Advances in Biological Chemistry
Vol.06 No.05(2016), Article ID:71593,17 pages
10.4236/abc.2016.65014
Coumarin and Biscoumarin Inhibit in Vitro Obesity Model
Farah Mukhtar1, Kimberly Stieglitz2, Shamsher Ali3, Asma Ejaz4, Muhammad Iqbal Choudhary5,6,7, Muhammad Imran Fakhri7, Uzma Salar7, Khalid Mohammed Khan7
1Department of Chemistry, University of Karachi, Karachi, Pakistan
2STEM Biotechnology Division, Roxbury Community College, Roxbury, MA, USA
3Department of Environmental Health, Harvard University TH Chan School of Public Health, Harvard-MIT Division of Health Sciences and Technology, Department of Medicine, Brigham and Women’s Hospital, Boston, MA, USA
4Vascular Surgery Research, Harvard Medical School, Boston, MA, USA
5Dr. Panjwani Center for Molecular Medicine and Drug Research, International Center for Chemical and Biological Sciences, University of Karachi, Karachi, Pakistan
6Department of Biochemistry, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
7H. E. J. Research Institute of Chemistry, International Center for Chemical and Biological Sciences, University of Karachi, Karachi, Pakistan

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: August 27, 2016; Accepted: October 25, 2016; Published: October 28, 2016
ABSTRACT
Coumarin, sulphonated coumarin, and biscoumarin compounds were examined for their effects on suppressing the adipocyte differentiation in 3T3-L1 cells. Many of them inhibited the adipocyte differentiation in a dose dependent manner, amongst them compounds (7), (28), and (33) significantly suppressed the adipogenic differentiation, and also exhibited lipolytic effect on mature adipocytes. The active compounds potentially imitate the AMP-activated protein kinase (AMPK) ligands, there- fore, binding of these compounds with AMPK possibly shuts down the anabolic pathways. Furthermore, these compounds were docked into binding pockets with reasonable predicted binding constants in the low micromolar range. These results indicate that compounds (7), (28), and (33) inhibit adipogenic development in pre- adipocytes, having lipolytic effect on mature adipocytes, and can be potent activators of human AMPK.
Keywords:
Coumarin, Sulphonated Coumarin, Biscoumarin, Adipocyte Differentiation and Docking Studies

1. Introduction
1.1. Obesity
Obesity has become a serious health problem that constitutes the greatest threat to global human well-being. Many factors involve in the progression of obesity, for instance, genetic variation, endocrine disorders, nutritional and medicinal changes, to name a few. This leads to several pathological conditions, such as diabetes, hypertension, hyper-lipidemia, and cardiovascular diseases [1] - [3] . The prevalence of obesity epidemic varies in different races and communities of the world, mainly associated with heredity, gender, dietary patterns, life style and behavior.
Adipose tissue is one of the main connective tissues that are metabolically crucial for insulin sensitivity, and energy homeostasis. Its primary function is to store excess nutrients as triacylglycerols, and to release free fatty acids during fasting. Adipose tissues secrete more than 50 hormones and signaling molecules, collectively called adipokines, which are involved in energy homeostasis, glucose metabolism, and immunity [4] , vasculature and metabolic changes associated with body weight change. More specifically, adipokines can exhibit either pro-inflammatory or anti-inflammatory properties, thereby contributing to insulin resistance. In obesity, adipocytes undergo abnormal growth characterized by increased numbers of fat cells (hyperplasic) or increase in adipocyte volume (hypertrophic), resulted in insulin resistance, lipid and other consequent disorders. Pathological adipocyte growth is based on the excessive mitogenesis and differentiation. Therefore inhibition of mitogenesis and differentiation of pre-adipocytes to adipocytes can prevent the initiation and progression of obesity [5] . With recognition of the epidemic of obesity, the need of effective treatments is increasing day by day in order to improve the quality of life because it is a degenerative disease, for which none of the current medications provide a cure. Thus, when treatment is stopped, patients regain body weight since the disease is still present. Only five new drugs have been registered over the last fifteen years, namely, dexfenfluramine (Redux®), sibutramine (Meridia®, Reductil®), orlistat (Xenical®) and rimonabant (Acomplia®), for the treatment of obesity. The antiobesity drug candidates in different phases of clinical trials include the centrally-acting monoaminergic food intake regulators (tesofensine, ATHX-105 and PRX-0703), hypothalamic neuropeptides (obinepitide), gut hormones (TKS 1225), and various combination products (empatic®, contrave® and pramlintide/ metreleptin) [6] - [8] . Besides the pharmacological treatment, the emphasis is now on behavior therapy, as adjunct therapy, that aims to modify the dietary habits and sedentary life style. It has been reported that some drugs have a potential to target metabolic pathways of adipocytes, liver and skeletal muscles in preclinical trials, but none has yet reached to the clinical trials.
1.2. Coumarin and Biscoumarin
Coumarins represent an important class of natural, and synthetic analogues of oxygen containing heterocycles. They have a typical benzopyrone structure with rich electron and good charge transport properties [9] . Coumarin backbone is extensively applied to synthesize diverse functional molecules for biological diagnosis, and as probes. A great deal of work has been directed not only towards the purification of naturally occurring coumarins from a variety of plants, animals, and microorganisms, but also towards the synthesis of coumarin analogues with novel structures and properties [10] - [12] . Coumarins and their derivatives as pharmacophores have attracted intense interest. These compounds have the ability to engage in non-covalent interactions (hydrophobic, π-π, and electrostatic interactions as well hydrogen bonding, metal coordination, van der waals forces etc.) with various active sites in bimolecules, and thus display a wide range of biological activities, such as anticoagulant [13] , antineurodegenerative [14] , antioxidant [15] , anticancer [16] , anti-inflammatory, anti-diabetes, antidepresive [15] , antimicrobial efficacies [17] , etc. 4-hydroxycoumarin derivatives of natural or synthetic origin has attracted much interest in several fields [18] - [20] . These compounds are reported to have diverse biological activities, such as coagulant, insecticidal, antihelmintic, hypnotic, HIV protease inhibition, and antifungal activities [21] - [24] . Biscoumarin (a 4-hydrox- ycoumarin dimer), another important class, has attracted great interest due to the novelity of its molecular structures and diverse biological properties. The presence of two intramolecular hydrogen bonds, and different substituents on the central linker methylene enables it to interact with various enzymes and receptors through weak bond interaction, thus exhibit versatile biological activities, for instance, antifungal, anti HIV, anticancer, antithrombotic, anticoagulant, antimicrobial and antioxidant [25] - [32] , urease inhibitory [33] , cytotoxicity and enzyme inhibitory activities [34] - [36] . In conclusion, coumarin derivatives in general, and biscoumarins, in particular, have received considerable attention because of their biological importance and numerous pharmacological activities.
2. Experimental
2.1. Materials
Swiss albino mouse 3T3-L1 fibroblast (CL-173) cells were purchased from the American Type Culture Collection (Manassas, VA, USA), while 3T3-NIH mouse fibroblast cells were obtained from Aga Khan University Hospital, Karachi, Pakistan. Fetal bovine serum (FBS) was purchased from Invitrogen (Grand Island, NY, USA) and Biowest (9871-5244). DMSO (≥99% purity), ethanol (99.5% pure absolute and non denatured alcohol), methanol (≥99% purity), isopropanol, 3-isobutyl-1-methylxanthine (IBMX), dexamethasone (DEX), insulin, penicillin (1000 U/mL), streptomycin (1000 U/mL), sodium bicarbonate, sodium hydroxide, curcumin (≥94 purity), epigallocatechin gallate (EGCG, ≥95 purity), and RIPA buffer were purchased from Sigma-Aldrich (St. Louis, MO, USA). DMEM (Dulbecco modified Eagle medium), DMEM-F12 (Dulbecco mo- dified eagle medium, nutrient F-12), phosphate buffer saline (PBS), and penicillin/ streptomycin were purchased from GIBCO BRL Life Technologies (Gaithersburg, MD, USA). Cell titer blue was purchased from Promega (Madison, UI, USA), while trypsin- EDTA was obtained from Hyclone (Logan, UT). Oil red O was purchased from Sigma- Aldrich (St. Louis, MO, USA). Protease inhibitor, phosphatase inhibitor, bovine serum albumin (F-V), nitrocellulose membranes, western blot filter papers, nitrocellulose membrane (0.45 µm, 8 cm × 12 cm), stripping buffer, and modified Lowry protein assay kit (P-123240) were purchased from Pierce, Life Technologies (Thermo Fischer Scientific Corporation, CA, USA). Other reagents used in the western blot i.e., glycine, trisbase, sodium chloride, tween-20, TEMED (N,N,N’,N’-tetramethylenediamine), ammonium per sulfate (APS), and sodium dodecyl sulfate (SDS) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Primary antibody (SCD-1, C12H5, Rabbit, mAm), and secondary antibody (FITC Alexa-labeled secondary anti-rabbit) was purchased from Cell Signaling Technology (Danvers, Ma, USA). Chemiluminescent blue prestained Mol. Wt. Marker mix (No. 26651, 18 - 220 KDa) was purchased from Pierce Life Technologies (Thermo Fischer Scientific Corporation, CA, USA). Polystyrene culture flasks (canted neck and filtered caps), stretop filters (0.22 µm, PVDF, non-pyrogenic, and stripettepipets were purchased from Corning (NY, USA). Syringe filters (0.22 µm, PVDF) were obtained from Millipore (Massachusetts, USA). Tissue treated plates (96- well, flat bottom) were purchased from Orange Scientific Company (Braine-l’ Allued, Belgium), while 24- and 6-well plates were purchased from Jet Biofilm (China, Cat. No. TCP 011006 and TCP 011024, respectively).
2.2. In Vitro Assays
2.2.1. Antiadipogenesis Assay
1) Method
The antiadipogenic effect of test compounds was evaluated by using the in vitro adipogenesis assay on 3T3-L1. 3T3-L1 mouse embryo fibroblasts pre-adipocytes were obtained from American Type Culture Collection (Manassas, VA, USA). Cells were grown in Dulbecco’s modified eagle medium-F-12 (DMEM F-12) containing 10% fetal bovine serum (FBS) and 2% antibiotic (pencillin/streptomycin), incubated at 37˚C in a humidified 5% CO2 atmosphere until 70% - 80% confluency. Cell suspension (2 × 103 cells/mL) was seeded in the 96-well plate and incubated for confluency. Two days of post confluency, the cells were stimulated to differentiate with DMEM-F-12 containing 10% fetal bovine serum (FBS), 5 μg/mL insulin, 0.5 mM 3-isobutyl-1-methylxanthine, and 0.25 mM dexamethasone for 96 h, at which time >90% of cells were mature adipocytes with accumulated fat droplets. Compounds were dissolved in dimethylsulfoxide and added to cell culture medium at concentrations of 50, 20, 15, 10, 5 μg/mL. The final concentration of dimethylsulfoxide was 0.001%. After 4 days of induction of differentiation medium, the medium was replaced with insulin medium (10% DMEM containing insulin). On day 6, the medium was changed with regular medium. Two days later, cell monolayer was washed with PBS and fixed with 10% formalin in PBS. Intracellular lipid contents were measured by staining the cells with Oil Red O. After 20 minutes, dye was aspirated and the absorbance was measured at 520 nm using Microplate Spectra Max 340 (Molecular Devices, CA, USA). At the end, IC50 values were calculated, and all experiments were performed in triplicate. Curcumin and epigallocatechin gallate were used as standards in this assay.
2) 3T3-L1 Cell Culture
B3T3-L1 Pre-adipocyte cell line was obtained from ATCC (American Type Culture Collection, Rockville, MD, USA). Cells were cultured in DMEM-F12 (Dulbecco modified eagle medium, nutrient F12), with 10% fetal bovine serum (FBS), 100 1U/mL penicillin, 100 μg/mL streptomycin and 2 mM glutamax. Cells were incubated at 37˚C with 5% CO2. Cells were examined under a phase contrast microscope, when cells reached 80% of confluence in culture flask, the medium was removed, and the cells were rinsed twice with PBS (Without Ca and Mg). Washing solution was replaced with 1 - 2 mL trypsin EDTA solution for 2 - 3 minutes until the cells were completely detached. The cell suspension was centrifuged at 1200 rpm and 25˚C for 10 minutes, cell pellet was resuspended in complete medium, and the number of cells was counted by using hemocytometer. Preparation of cells for assay: Fresh cultured cells were used for the test. Cell suspension 3T3-L1 (2 × 103 cells/mL) was prepared for seeding the 96-well plate. 100 μL of cell suspension was dispensed in each well of the 96-well plate, and placed in the incubator for 24 hours (37˚C, 90% humidity, 5% CO2 air). Plates were examined under the microscope to assure the even growth all across the micro titer plates. Preparation of test compounds: The test compounds were prepared immediately prior to use in aseptic condition. Stock solution was made with 0.5 mg/mL in 0.001% of DMSO.
3) Oil Red O Staining
3T3-L1 adipocytes were washed with PBS, fixed with 10% formalin in PBS for 1 hour, and then stained with Oil Red O (six parts of 0.6% Oil Red O in isopropanol and four parts of water) for 20 minutes. Excessive stain was removed by washing with deionized water three times, and the stained cells were dried and then oil droplets were dissolved in 100 μL of isopropanol, and quantified by measuring the absorbance at 520 nm. The method has been followed as described by Ejaz A. [37] . Briefly, IC50 Values were calculated by using EZ-Fit enzyme kinetics program (Perellela Scientific, Inc., Amherst, Massachusetts, USA).
4) Protein Extraction and Western Blot
In order to determine the changes in the protein expression of enzymes involved in adipogenesis, cells were washed with ice cold PBS and scrapped with the RIPA lysis buffer (150 mM sodium chloride, 1.0% NP-40 or Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecylosulphate, 50 mM Tris). The supernatant was centrifuged at 15,000 g for 15 minutes at 4˚C. The protein concentration was measured by modified Lowry protein assay. The western blotting was performed using SCD-1, antibody involved in fatty acid metabolism. The expression of SCD-1 was normalized with β-actin expression. The membranes were scanned by using Versa Doc Scanner, and calculations and correction of bands were done by Image Studio Lite software (LI-COR, Biosciences, Lincoln, Nebraska, USA).
2.2.2. Cytotoxicity Assay (MTT Assay)
1) Method
The cell growth inhibitory effect of test compounds was determined by using the MTT assay on 3T3-NIH. For the assay, cells were grown in DMEM containing 10% FBS and 2% antibiotic (Penicillin/streptomycin), maintained at 37C with 5% CO2 level for 24 hours incubation to allow for cell adherence. Various concentrations of sample (50, 25, 12.5 μg/mL) were added into the well and incubated for 48 hours. A 50 μL MTT (3- (4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium bromide) (2 mg/mL) were added to the well, 4 hours before the end of incubation. Medium and reagents were aspirated and 100 μL DMSO was added and mixed thoroughly for 15 minutes to dissolve the formazan crystals. The absorbance was measured at 570 nm using microplate Spectra Max 340 (Molecular Devices, CA, USA). At the end, IC50 values were calculated and at least three independent experiments were carried out for each sample. Cycloheximide was used in this assay as a positive control.
2) 3T3-NIH Cell Culture
3T3-NIH Cell line was obtained from Aga Khan University Hospital, Karachi, Pakistan. Cells were cultured in Dulbecco modified eagle medium (DMEM), with 10% fetal bovine serum (FBS), 100 1U/mL penicillin, 100 μg/mL streptomycin and 2 mM glutamax. Cells were incubated at 37˚C with 5% CO2. Cells were examined on a daily basis for checking the confluency, under a phase contrast microscope. When the cells reached 80% of confluence in culture flask, the medium was removed, and the cells were rinsed twice with PBS (Without Ca and Mg). Washing solution was replaced with 1 - 2 mL trypsin EDTA solution for 2 - 3 minutes until the cells were completely detached. The cell suspension was centrifuged for 10 minutes at 1200 rpm and 25˚C. The cell pellet was resuspended in complete medium, and the number of cells was counted by using hemocytometer. Preparation of cells for assay: Fresh cultured cells were used for the test. Cell suspension of 3T3-NIH (1 × 105 cells/mL) was prepared for seeding the 96-well plate. 100 μL of cell suspension was dispensed in each well of the 96-well tissue culture microtiter plate, and placed in the incubator for 24 hours (37˚C, 90% humidity, 5% CO2 air). Plates were examined under the microscope to assure the even growth all across the microtiter plates. Preparation of test compounds: The test compound solutions were prepared immediately prior to use in aseptic condition. Stock solution was made with 0.5 mg/mL in 0.01% of DMSO.
3. Results
3.1. Chemistry
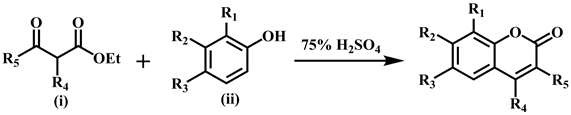
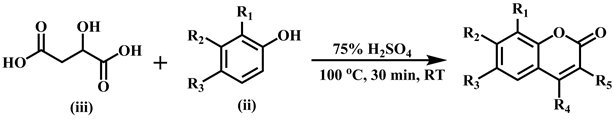
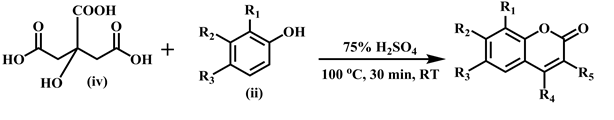
Considerable increase of attention towards the synthesis of coumarins and their derivatives has been seen in last few decades. Pechmann reaction is an acid catalyzed and high yielding method among the various reported methods in literature. Coumarin derivatives 1 - 24 were synthesized by Khan et al. by adopting following two methods as reported earlier [12] .
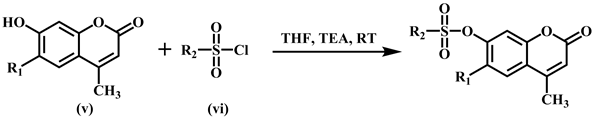
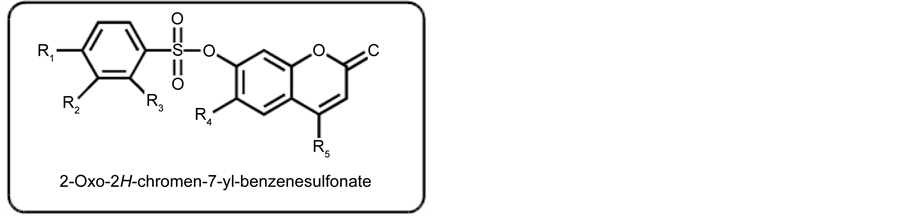
The synthesis of sulfonated coumarins 25-30 was carried out by reacting 7-hydroxy substituted coumarin derivatives (v) with the substituted sulfonyl chlorides (vi) in the presence of triethyl amine at room temperature, and tetrahydrofuran (THF) was used as solvent. The reaction proceeding was checked by monitoring TLC and crystallized from methanol.
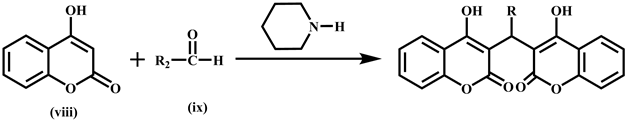
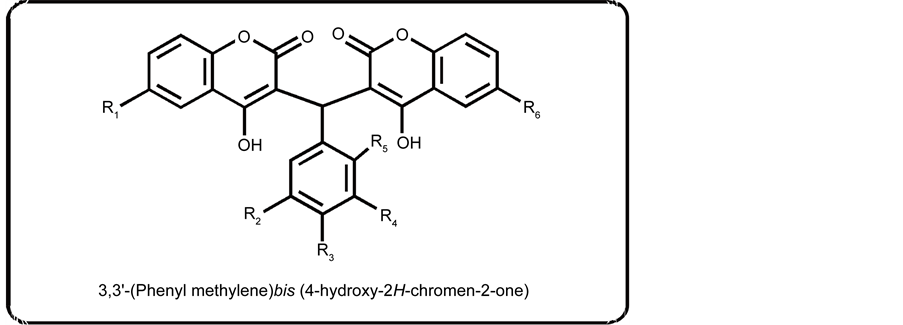
The synthesis of biscoumarins 31-38 was done by reacting two moles of 4-hydrox- ycoumarin (viii) with one mole of aldehyde (ix) in ethanol at room temperature in the presence of catalytic amount of piperidine to afford excellent yields [33] .
3.2. In Vitro Antiadipogenesis Assay and Evaluation of Cytotoxic Effect of Test Compounds by MTT Assay
3.2.1. Coumarins
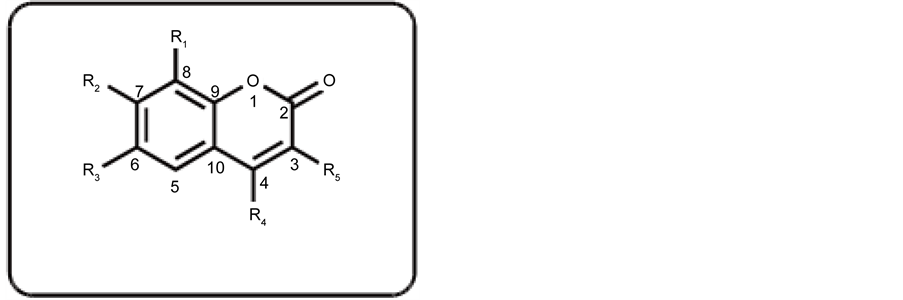
The inhibitory activity of compounds (1 - 24) on adipocyte differentiation was evaluated by in vitro antiadipogenesis assay. The basic structure of the coumarins is shown in Scheme 1.
Among the various coumarins tested, compounds (7) [7,8-dihydroxy-4-methyl-2H- chromen-2-one] (5) [7-hydroxy-4,8-dimethyl-2H-chromen-2-one] showed the most potent activity, followed by compounds (10), (11), and (14). Compounds (10) [ethyl6, 8-dichloro-2-oxo-2H-chromene-3-carboxylate], (11) [7-hydroxy-4-methyl-2H-chromen- 2-one], and (14) [2-oxo-2H-chromen-3-carboxamide] showed a good inhibitory effect on differentiation of adipocytes with IC50 values of 37.79, 26.46, and 29.61 µM respectively.
Scheme 1. Basic skeleton of coumarins.
The absence of second hydroxyl group at C-8 in compound (11) decreases the activity as compared to compound (7), while the substitution of ?CONH2 at C-3 in compound (14) increases the inhibitory effect to some extent, as compared to compound (7). These compounds were found to be non cytotoxic in the MTT assay. Compounds (9) [3-acetyl-8-methoxy-2H-chromen-2-one], (1) [4-hydroxy-6-methyl-2H-chromen-2- one], (21) [ethyl-8-ethoxy-2-oxo-2H-chromene-3-carboxylate], (24) [3-acetyl-6-methoxy- 2H-chromene-2-one], (10) [ethyl 6,8-dichloro-2-oxo-2H-carboxylate], and (8) [7-hydroxy- 8-methyl-2H-chromen-2-one] showed a moderate anti-adipogenic activity. Other compounds of this series showed low to weak inhibitory activity. The compounds of this series found to be non-cytotoxic, except compound (18). Interestingly compound (12) showed low IC50 value in antiadipogenesis assay, not only due to inhibitory effect but also loss of cell adherence quickly. Further studies on different parameters for this compound are under way (Table 1).
3.2.2. Sulphonated Coumarins
Antiadipogenic activity of compounds (25 - 30), and standards i.e. curcumin and epigallocatechin gallate were determined by in vitro adipogenesis assay. The basic skeleton of sulphonated coumarins is shown in Scheme 2. Antiadipogenic activity: The adipogenic inhibitory effect of sulphonated biscoumarins along in comparison to the standards was in the order of EGCG > Curcumin > compounds (28) > (30) > (29) > (25) > (26) > (27) as shown in Table 2.
3.2.3. Biscoumarins
The inhibitory activity of compounds (31 - 38) on adipocyte differentiation was evaluated by in vitro antiadipogenesis assay. The basic skeleton of biscoumarins is shown in Scheme 3. Hence in in vitro screening of three pharmacologically important classes, several compounds i.e., (5), (7), (11), (12), (14), (28), and (33) showed a significant inhibitory effect on adipogenic differentiation (Tables 1-3). It was also observed that these compounds also have lipolytic effect on mature adipocytes (see Figure 1 and Figure 2). In order to further investigate the effect of these compounds at the molecular level, compounds (5), (7), (11), and (33) were examined for mRNA levels of genes
Table 1.AntiadipogenesisandMTTassay (IC50 values in µM)ofcompounds (1-24),and standards.
Antiadipogenesis assay: Standards IC50 (µM) ± SEM values: Curcumin = 18.89 ± 1.75, EGCG: 16.34 ± 0.66, aSEM: standard error of means of three experiments. MTT assay: Standard IC50 (µM) ± SEM values: Cycloheximide = 0.26 ± 0.12, aSEM: standard error of means of three experiments, ¥NA: not active.
Scheme 2. Basic skeleton of sulphonated coumarins.
Scheme 3. Basic skeleton of biscoumarins.
Table 2. Antiadipogenesis and MTT assay (IC50 values in µM) of compounds (25 - 30), and standards.
Antiadipogenesis assay: Standards IC50 (µM) ± SEM values: Curcumin = 18.89 ± 1.75, EGCG: 16.34 ± 0.66, aSEM: standard error of means of three experiments. MTT assay: Standard IC50 (µM) ± SEM values: Cycloheximide = 0.26 ± 0.12, aSEM: standard error of means of three experiments, ¥NA: not active.
involved in adipocyte differentiation and lipid synthesis in 3T3-L1 pre-adipocyte cells by western blot analysis (Figure 3). This was based on the previous report that the mRNA level of sterol-regulatory element-binding protein-1c (SREPB-1c) and stearoyl-CoA desaturase-1 (SCD-1, the major regulators of lipogenesis), is markedly increased by
Table 3. Anti-adipogenesis and MTT assay (IC50 values in µM) of compounds (31 - 38), and standards.
Antiadipogenesis assay: Standards IC50 (µM) ± SEM values: Curcumin = 18.89 ± 1.75, EGCG: 16.34 ± 0.66, aSEM: standard error of means of three experiments. MTT assay: Standard IC50 (µM) ± SEM values: Cycloheximide = 0.26 ± 0.12, aSEM: standard error of means of three experiments, ¥NA: not active.
Figure 1. Inhibitory effect of fat accumulation in 3T3-L1 cells by standards and test compounds (7), (5), (10), (11), (12), and (14) along with positive and negative control.3T3-L1 fibroblasts were incubated with standards (curcumin and epigallocatechingallate) and test compounds (7), (5), (10), (11), and (14). Microscopic images of Oil Red O staining of adipocytes were obtained at Day 8 of differentiation, Magnification: 40×.
Figure 2. Inhibitory effect of fat accumulation in 3T3-L1 cells by standards and test compounds (33), (36), (34), and (37) along with positive and negative control. 3T3-L1 fibroblasts were incubated with standards (curcumin and epigallocatechingallate) and test compounds (33), (36), (34), and (37). Microscopic images of Oil Red O staining of adipocytes were obtained at Day 8 of differentiation, Magnification: 40×.

Figure 3. (a): Effect of compounds (5), (7), (11), and (33) on expression of SCD-1, (b): dose dependent effect of compound (7).
MDI (Mixture of Differentiation Inducers). SCD-1 was shown to be essential for the onset of diet-induced body weight gain. Compound (7) remarkably down regulated the expression of SCD-1 in a dose dependent manner. Compound (5) also significantly suppressed the expression of SCD-1, while compound (33) suppressed the expression of SCD-1 to a lesser extent, and compound (11) did not show any suppressive effect (Figure 3). Hence it can be concluded that compound (7) [7,8-dihydroxy-4-methyl- 2H-chromen-2-one] showed a remarkable inhibition of adipocyte differentiation, lipolytic effect, and non cytotoxic effect, and was capable of suppressing the SCD-1 expression, a key lipogenic enzyme (Figure 3). Thus, it will be of interest to further study this compound at molecular level and to evaluate its effect on adiposity in in vivo model. The efficacy of the compounds (7) [7,8-dihydroxy-4-methyl-2H-chromen-2-one], (11) [7-hydroxy-4-methyl-2H-chromen-2-one], and (33) [3,3’-((2-Nitrophenyl)methylene)- bis (4-hydroxy-6-methyl- 2H-chromen-2-one)] could be tested against other key lipogenic enzymes, e.g. ACAT (acetyl-CoA acetyl transferases), DGAT (diglyceride acyl transferases) etc. This will provide further insight into practical approaches towards discovering new drugs against obesity disorders.
4. Discussion
Possible Mechanism of Active Coumarin via AMPK Activation
The active compounds (7), (28), and (33) potentially imitate the AMPK ligands. It is assumed that when these compounds bind with AMPK, they possibly shut down the anabolic pathways, as shown in Figure 4. In response, the catabolic pathways down- regulate the activity of key enzymes of intermediary metabolism. Fatty acid oxidation is enhanced by regulating the concentration of malonyl coenzyme A (MCA) through phosphorylation of acetyl-CoA carboxylase (ACC). MCA has an inhibitory effect on carnitine palmitoyl transferase-1 (CPT-1). In adipose tissues, these changes are associated with decrease in the activity of glycerol-3-phosphate acyltransferase (GPAT). In addition to increasing fatty acid oxidation, activation of AMPK in adipose tissue decreases the fatty acid esterification through inhibition of GPAT activity. Therefore, the activation of AMPK leads to the inhibition of cytosolic accumulation of lipids in adipocytes. It also regulates the ligand activated transcriptional factor peroxisome proliferator-activated receptors gamma which is the central regulator of adipogenesis and enhances lipid storage in adipocytes as seen in Figure 4.
In order to further investigate the possibility of the basic coumarins, sulphonated
Figure 4. A schematic diagram that depicts the probable biochemical pathway to decreased fatty acid esterification.
coumarins, and biscoumarins activating AMPK, comparative docking studies using Autodock [38] , were performed with the 3 dimensional coordinates from the Protein Data Bank (PDB) of human AMPK. As shown in Figure 5, PDB code 2YA3 [39] and PDB code 4CFF [40] of human AMPK were co-crystallized with coumarin-ADP,



Figure 5. A docking results from Autodock [38] automated docking program of adipogenesis inhibitory compounds tested in the anti- adipogenesis assay (7), (28), and (33). These compounds were docked into the binding pockets of AMPK PDB codes 2YA3 [39] and 4CFF [40] . (a) and (b) 2YA3 [39] , middle (c) and (d) and lower panels (e) are 4CFF [40] . In (a), (c), and (e), compounds (7), (28), and (33) are replacing the original co-crystallized small molecule activators coumarin-ADP, STU (Staurosporine), and C1V (a thienopyridone derivative) respectively.
C1V(3-[4-(2-hydroxyphenyl) phenyl]-4-oxi-danyl-6-oxidanylidene-7H-thieno [2,3-b] pyridine-5-carbonitrile, and staurosporine, respectively. The compounds (7), (28), and (33) were docked into the binding pockets with reasonable predicted binding constants in the low micromolar range.
Taken together these results suggest that these compounds are potent activators of human AMPK (Figure 5).
Acknowledgements
The authors acknowledge to the Higher Education Commission (HEC), Pakistan, for the financial support for this research study under the National Research Program for Universities (Project No. 20-1910).
Cite this paper
Mukhtar, F., Stieglitz, K., Ali, S., Ejaz, A., Choudhary, M.I., Fakhri, M.I., Salar, U. and Khan, K.M. (2016) Coumarin and Biscoumarin Inhibit in Vitro Obesity Model. Advances in Biolo- gical Chemistry, 6, 152-168. http://dx.doi.org/10.4236/abc.2016.65014
References
- 1. Carmeliet, P. (2005) Angiogenesis in Life, Disease and Medicine. Nature, 438, 932-936.
https://doi.org/10.1038/nature04478 - 2. Dvorak, H.F. (2005) Angiogenesis: Update 2005. Journal of Thrombosis and Haemostasis, 3, 1835-1842.
https://doi.org/10.1111/j.1538-7836.2005.01361.x - 3. Ferrara, N. and Kerbel, R.S. (2005) Angiogenesis as a Therapeutic Target. Nature, 438, 967- 974.
https://doi.org/10.1038/nature04483 - 4. Waki, H. and Tontonoz, P. (2007) Endocrine Functions of Adipose Tissue. Annual Review of Pathology, 2, 31-56.
https://doi.org/10.1146/annurev.pathol.2.010506.091859 - 5. Rayalam, S., Cella-Fera, M.A. and Baile, C.A. (2008) Phytochemicals and Regulation of the Adipocyte Life Cycle. Journal of Nutritional Biochemistry, 19, 717-726.
https://doi.org/10.1016/j.jnutbio.2007.12.007 - 6. Heal, D.J., Gosden, J. and Smith, S.L. (2009) Regulatory Challenges for New Drugs to Treat Obesity and Comorbid Metabolic Disorders. British Journal of Clinical Pharmacology, 68, 861-874.
https://doi.org/10.1111/j.1365-2125.2009.03549.x - 7. Rodgers, R.J., Tschop, M.H. and Wilding, P.H. (2012) Anti-Obesity Drugs: Past, Present and Future. Disease Models & Mechanisms, 5, 621-626.
https://doi.org/10.1242/dmm.009621 - 8. Bray, G.E. and Frank, L.G. (1999) Current and Potential Drugs for Treatment of Obesity. Endocrine Reviews, 20, 805-875.
https://doi.org/10.1210/edrv.20.6.0383 - 9. Peng, X.M., Damu, G.L.V. and Zhou, C.H. (2013) Current Developments of Coumarin Compounds in Medicinal Chemistry. Current Pharmaceutical Design, 19, 3884-3930.
https://doi.org/10.2174/1381612811319210013 - 10. Salar, U., Khan, K.M., Jabeen, A., Faheem, A., Fakhri, M.I., Saad, S.M., Perveen, S. and Taha, M. (2016) Coumarin Sulfonates: As Potential Leads for ROS Inhibition. Bioorganic Chemistry, 69, 37-47.
https://doi.org/10.1016/j.bioorg.2016.09.006 - 11. Salar, U., Taha, M., Khan, K.M., Ismail, N.H., Imran, S., Perveen, S., Gul, S. and Wadood, A. (2016) Syntheses of New 3-Thiazolylcoumarin Derivatives, in Vitro α-Glucosidase Inhibitory Activity, and Molecular Modeling Studies. European Journal of Medicinal Chemistry, 122, 196-204.
https://doi.org/10.1016/j.ejmech.2016.06.037 - 12. Khan, K.M., Fakhri, M.I., Shaikh, N.N., Saad, S.M., Hussain, S., Perveen, S. and Choudhary, M.I. (2014) β-Glucuronidase Inhibitory Studies on Coumarin Derivatives. Medicinal Chemistry, 10, 778-782.
https://doi.org/10.2174/1573406410666140311093352 - 13. Gomez-Outes, A., Suarez-Gea, M.L., Calvo-Rojas, G., Lecumberri, R., Rocha, E., Pozo-Hernandez, C., Terleira-Fernandez, A.I. and Vargas-Castrillon, E. (2012) Discovery of Anticoagulant Drugs: A Historical Perspective. Current Drug Discovery Technologies, 9, 83- 104.
https://doi.org/10.2174/1570163811209020083 - 14. Anand, P., Singh, B. and Singh, N. (2012) A Review on Coumarins as Acetylcholineesterase Inhibitors for Alzheimer’s Disease. Bioorganic & Medicinal Chemistry, 20, 1175-1180.
https://doi.org/10.1016/j.bmc.2011.12.042 - 15. Kostova, I., Bhatia, S., Grigorov, P., Balkansky, S.S., Parmar, V.K., Prasad, A. and Saso, L. (2011) Coumarins as Antioxidants. Current Medicinal Chemistry, 18, 3929-3951.
https://doi.org/10.2174/092986711803414395 - 16. Riveiro, M.E., Kimpe, N.D. and Moglioni, A., Vazquez, R., Monczor, F., Shayo, C. and Da vio, C. (2010) Coumarins: Old Compounds with Novel Promising Therapeutic Perspectives. Current Medicinal Chemistry, 17, 1325-1338.
https://doi.org/10.2174/092986710790936284 - 17. Wu, L., Wang, X., Xu, W., Farzaneh, F. and Xu, R. (2009) The Structure and Pharmacological Functions of Coumarins and Their Derivatives. Current Medicinal Chemistry, 16, 4236-4260.
https://doi.org/10.2174/092986709789578187 - 18. Cravotto, G., Nano, G.M., Palmisano, S.G. and Tagliapietra, S. (2003) The Chemistry of Coumarin Derivatives, Part XIII. The Reactivity of 4-Hydroxycoumarin under Heterogenous High Intensity Sonochemical Conditions. Synthesis, 8, 1286-1291.
- 19. Attaur-Rahman, Shabbir, M., Ziauddin, S.S., Jabar, A. and Choudhary, M.I. (1997) Cinnamates and Coumarins from the Leaves of Murraya paniculata. Phytochemistry, 44, 683-685.
https://doi.org/10.1016/S0031-9422(96)00617-6 - 20. Al-Amiery, A.A., Al-Bayati, R.I.H., Saour, K.Y. and Radi, M.F. (2012) Cytotoxicity, Anti-oxidant, and Antimicrobial Activities of Novel 2-Quinolone Derivatives Derived from Coumarin. Research on Chemical Intermediates, 38, 559-569.
https://doi.org/10.1007/s11164-011-0371-2 - 21. Kontoggiorgis, C. and Hadjipavolou-Litiana, D. (2003) Biological Evaluation of Several Derivatives Desined Possible Anti-Inflammatory/Antioxidant Agents. Journal of Enzyme Inhibition and Medicinal Chemistry, 18, 63-69.
https://doi.org/10.1080/1475636031000069291 - 22. Smyth, T., Ramachandran, V.N. and Smyth, W.F. (2009) A Study of Antimicrobial Activity of Naturally Occurring and Synthetic Coumarins. International Journal of Antimicrobial Agents, 33, 421-426.
https://doi.org/10.1016/j.ijantimicag.2008.10.022 - 23. Liu, W., Hua, J., Zhou, J., Zhang, H., Zhu, H., Cheng, Y. and Gust, R. (2012) Synthesis and in Vitro Antitumor Activity of Novel Scopoletin Derivatives. Bioorganic & Medicinal Chemistry Letters, 22, 5008-5012.
https://doi.org/10.1016/j.bmcl.2012.06.014 - 24. Iranshahi, M., Askari, M., Sahebkar, A. and Hadjipavlou-Litiana, D. (2009) Evaluation of Antioxidant, Anti-Inflammatory and Lipoxygenase Inhibitory Activities of the Prenylated Coumarin Umbelliprenin. DARU Journal of Pharmaceutical Sciences, 17, 99-103.
- 25. Borges, F., Roleira, F., Milhazes, N., Santana, L. and Uriarte, E. (2005) Simple Coumarins and Analogues in Medicinal Chemistry: Occurrence, Synthesis and Biological Activity. Current Medicinal Chemistry, 12, 887-916.
https://doi.org/10.2174/0929867053507315 - 26. Manolov, I. and Danchev, N. (2003) Synthesis and Pharmacological Investigations of Some 4-Hydroxycoumarin Derivatives. Archiv der Pharmazie, 336, 83-94.
https://doi.org/10.1002/ardp.200390010 - 27. Jung, J.C. and Park, O.S. (2009) Synthetic Approaches and Biological Activities of 4-Hydroxycoumarin Derivatives. Molecules, 14, 4790-4803.
https://doi.org/10.3390/molecules14114790 - 28. Manolov, I., Maichle-Moessmer, C. and Dancher, N. (2006) Synthesis, Structure, Toxicological and Pharmacological Investigations of 4-Hydroxycoumarin Derivatives. European Journal of Medicinal Chemistry, 41, 882-890.
https://doi.org/10.1016/j.ejmech.2006.03.007 - 29. Su, C.X., Mouscadet, J.F., Chiang, C.C., Tsai, H.J. and Hsu, L.Y. (2006) HIV-1 Integrase Inhibition of Biscoumarin Analogues. Chemical and Pharmaceutical Bulletin, 54, 682-686.
https://doi.org/10.1248/cpb.54.682 - 30. Zhao, H., Namati, N., Hong, H., Mazumder, A., Shaomeng, W., Sanjay, S., George, W.A.M., Yves, P. and Terrence, R.B. (1997) Coumarin-Based Inhibitors of HIV-Integrase. Journal of Medicinal Chemistry, 40, 242-249.
https://doi.org/10.1021/jm960450v - 31. Nolan, K.A., Zhao, H., Faulder, P.F., Frenkel, A.D., Timson, J.D., Siegel, D., Ross, D., Burke Jr., T.R., Stratford, I.J. and Bryce, A.R. (2007) Coumarin-Based Inhibitors of Human NAD(P)H: Quinone Oxidoreductase-1. Identification, Structure-Activity, Off-Target Effects and in Vitro Human Pancreatic Cancer Toxicity. Journal of Medicinal Chemistry, 50, 6316-6325.
https://doi.org/10.1021/jm070472p - 32. Hamdi, N., Puetra, M.C. and Valerga, P. (2008) Synthesis, Structure, Antimicrobial and Antioxidant Investigations of Dicoumarol and Related Compounds. European Journal of Medicinal Chemistry, 43, 2541-2548.
https://doi.org/10.1016/j.ejmech.2008.03.038 - 33. Khan, K.M., Iqbal, S., Lodhi, M.A., Maharvi, G.M., Zia-Ullah, Choudhary, M.I., Attaur-Rahman and Perveen, S. (2004) Biscoumarin: New Class of Urease Inhibitors, Economical Synthesis and Activity. Bioorganic & Medicinal Chemistry, 12, 1963-1968.
https://doi.org/10.1016/j.bmc.2004.01.010 - 34. Choudhary, M.I., Fatima, N., Khan, K.M., Jalil, S., Iqbal, S. and Attaur-Rahman (2006) New Biscoumarin Derivatives-Cytotoxicity and Enzyme Inhibitory Activities. Bioorganic & Medicinal Chemistry, 14, 8066-8072.
https://doi.org/10.1016/j.bmc.2006.07.037 - 35. Kostova, I., Momekov, G., Zaharieva, M. and Karaivanova, M. (2005) Cytotoxic Activity of New Lanthanum (III) Complexes of Bis-Coumarins. European Journal of Medicinal Chemistry, 40, 542-551.
https://doi.org/10.1016/j.ejmech.2004.12.007 - 36. Tabatabaeian, K., Heidari, H., Khorshidi, A., Ghani, M.M. and Mahmoodi, N.O. (2012) Synthesis of Biscoumarin Derivatives by the Reaction of Aldehydes and 4-Hydroxycoumarin Using Ruthenium (III) Chloride Hydrate as a Versatile Homogenous Catalyst. Journal of the Serbian Chemical Society, 77, 407-413.
https://doi.org/10.2298/JSC110427189T - 37. Ejaz, A., Wu, D., Kwan, P. and Meydani, M. (2009) Curcumin Inhibits Adipogenesis in 3T3-L1 Adipocytes and Angiogenesis and Obesity in C57/BL Mice. Journal of Nutrition, 139, 919-925.
https://doi.org/10.3945/jn.108.100966 - 38. Morris, G.M., Huey, R., Lindstrom, W., Sanner, M.F., Belew, R.K., Goodsell, D.S. and Olson, A.J. (2009) Autodock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. Journal of Computational Chemistry, 16, 2785-2791.
https://doi.org/10.1002/jcc.21256 - 39. Xiao, B., Sanders, M.J., Underwood, E., Heath, R., Mayer, F., Carmena, D., Jing, C., Walker, P.A., Eccleston, J.F., Haire, L.F., Saiu, P., Howell, S.A., Aasland, R., Martin, S.R., Carling, D. and Gamblin, S.J. (2011) Structure of Mammalian Ampk and Its Regulation by Adp. Nature, 472, 230.
https://doi.org/10.1038/nature09932 - 40. Xiao, B., Sanders, M.J., Carmena, D., Bright, N.J., Haire, L.F., Underwood, E., Patel, B.R., Heath, R.B., Walker, P.A., Hallen, S., Giordanetto, F., Martin, S.R., Carling, D. and Gamblin, S.J. (2013) Structural Basis of Ampk Regulation by Small Molecule Activators. Nature Communications, 4, 3017.
https://doi.org/10.1038/ncomms4017







