells in the mucosal epithelium in response to allergens, thus amplifying allergic symptoms and maintaining chronic inflammation [17]. Supportive evidence is derived from the H4 receptor-mediated synergistic sequential action of histamine and CXCL12, the chemokine that is constitutively expressed in skin and airway epithelium and plays a key role in allergic airway disorders, to induce migration of mast cells precursors in vitro [55]. Moreover, the H4 receptor seems to regulate only locally mast cell redistribution in the oesophageal mucosal epithelium followed by infiltration of eosinophils in ovalbumin-challenged guinea pigs [73].
H4R—deficient mice and mice treated with H4R antagonists exhibited decreased allergic lung inflammation, with decreases in Th2 responses, including decreases in IL-4, IL-5, IL-13, IL-6 and IL-17 levels. H4 receptor play a role in modulating TH2 allergic responses, by influencing CD4+ T cell activation attributed to decreased cytokine and chemokine production by dendritic cells [29]. Cowden et al. (2010) [74] demonstrated, that therapeutic H4R antagonism can significantly ameliorate allergen induced, Th2 cytokine driven pathologies such as lung remodeling and airway dysfunction. In the mice sensitized to ovalbumin, therapeutic H4R antagonism inhibited T cell infiltration in to the lung and decreased Th2 cytokines IL-13 and IL-5. IL-13 dependent remodeling parameters, such as goblet cell hyperplasia and lung collagen were reduced. Intervention with H4R antagonist also improved measures of central and peripheral airway dysfunction. Most recently the H4R has been shown to by functionally expressed on human Th2 cells and the expression level is upregulated by IL-4. In another study, inhibition of airway resistance and inflammation, mediated through the recruitment of CD25 + FoxP3+ T regulatory (Treg) cells was observed by using the selective H4 receptor agonist 4-methylhistidine administered intratracheally into the lungs of asthmatic mice [75].
In contrast to H1R antagonists, H4R antagonists were equally effective during the sensitization and the alergen challenge phase of a mouse asthma model [29]. The H4R may account for effects of histamine that are not blocked by H1R antagnists an asthmatic responses and, in addition, there may been interaction between the two receptors [76]. Increased eosinophile numbers are found in asthmatic lungs and the H4R has been shown to mediate eosinophil chemotaxis [63]. A recent study demonstrated, that in an acute mouse asthma model, the H1R antagonist mepyramine and the H4R antagonist JNJ7777120 exhibited synergistic inhibitory effects on eosinophil accumulation in bronchoalveolar lavage fluid [77].
The H4R may have other effects that could contribute to asthma. In mice, the H4R mediates IL-4 and IFN-γ production from invariant NK T cells and such cells have been implicated in the pathogenesis of asthma in humans [78].
The H4R mediates the migration of lung fibroblasts, which are important contributors of lung remodeling and other fibrotic lung disorders. Histamine augmented the migration of human fetal lung fibroblasts induced by fibronectin and this effect could be blocked by the H4R antagonist JNJ7777120 [79].
5. ALLERGIC RHINITIS
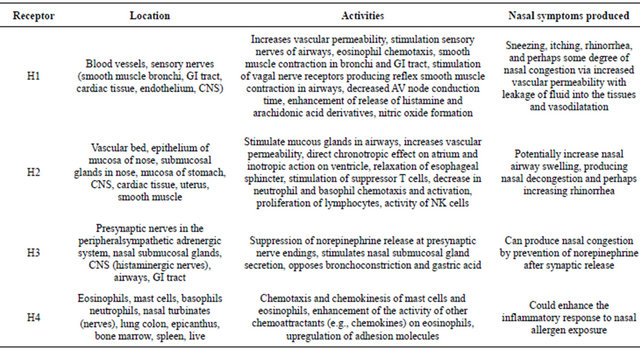
Histamine plays an important role in eliciting nasal symptoms of allergic rhinitis, such as sneezing, itch, rhinorrhea and nasal obstruction [80]. All histamine receptors subtypes (H1, H2, H3 and H4) play a role in allergic rhinitis. Nakaya et al. [81] demonstrated, that in human nasal mucosa, H1R was localised primarily in the epitelium, vessels and nerves, H2R was localized primarily in the epitelium and the glands, and the H3R and H4R were localized primarily in the nerves. Table 1. summarize location, activities of various histamine receptor subtypes in relationship to nasal symptoms of allergic rhinitis. Histamine H1 receptor antagonists, such as chlorpheniramine and ketotifen, have been used as the first choice in the treatment of nasal allergy. Although histamine H1 receptor antagonists inhibited nasal symptoms induced by antigen-antibody reaction in rats and mice [82], it has been pointed out that these histamine H1 receptor antagonists did not completely inhibit nasal symptoms [83]. JNJ7777120 is reported to be a selective histamine H4 receptor antagonist from the findings that the compound showed at least 1000-fold selectivity over other histamine receptors [17]. Repeated intranasal administration of JNJ7777120 significantly inhibited nasal symptoms of allergic rhinitis in mice. Single and repeated oral administrations of JNJ7777120 also significantly inhibited nasal symptoms. In addition, repeated oral administration significantly decreased serum total IgE. Shapira et al. (1991) reported that IL-4 induced B cells to switch to IgE production. On the other hand, IFN-γ is known to inhibit the switching of B cells to IgE production. JNJ7777120 caused a significant decrease in the levels of IL-4 and a significant increase in the levels of IFN-gamma in nasal lavage fluid. JNJ 7777120, could relieve symptoms and inflammatory conditions in allergic rhinitis in rat, the effect was weak compared with H1 receptor antagonist—Loratadine [84].
6. CONCLUDING REMARKS
Histamine plays an important role as neurotransmitter and chemical mediator in multiple physiological and pathophysiological processes in central and peripheral tissues. In the last century the extensive study of its
Table 1. Overview of 4 histamine receptors.
actions in the human body, resulted in the identification of four G protein-coupled receptor (GPCR) subtypes (H1R-H4R), mediating numerous effects. The successful application of H1R and H2R antagonists/inverse agonists in the treatment of allergic conditions and gastric ulcer proved that these two receptors are excellent drug targets.
Growing pool of evidences about action and eficacy of H4R antagonists makes it very interesting to wait for the results of clinical trials. As it could be seen from the literature and novel patent applications there is huge reinforce of H4R antagonists in inflammatory diseases such as pruritus, asthma, inflammatory pain and allergic rhinitis. Targeting histamine mediated pathways could be now more effective and influence pathomechanisms of certain diseases covering all known subtypes of histamine receptors.
REFERENCES
- Togias, A. (2001) Systemic cross-talk between the lung and the nose. American Journal of Respiratory and Critical Care Medicine, 164, 726-727.
- Susiva, C. (2009) Antihistamine: Anti-allergic effects. Siriraj Medical Journal, 61, 66-67.
- Lewis, T. and Grant, T. (1924) Vascular reactions of the skin to injury. Heart, 11, 209-265.
- Popielski, L. (1920) β-Imidazolylathylamin und die Organextrakte Erster Teil: β-Imidazolylathylamin mechtiger Errezer der Magendrucken. Pfluegers Archiv, 178, 214-236. doi:10.1007/BF01722024
- Steinhoff, M., Griffiths, C., Church, M. and Lugar, T.A. (2004) Histamine. In: Burns, T., Breathnach, S., Cox, N. and Griffiths, C., Eds., Rook’s Textbook of Dermatology, Blackwell Science, Oxford, 9, 50-52.
- Endo, Y. (1982) Simultaneous induction of histidine and ornithine decarboxylases and changes in their product amines following the injection of Escherichia coli lipopolysaccharide into mice. Biochemical Pharmacology, 31, 1643-1647. doi:10.1016/0006-2952(82)90394-X
- Maintz, L. and Novak, N. (2007) Histamine and histamine intolerance. The American Journal of Clinical Nutrition, 85, 1185-1196.
- Saxena, S.P., Brandes, L.J., Becker, A.B., Simons, K.J., LaBella, F.S. and Gerrard, J.M. (1989) Histamine is an intracellular messenger mediating platelet aggregation. Science, 243, 1596-1599. doi:10.1126/science.2928797
- Shiraishi, M., Hirasawa, N., Oikawa, S., Kobayashi, Y. and Ohuchi, K. (2000) Analysis of histamine-producing cells at the late phase of allergic inflammation in rats. Immunology, 99, 600-606. doi:10.1046/j.1365-2567.2000.00986.x
- Szeberenyi, J.B, Pallinger, E., Zsinko, M., Pos, Z., Rothe, G., Orso, E., Szeberenyi, S., Schmitz, G., Falus, A. and Laszlo, V. (2001) Inhibition of effects of endogenously synthesized histamine disturbs in vitro human dendritic cell differentiation. Immunology Letters, 76, 175-182. doi:10.1016/S0165-2478(01)00184-5
- Vlieg-Boerstra, B.J., van der Heide, S., Oude Elberink, J.N., Kluin-Nelemans, J.C. and Dubois, A.E. (2005) Mastocytosis and adverse reactions to biogenic amines and histamine-releasing foods: What is the evidence? The Netherlands Journal of Medicine, 63, 244-249.
- Ring, J. (2004) Angewandte allergologie. Urban & Vogel, Munich.
- White, M.V., Slater, J. and Kaliner, M. (1987) Histamine and asthma. The American Review of Respiratory Disease, 57, 1165-1176.
- Nakamura, T., Itadani, H., Hidaka, Y., Ohta, M. and Tanaka, K. (2000) Molecular cloning and characterization of a new human histamine receptor, HH4R. Biochemical and Biophysical Research Communications, 279, 615-620. doi:10.1006/bbrc.2000.4008
- Milligan, G., Bond, R. and Lee, M. (1995) Inverse agonism: Pharmacological curiosity or potential therapeutic strategy? Trends in Pharmacological Sciences, 16, 10-13. doi:10.1016/S0165-6147(00)88963-4
- Ash, A.S. and Schild, H.O. (1997) Receptors mediating some actions of histamine. 1966. British Journal of Pharmacology, 120, 302-314. doi:10.1111/j.1476-5381.1997.tb06811.x
- Thurmond, R.L, Desai, P.J., Dunford, P.J., et al. (2004) A potent and selective histamine H4 receptor antagonist with anti-inflammatory properties. The Journal of Pharmacology and Experimental Therapeutics, 309, 404-413. doi:10.1124/jpet.103.061754
- Jutel, M., Akdis, M. and Akdis, C.A. (2009) Histamine, histamine receptors and their role in immune pathology. Clinical & Experimental Allergy, 39, 1786-1800. doi:10.1111/j.1365-2222.2009.03374.x
- LaBella, F.S., Queen, G.M. and Brandes, L.J. (2000) Interactive binding at cytochrome P-450 of cell growth regulatory bioamines, steroid hormones, antihormones, and drugs. Journal of Cellular Biochemistry, 76, 686-694. doi:10.1002/(SICI)1097-4644(20000315)76:4<686::AID-JCB16>3.0.CO;2-V
- Brandes, L.J., Queen, G.M. and LaBella, F.S. (2000) N,N-diethyl-2-[4phenoxy] ethanamine (DPPE) a chemopotentiating and cytoprotective agent in clinical trials: Interaction with histamine at cytochrome P450 3A4 and other isozymes that metabolize antineoplastic drugs. Cancer Chemotherapy and Pharmacology, 45, 298-304. doi:10.1007/s002800050044
- Paesen, G.C., Adams, P.L., Harlos, K., Nuttall, P.A. and Stuart, D.I. (1999) Tick histamine-binding proteins: Isolation, cloning, and three-dimensional structure. Molecular cell, 3, 661-671. doi:10.1016/S1097-2765(00)80359-7
- Raithel, M., Ulrich, P., Hochberger, J. and Hahn, E.G. (1998) Measurement of gut diamine oxidase activity. Diamine oxidase as a new biologic marker of colorectal proliferation? Annals of the New York Academy of Sciences, 859, 262-266. doi:10.1111/j.1749-6632.1998.tb11142.x
- Kusche, J., Bieganski, T., Hesterberg, R., Stahlknecht, C.D., Feussner, K.D., Stahlenberg, I. and Lorenz, W. (1980) The influence of carcinoma growth on diamine oxidase activity in human gastrointestinal tract. Agents and Actions, 10, 110-113. doi:10.1007/BF02024191
- Kalchmair, B., Klocker, J., Perkmann, R., Biebl, M., Bodner, G., Kolbitsch, C., Fraedrich, G. and Schwelberger, H.G. (2003) Alterations in plasma amine oxidase activities in a compartment syndrome model. Inflammation Research, 52, 67-68. doi:10.1007/s000110300058
- Maslinski, C. and Fogel, W.A. (1991) Catabolism of histamine. In: Uvnäs, B., Ed., Handbook of Experimental Pharmacology. Histamine and Histamine Antagonists, Springer, Berlin, 97, 165-189. doi:10.1007/978-3-642-75840-9_14
- Schwelberger, H.G., Hittmair, A. and Kohlwein, S.D. (1998) Analysis of tissue and subcellular localization of mammalian diamine oxidase by confocal laser scanning fluorescence microscopy. Inflamatory Research, 47, 60- 61. doi:10.1007/s000110050273
- Hill, S.J., Ganellin, C.R., Timmerman, H., Schwartz, J.C., Shankley, N.P., Young, J.M., Schunack, W., Levi, R. and Haas, H.L. (1997) International union of pharmacology. XIII. Classification of histamine receptors. Pharmacological reviews, 49, 253-278.
- Ikawa, Y., Suzuki, M., Shiono, S., Ohki, E., Moriya, H., Negishi, E. and Ueno, K. (2005) Histamine H4 receptor expression in human synovial cells obtained from patients suffering from rheumatoid arthritis. Biological & Pharmaceutical Bulletin, 28, 2016-2018. doi:10.1248/bpb.28.2016
- Dunford, P.J., O’Donnell, N., Riley, J.P., Williams, K.N., Karlsson, L. and Thurmond, R.L. (2006). The histamine H4 receptor mediates allergic airway inflammation by regulating the activation of CD4+ T cells. The Journal of immunology, 176, 7062-7070. doi:10.1006/bbrc.2001.4976
- Cogé, F., Guénin, S.P., Rique, H., Boutin, J.A. and Galizzi, J.P. (2001) Structure and expression of the human histamine H4-receptor gene. Biochemical and Biophysical Research Communications, 284, 301-309.
- Gantner, F., Sakai, K., Tusche, M.W., Cruikshank, W.W., Center, D.M. and Bacon, K.B. (2002) Histamine H4 and H2 receptors control histamine-induced interleukin-16 release from human CD8+ T cells. The Journal of Pharmacology and Experimental Therapeutics, 303, 300-307. doi:10.1124/jpet.102.036939
- Gutzmer, R., Diestel, C., Mommert, S., Köther, B., Stark, H., Wittmann, M. and Werfel, T. (2005) Histamine H4 receptor stimulation suppresses IL-12p70 production and mediates chemotaxis in human monocyte-derived dendritic cells. The Journal of Immunology, 174, 5224-5232.
- Kakavas, S., Zampeli, E., Papamichael, K., Delitheos, B. and Tiligada, E. (2006) The mast cell pathway to inflammation and homeostasis: Pharma-cological insights. Current medicinal chemistry. Anti-Inflammatory & AntiAllergy Agents, 5, 323-334. doi:10.2174/187152306778772793
- Dunford, P.J., Williams, K.N., Desai, P.J., Karlsson, L., McQueen, D. and Thurmond, R.L. (2007) Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. The Journal of Allergy and Clinical Immunology, 119, 176- 183. doi:10.1016/j.jaci.2006.08.034
- Zhang, M., Thurmond, R.L. and Dunford, P.J. (2007) The histamine H4 receptor: A novel modulator of inflammatory and immune disorders. Pharmacology & Therapeutics, 113, 594-606. doi:10.1016/j.pharmthera.2006.11.008
- Oda, T., Morikawa, N., Saito, Y., Masuho, Y. and Matsumoto, S. (2000) Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. The Journal of Biological Chemistry, 275, 36781-36786. doi:10.1074/jbc.M006480200
- Zhu, Y., Michalovich, D., Wu, H., et al. (2001) Cloning, expression, and pharmacological characterization of a novel human histamine receptor. Molecular Pharmacology, 59, 434-441.
- Nguyen, T., Shapiro, D.A., George, S.R., Setola, V., Lee, D.K., Cheng, R., Rauser, L., Lee, S.P., Lynch, K.R., Roth, B.L. and O’Dowd, B.F. (2001) Discovery of a novel member of the histamine receptor family. Molecular Pharmacology, 59, 427-433.
- Morse, K.L., Behan, J., Laz, T.M., et al. (2001) Cloning and characterization of a novel human histamine receptor. The Journal of Pharmacology and Experimental Therapeutics, 296, 1058-1066.
- Liu, C., Ma, X., Jiang, X., Wilson, S.J., Hofstra, C.L., Blevitt, J., Pyati, J., Li, X., Chai, W., Carruthers, N. and Lovenberg, T.W. (2001) Cloning and pharmacological characterization of a fourth histamine receptor (H(4)) expressed in bone marrow. Molecular Pharmacology, 59, 420-426.
- O’Reilly, M.R., Alpert, S., Jenkinson, R.P., et al. (2002) Identification of a histamine H4 receptor on human eosinophils: Role in eosinophil chemotaxis. Journal of Receptor and Signal Transduction Research, 22, 431- 448. doi:10.1081/RRS-120014612
- Cogé, F., Guénin, S.P., Audinot, V., Renouard-Try, A., Beauverger, P. and Macia, C. (2001) Genomic organization and characterization of splice variants of the human histamine H3 receptor. The Biochemical Journal, 355, 279-288. doi:10.1042/0264-6021:3550279
- Liu, C., Wilson, S.J., Kuei, C. and Lovenberg, T.W. (2001) Comparison of human, mouse, rat, and guinea pig histamine H4 receptors reveals substantial pharmacological species variation. The Journal of Pharmacology and Experimental Therapeutics, 299, 121-130.
- Oda, T., Matsumoto, S., Masuho, Y., Takasaki, J., Matsumoto, M., Kamohara, M., Saito, T., Ohishi, T., Soga, T., Hiyama, H., Matsushime, H. and Furuichi, K. cDNA cloning and characterization of porcine histamine H4 receptor. Biochimica et Biophysica Acta, 1575, 135- 138.
- Leurs, R., Chazot, P.L., Shenton, F.C., Lim, H.D. and de Esch, I.J. (2009) Molecular and biochemical pharmacology of the histamine H 4 receptor. British Journal of Pharmacology, 157, 14-23. doi:10.1111/j.1476-5381.2009.00250.x
- Buckland, K.F., Williams, T.J. and Conroy, D.M. (2003) Histamine induces cytoskeletal changes in human eosinophils via the H4 receptor. British Journal of Pharmacology, 140, 1117-1127. doi:10.1038/sj.bjp.0705530
- Hofstra, C.L., Desai, P.J., Thurmond, R.L. and FungLeung, W.P. (2003) Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. The Journal of Pharmacology and Experimental Therapeutics, 305, 1212-1221. doi:10.1124/jpet.102.046581
- De Esch, I.J., Thurmond, R.L., Jongejan, A. and Leurs, R. (2005) The histamine H4 receptor as a new therapeutic target for inflammation. Trends in Pharmacological Sciences, 26, 462-469.
- Rosethorne, E.M. and Charlton, S.J. (2011) Agonistbiased signaling at the histamine H4 receptor: JNJ- 7777120 recruits β-arrestin without activating G proteins. Molecular Pharmacology, 79, 749-757. doi:10.1124/mol.110.068395
- Seifert, R., Schneider, E.H., Dove, S., Brunskole, I., Neumann, D., Strasser, A. and Buschauer, A. (2011) Paradoxical stimulatory effects of the “standard” histamine H4 receptor antagonist JNJ7777120: The H4 receptor joins the club of 7 transmembrane domain receptors exhibiting functional selectivity. Molecular Pharmacology, 79, 631-638. doi:10.1124/mol.111.071266
- Schneider, E.H., Schnell, D., Papa, D. and Seifert, R. (2009) High constitutive activity and a Gproteinindependent high-affinity state of the human histamine H 4 receptor. Biochemistry, 48, 1424-1438. doi:10.1021/bi802050d
- Brunskole, I. (2011) Molecular and cellular analysis of aminergic G protein-coupled receptors: Histamine H2, H4 and β2-adrenergic receptors, a scientific paradigm. Dissertation, Universität Regensburg, Regensburg.
- Horr, B., Borck, H., Thurmond, R., Grösch, S. and Diel, F. (2006) STAT1 phosphorylation and cleavage is regulated by the histamine (H4) receptor in human atopic and non-atopic lymphocytes. International Immunopharmacology, 6, 1577-1585. doi:10.1016/j.intimp.2006.06.005
- Michel, I., Borck, H., McElligott, S., Krieg, C. and Diel, F. (2008) Histamine receptor H4R-selective ligands influence the STAT6 Transcription Activation Domain (TAD) and the DNA-binding. Inflammation Research, 57, 47-48. doi:10.1007/s00011-007-0623-1
- Godot, V., Arock, M., Garcia, G., Capel, F., Flys, C., Dy, M., Emilie, D. and Humbert, M. (2007) H4 histamine receptor mediates optimal migration of mast cell precursors to CXCL12. The Journal of Allergy and Clinical Immunology, 120, 827-834. doi:10.1016/j.jaci.2007.05.046
- Lippert, U., Artuc, M., Grützkau, A., Babina, M., Guhl, S., Haase, I., Blaschke, V., Zachmann, K., Knosalla, M., Middel, P., Krüger-Krasagakis, S. and Henz, B.M. (2004) Human skin mast cells express H2 and H4, but not H3 receptors. The Journal of Investigative Dermatology, 123, 116-123. doi:10.1111/j.0022-202X.2004.22721.x
- Barnard, R., Barnard, A., Salmon, G., Liu, W. and Sreckovic, S. (2008) Histamine-induced actin polymerization in human eosinophils: An imaging approach for histamine H4 receptor. Cytometry. Part A: The Journal of the International Society for Analytical Cytology, 73, 299-304.
- Damaj, B.B., Becerra, C.B., Esber, H.J., Wen, Y. and Maghazachi, A.A. (2007) Functional expression of H4 histamine receptor in human natural killer cells, monocytes, and dendritic cells. The Journal of Immunology, 179, 7907-7915.
- Ikawa, Y., Shiba, K., Ohki, E., Mutoh, N., Suzuki, M., Sato, H. and Ueno, K. (2008) Comparative study of histamine H4 receptor expression in human dermal fibroblasts. The Journal of Toxicological Sciences, 33, 503-508. doi:10.2131/jts.33.503
- [61] Morini, G., Becchi, G., Shenton, F.C., Chazot, P.L. and Grandi, D. (2008) Histamine H3 and H4 receptors are expressed on distinct endocrine cell types in the rat fundic mucosa. Inflammation Research, 57, 57-58. doi:10.1007/s00011-007-0628-9
- [62] Lim, H.D., Smits, R.A., Leurs, R. and De Esch, I.J. (2006) The emerging role of the histamine H4 receptor in antiinflammatory therapy. Current Topics in Medicinal Chemistry, 6, 1365-1373. doi:10.2174/15680266106061365
- [63] Nakayama, T., Kato, Y., Hieshima, K., Nagakubo, D., Kunori, Y., Fujisawa, T. and Yoshie, O. (2004) Liverexpressed chemokine/CC chemokine ligand 16 attracts eosinophils by interacting with histamine H4 receptor. The Journal of Immunology, 173, 2078-2083.
- [64] Ling, P., Ngo, K., Nguyen, S., Thurmond, R.L, Edwards, J.P., Karlsson, L. and Fung-Leung, W.P. (2004) Histamine H4 receptor mediates eosinophil chemotaxis with cell shape change and adhesion molecule upregulation. British Journal of Pharmacology, 142, 161-171. doi:10.1038/sj.bjp.0705729
- [65] Takeshita, K., Sakai, K., Bacon, K.B. and Gantner, F. (2003) Critical role of histamine H4 receptor in leukotriene B4 production and mast cell-dependent neutrophil recruitment induced by zymosan in vivo. The Journal of Pharmacology and Experimental Therapeutics, 307, 1072-1078. doi:10.1124/jpet.103.057489
- [66] Jutel, M., Watanabe, T., Klunker, S., Akdis, M., Thomet, O.A., Malolepszy, J., Zak-Nejmark, T., Koga, R., Kobayashi, T., Blaser, K. and Akdis, C.A. (2001) Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature, 413, 420- 425. doi:10.1038/35096564
- [67] Dijkstra, D., Leurs, R., Chazot, P., Shenton, F.C., Stark, H., Werfel, T. and Gutzmer, R. (2007) Histamine downregulates monocyte CCL2 production through the histamine H4 receptor. The Journal of Allergy and Clinical Immunology, 120, 300-307. doi:10.1016/j.jaci.2007.03.024
- [68] Dijkstra, D., Stark, H., Chazot, P.L., Shenton, F.C., Leurs, R., Werfel, T. and Gutzme, R. (2008). Human inflammatory dendritic epidermal cells express a functional histamine H 4 receptor. The Journal of Investigative Dermatology, 128, 1696-1703. doi:10.1038/sj.jid.5701250
- [69] Busse, W.W. and Swenson, C.A. (1989) The relationship between plasma histamine concentrations and bronchial obstruction to antigen challenge in allergic rhinitis. The Journal of Allergy and Clinical Immunology, 84, 658- 666. doi:10.1016/0091-6749(89)90293-5
- [70] Thurmond, R.L., Gelfand, E.W. and Dunford, P.J. (2008) The role of histamine H1 and H4 receptors in allergic inflammation: The search for new antihistamines. Nature Reviews. Drug Discovery, 7, 41-53. doi:10.1038/nrd2465
- [71] Van Ganse, E., Kaufman, L., Derde, M.P., Yernault, J.C., Delaunois, L. and Vincken, W. (1997) Effects of antihistamines in adult asthma: A meta-analysis of clinical trials. The European Respiratory Journal, 10, 2216-2224. doi:10.1183/09031936.97.10102216
- [72] Leopold, J.D., Hartley, J.P. and Smith, A.P. (1979) Effects of oral H1 and H2 receptor antagonists in asthma. The European Respiratory Journal, 8, 249-251.
- [73] Neumann, D., Beermann, S. and Seifert, R. (2010) Does the histamine H4 receptor have a proor anti-inflammatory role in murine bronchial asthma? Pharmacology, 85, 217-223. doi:10.1159/000285088
- [74] Yu, S., Stahl, E., Li, Q. and Ouyang, A. (2008) Antigen inhalation induces mast cells and eosinophils infiltration in the guinea pig esophageal epithelium involving histamine-mediated pathway. Life Sciences, 82, 324-330. doi:10.1016/j.lfs.2007.12.002
- [75] Cowden, J.M., Riley, J.P., Ma, J.Y., Thurmond, R.L. and Dunford, P.J. (2010) Histamine H4 receptor antagonism diminishes existing airway inflammation and dysfunction via modulation of Th2 cytokines. Respiratory Research, 11, 86. doi:10.1186/1465-9921-11-86
- [76] Morgan, R.K., McAllister, B., Cross, L., Green, D.S., Kornfeld, H., Center, D.M. and Cruikshank, W.W. (2007) Histamine 4 receptor activation induces recruitment of FoxP3+ T cells and inhibits allergic asthma in a murine model. The Journal of immunology, 178, 8081-8089.
- [77] Yu, F., Bonaventure, P. and Thurmond, R.L. (2010) The future antihistamines: Histamine H(3) and H(4) receptor ligands, Advances in Experimental Medicine and Biology, 709, 125-140. doi:10.1007/978-1-4419-8056-4_12
- [78] Deml, K.F., Beermann, S., Neumann, D., Strasser, A. and Seifert, R. (2009) Interactions of histamine H1-receptor agonists and antagonists with the human histamine H4- receptor. Molecular Pharmacology, 76, 1019-1030. doi:10.1124/mol.109.058651
- [79] Pichavant, M., Matangkasombut, P., Dekruyff, R.H. and Umetsu, D.T. (2009) Natural killer T cells regulate the development of asthma. Expert Review of Clinical Immunology, 5, 251-260. doi:10.1586/eci.09.7
- [80] Leite-de-Moraes, M.C., Diem, S., Michel, M.L., Ohtsu, H., Thurmond, R.L., Schneider, E. and Dy, M. (2009) Cutting edge: Histamine receptor H4 activation positively regulates in vivo IL-4 and IFN-gamma production by invariant NKT cells. The Journal of Immunology, 182, 1233-1236.
- [81] Taylor-Clark, T.E. (2008) Insights into the mechanisms of histamine-induced inflammation in the nasal mucosa. Pulmonary Pharmacology & Therapeutics, 21, 455-460. doi:10.1016/j.pupt.2007.08.002
- [82] Nakaya, M., Takeuchi, N. and Kondo, K. (2004) Immunohistochemical localization of histamine receptor subtypes in human inferior turbinates. The Annals of Otology, Rhinology, and Laryngology, 113, 552-557.
- [83] Sugimoto, Y., Kawamoto, E., Chen, Z. and Kamei, C. (2000) A new model of allergic rhinitis in rats by topical sensitization and evaluation of H (1)-receptor antagonists. Immunopharmacology, 48, 1-7. doi:10.1016/S0162-3109(00)00173-9
- [84] Howarth, P. (2002) Antihistamines in rhinoconjunctivitis. Clinical Allergy and Immunology, 17, 179-220.
- [85] Takahashi, Y., Kagawa, Y., Izawa, K., Ono, R., Akagi, M. and Kamei, C. (2009) Effect of histamine H4 receptor antagonist on allergic rhinitis in mice. International Immunopharmacology, 9, 734-738. doi:10.1016/j.intimp.2009.02.011
NOTES
*Corresponding author.

