Advances in Biological Chemistry
Vol.4 No.1(2014), Article ID:42706,5 pages DOI:10.4236/abc.2014.41002
C-reactive protein and erythrocyte sedimentation rate: Continuing role for erythrocyte sedimentation rate
![]()
Clinical Pathology, Truman Medical Center, Kansas City, USA
Email: gurmukhsinghmdphd@yahoo.com
Copyright © 2014 Gurmukh Singh. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Gurmukh Singh. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received 21 November 2013; revised 27 December 2013; accepted 9 January 2014
KEYWORDS
CRP; ESR; Osteomyelitis; Inflammation
ABSTRACT
Background: Erythrocyte Sedimentation Rate (ESR) and C-reactive protein (CRP) tests are often done to detect or monitor patients with suspected inflammatory disorders. The objective of the study was to ascertain if the manual ESR test added value to the information available from automated CRP results alone. Methods: In this retrospective, observational study at a safety-net hospital, the ESR and CRP values were compared in 4527 instances when both tests were done. In 150 instances, involving 97 patients; when ESR was >60 mm/hr and CRP was ≤1.0 mg/dL, the medical records were reviewed to discern the cause of disparity between the ESR and CRP results and to assess the utility of continued use of the ESR test. Results: Review of medical records did not reveal an explanation for elevated ESR in 20 patients with normal CRP results. In the remaining 77 (79%) patients, an inflammatory disorder was noted despite a normal CRP value; in 27 (28%) patients, the disorder was osteomyelitis. Presence of skin necrosis was also a prominent factor in the discrepant results. Conclusions: ESR has value in detecting inflammatory disorders that may not be obvious by clinical examination or CRP results. ESR has a particularly useful role in patients with suspected bone lesions and osteomyelitis.
1. INTRODUCTION
Erythrocyte sedimentation rate (ESR) is one of the oldest laboratory tests and is still in use. ESR is elevated in some physiologic states, such as pregnancy, and many pathologic states, usually due to inflammation, anemia, paraproteinemia, elevated fibrinogen, cold agglutinins, and in some instances for unknown reasons [1-3]. The molecular basis for elevation of ESR in many circumstances is not known; however, empirically, elevated ESR has been associated with inflammation due to infections as well as non-infectious causes, such as rheumatoid arthritis, systemic lupus erythematosus, tissue necrosis, and inflammatory bowel disease [2-4].
Historically, ESR was performed by observing the rate of settling or red cell, in a vertically held long tube, under the influence of gravity. The testing method has evolved from the classic Westergren method to centrifugation in a capillary tube with laser detection of the rate of settling. The new test can be completed in about five minutes or less as compared to the one-hour observation in the classic method [1,5]. Test results for ESR are usually reported as mm/hour. Normal values or reference ranges vary by age and gender and vary from <15 mm/ hour to <40 mm/hour [1,6].
C-reactive protein (CRP) is one of the acute phase reactants whose blood levels increase in response to inflammation. Conventionally CRP values are reported as mg/dL to distinguish the results from high sensitivity CRP results that are reported in mg/L; the latter is used as one of the risk markers for atherosclerotic cardiovascular disease and is thought to reflect vascular inflammatory lesions [7]. Serum CRP levels in a “normal” population have a skewed distribution, i.e., not a Gaussian distribution. Levels <0.3 mg/dL are considered normal; however, “normal” people, i.e., people without known pathologic lesions, have levels between 0.1 and 1.0 mg/dL. Like ESR, serum CRP levels tend to increase with age. CRP tends to respond more rapidly to changing clinical conditions and may be a better analyte for the follow-up studies when both ESR and CRP are elevated and monitoring of the condition or response to treatment is needed [4,8].
Given the high prevalence of obesity in the US population, it is worth pointing out that obesity is considered an inflammatory state and obesity alone is associated with increased levels for both ESR and CRP [9].
Even though both ESR and CRP levels reflect similar pathologies, there are known disparities in the results of these two analytes. Discordance between ESR and CRP has been documented and the disparity depends, in part, on serum albumin, renal insufficiency, anemia and noninfectious inflammatory disorders [10-13].
Assay for CRP is automated on commonly used chemistry analyzers, whereas ESR is still a manual test. Though not applicable in this study, for research studies, CRP has the added advantage in that stored serum samples can be tested retrospectively while this option is not available for ESR. The faster response rate of CRP to changing clinical conditions has led some institutions to replace ESR with CRP [14].
If it could be ascertained that ESR does not add value to CRP test results, the elimination of this ancient, manual test promises to facilitate workflow in the laboratories and reduce expenses and this is the rationale for the investigation.
2. METHODS
We examined the laboratory records for ESR and CRP tests done from January 1, 2012 through August 15, 2013. Instances in which both tests were done on a given patient on the same day were identified and separated. The values of ESR > 30 mm/hr and CRP > 1.0 mg/dL were determined to be abnormal, from a combination of manufactures suggestions, local observations and data in the literature. The reference ranges for both ESR and CRP have wide variability and are dependent on age and gender. Normal values for ESR range from <15 mm/hr in men younger than 50 years and greater than 40 for women older than 85 year. For CRP the range is even wider depending on whether it is used as a risk marker for cardiovascular disease or for gross inflammatory lesions and the reference values range from 1.5 mg/dL to 3.0 mg/dL [1,15]. Using these parameters, we identified discordant pairs of results, i.e., instances in which one test was normal and the other abnormal. As mentioned earlier, due to the near universal prevalence of overweight/obesity in the US, we chose the upper limits of both ESR and CRP as cut offs for designating normal levels. Within the discordant pairs, we further isolated instances in which ESR was abnormal and CRP was normal. From this subpopulation we identified instances in which ESR was >60 mm/hr and CRP was ≤1.0 mg/dL. The medical records of this sub-group were reviewed to discern the cause of disparity in the results. The main diagnoses, as well as, diagnoses or findings associated with inflammation were recorded. In patients with multiple observations, the results were pooled because in almost all instances the multiple tests were done over a period of a few days. ESR value of 60 mm/hr was chosen, in contract to the usual upper limit of 30 mm/hr, to include only patients with marked disparity between the ESR and CRP results.
ESR was determined by centrifugation of about 100 μL of blood in a pre-marked capillary tube, using the ESR STATTM Plus analyzer. CRP determination was done by an immunoassay using UniCel DxC 800i analyzer and reagents from Beckman-Coulter.
This study was conducted in a two campus medical center with 520 beds. The medical center is affiliated with a medical school and is the main teaching hospital for the medical school. One campus is located in the inner city in Midwest USA and is a level-one trauma center. The second campus is located in a suburban setting and provides mainly family medicine and long-term care services. The medical centers serve as the safety net hospital for the county.
The Institutional Review Board of the Medical School and Privacy Board of the Medical Centers approved the study.
3. RESULTS
The numbers of various test categories are shown in Figure 1. Out of a total of 10,554 tests for both CRP and ESR, there were 9054 instances in which both tests were done on the same day (4527 pairs). In 3239 of the 4527 pairs (71.5%) the results for ESR and CRP were concordant. Both analytes were normal in 1860 and both were abnormal in 1379 instances. In 1288 instances the results were discordant for the two analytes. In 777 of these 1288 cases, CRP was abnormal and ESR was normal and in 511 instances CRP was normal and ESR was elevated. The 511 cases with elevated ESR and normal CRP were further selected to identify cases with ESR at twice the upper limit of designated normal, i.e., >60 mm/hour, and normal CRP, which revealed 151 instances of interest.
In brief, there were 151 instances in which ESR > 60 mm/hr and CRP was ≤1.0 mg/dL. These tests were done in 98 patients and the medical record of one patient was not available. The site of main pathologic lesion in each of the 97 patients is shown in Table 1. Many patients had multiple sites with pathologic lesions in general and inflammation in particular.
Bone lesions, in most cases were osteomyelitis, often without any break in skin or discharge, e.g., vertebral osteomyelitis. Other lesions included, gangrene, lytic bone lesions in a patient with light chain myeloma, bone involvement in patients with septic arthritis following joint replacement surgery, and necrosis of bone implant.
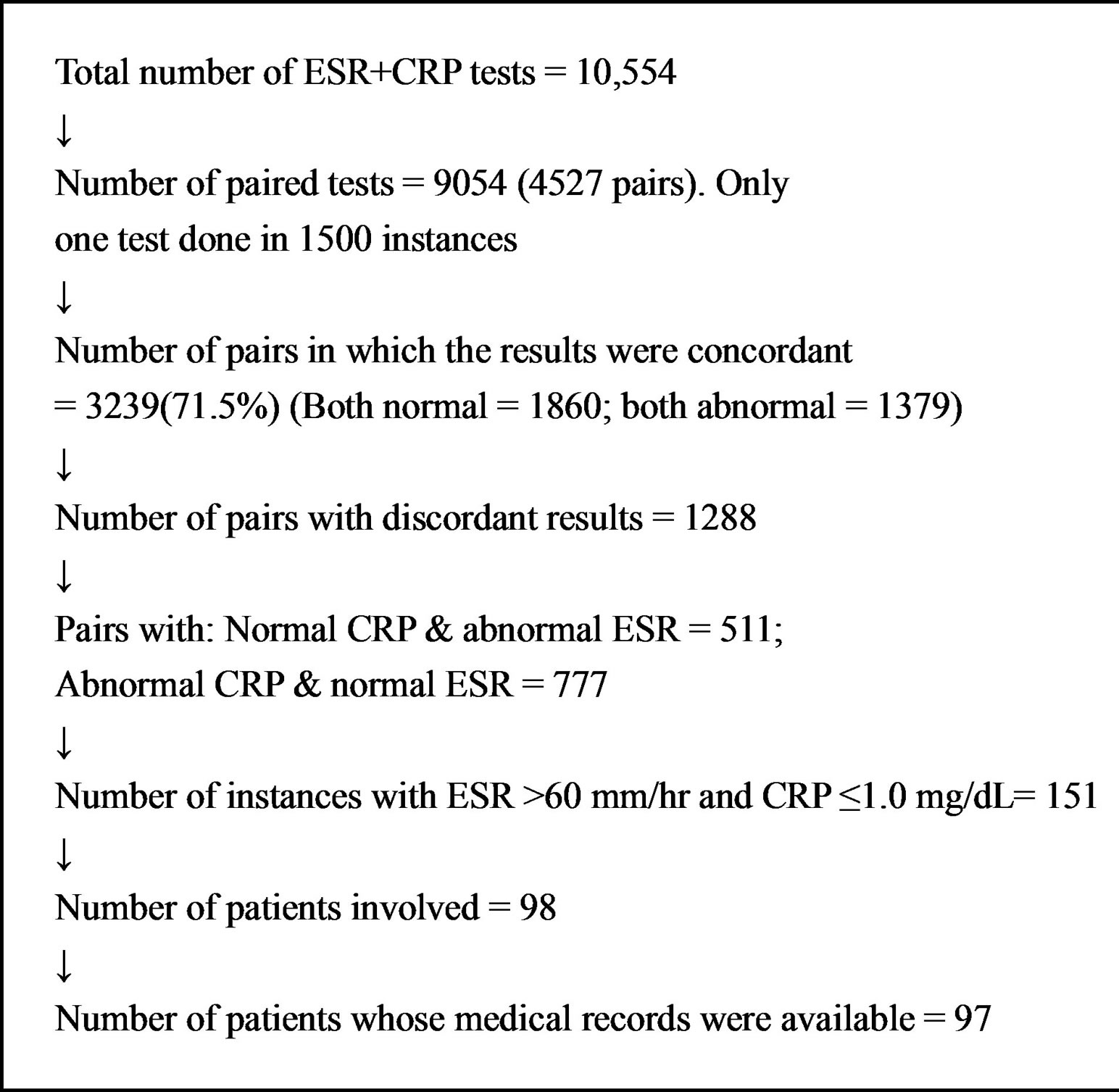
Figure 1. Flow diagram of case selection.
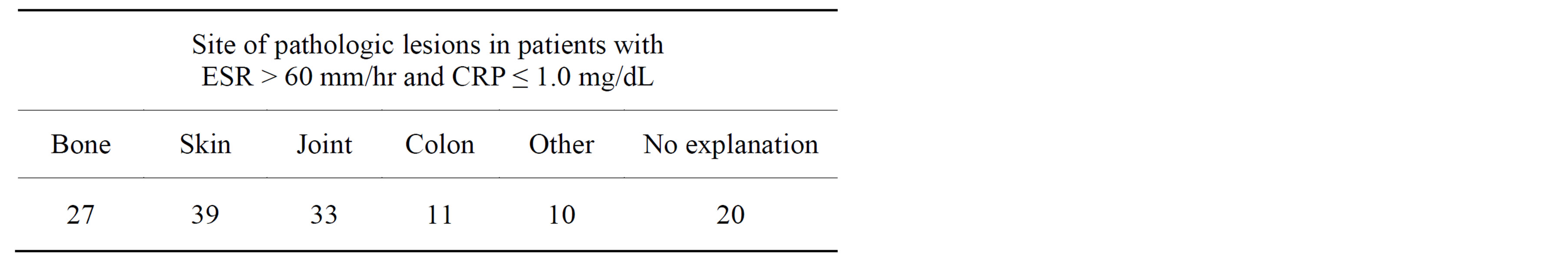
Table 1. Pathology in cases with ESR > 60 mm/hr and CRP ≤ 1.0 mg/dL.
Skin lesions were grossly visible and included cellulitis with or without venous stasis, skin necrosis in patients with vasculitis, gangrene due to vascular lesions and diabetes, and skin lesions of systemic lupus erythematosus. Skin necrosis was a prominent feature in this group of patients.
Rheumatoid arthritis was a common joint disease and other lesions were septic arthritis following joint replacement. Many patients had co-existing degenerative joint disease with or without synovitis and tendinitis.
Colon lesions included inflammatory bowel disease due to ulcerative colitis, Crohns disease, and diverticulitis.
Other lesions, present in one patient each, are listed in Table 2.
The main diagnoses/pathology, in the 20 patients in whom a commonly accepted cause of elevated ESR could not be ascertained, are listed in Table 3.
Virtually all patients had multiple diagnoses and the common diagnoses are listed in Table 4. The average age of the patients was 55.5 years, and the sample included 71 women and 26 men. The ratio of women:men is in keeping with the population served by the medical centers.
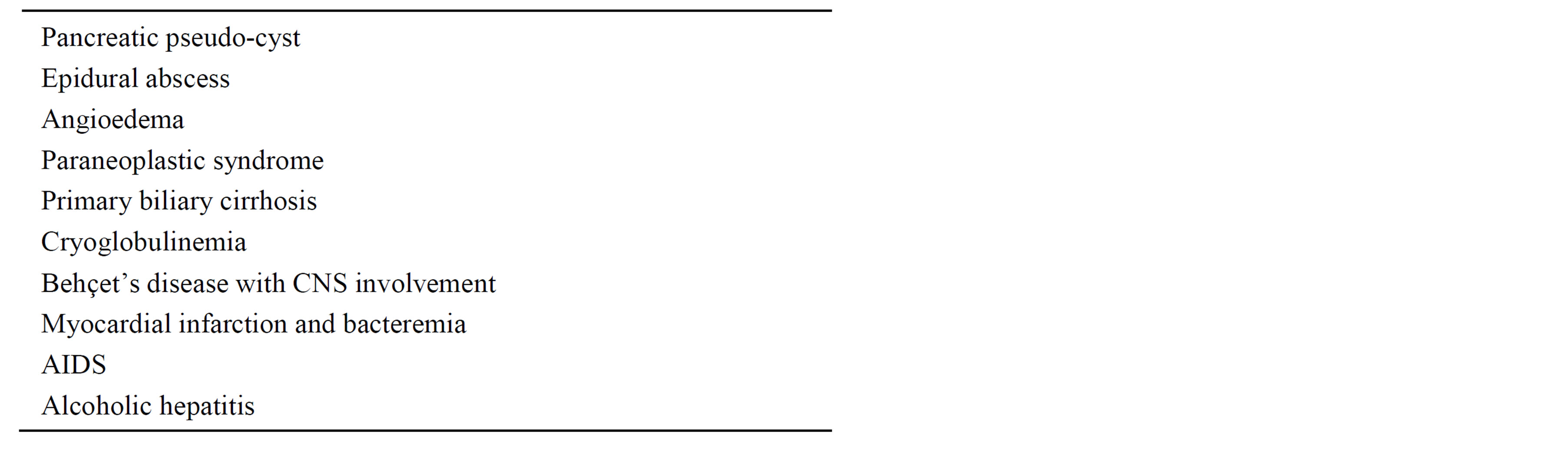
Table 2. Pathologic issues in patients in the “other” lesions category in Table 1, each lesion was noted in only one patient each.
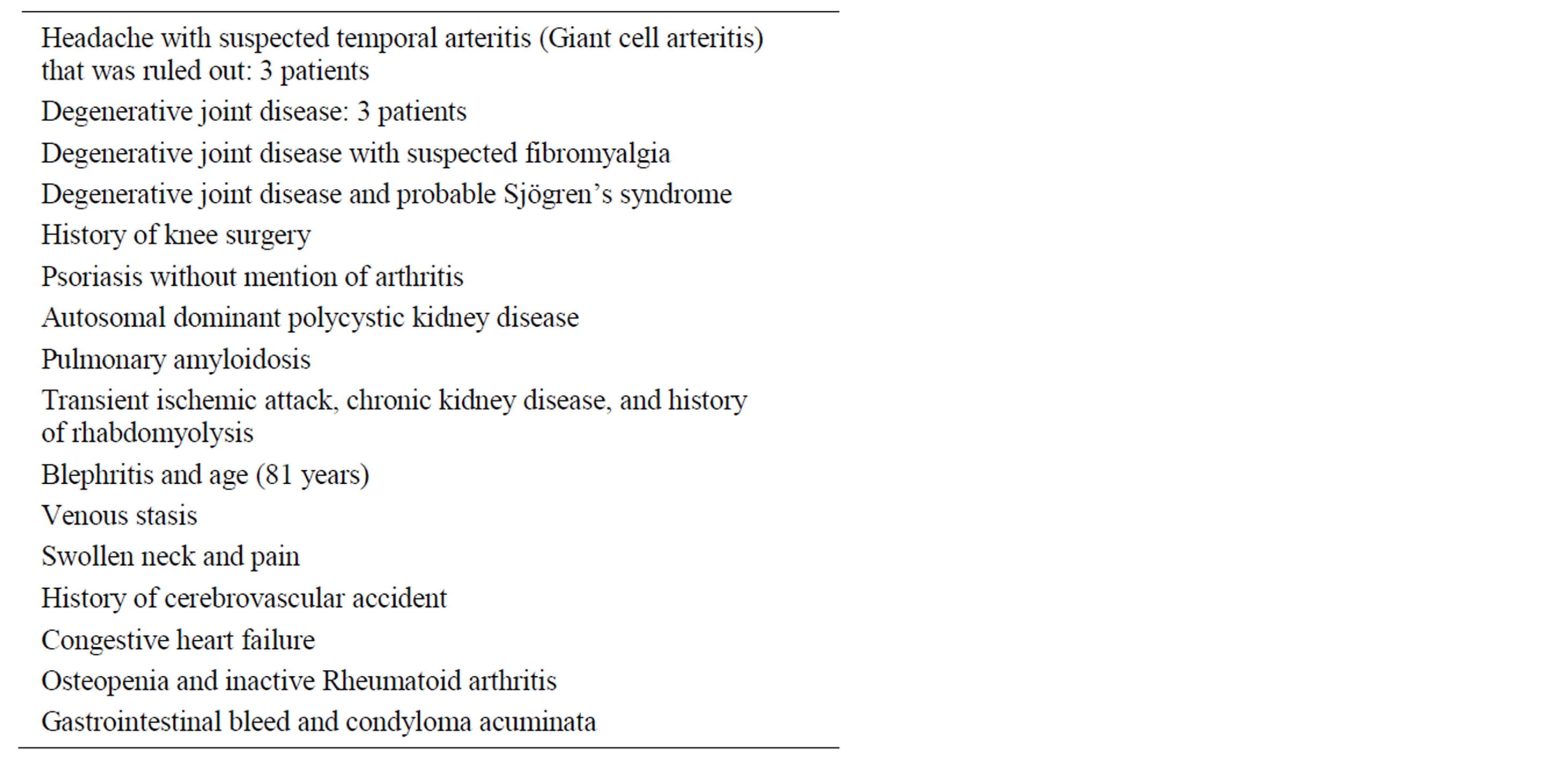
Table 3. Main diagnoses in patients without adequate explanation for elevated sedimentation rate.
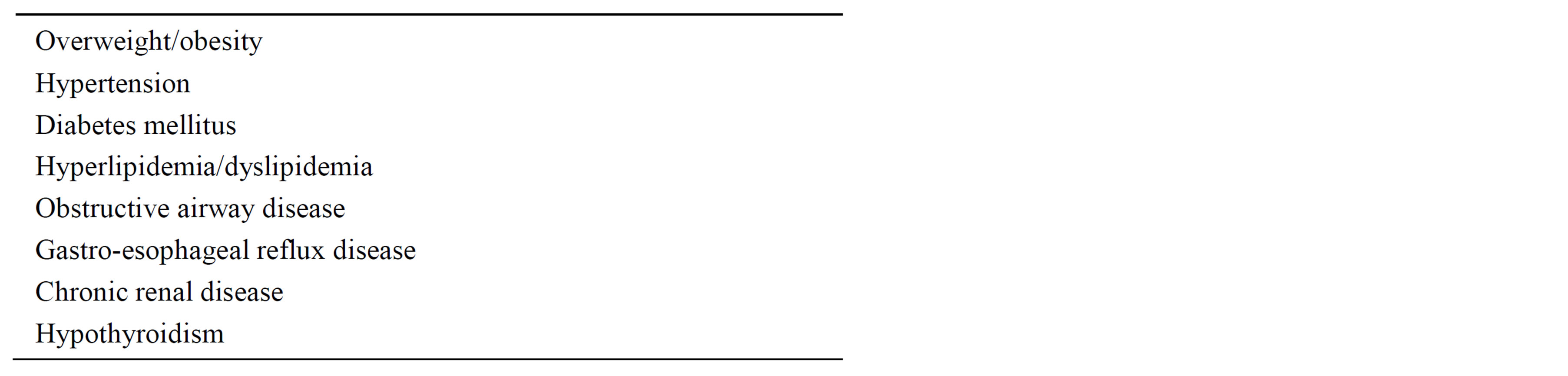
Table 4. Common diagnoses in the patient population under study.
4. DISCUSSION
Determination of the presence of inflammatory processes is of diagnostic value in many clinical circumstances, e.g., pneumonia, appendicitis, colitis, inflammatory joint disease, vasculitits, osteomyelitis, and systemic inflammatory lesions, bacteremia, and systemic lupus erythematosus etc. ESR is the older of the two commonly used tests as markers of inflammation. The method for determining ESR has undergone changes from the classic Westergren method and ESR can now be determined in minutes rather than hours, yet it remains a manual test and the molecular basis of the test has not been established.
Periodically, the usefulness and/or appropriateness of continuing to perform ESR is questioned, mostly by the laboratories, particularly with the availability of automated methods for quantification of an acute phase reactant, namely CRP [14]. The Laboratory Medicine section of this institution has initiated a systematic review of the test menu to ascertain outmoded tests that could be discontinued and an evaluation of the ESR was undertaken for that purpose. It is appropriate to disclose that the bias going into this study was to find evidence for discontinuing ESR.
The normal values for both ESR and CRP have not been fully standardized or harmomized, and vary among various laboratories. The reference range for ESR also depends on age and gender. ESR values increase with increasing age and are higher in women than in men. Given that the average age of our population was over 50 years and there were nearly three times as many women as men, we chose 30 mm/hour as the upper limit of normal for ESR. CRP of 1.0 mg/dL was chosen as the upper limit of normal, as stated earlier, to account for the high prevalence of obesity.
CRP was elevated more often than ESR. In the 4527 instances when both ESR and CRP were measured, the results were concordant in nearly 72% of the observations. In the 1288 instances of disparity between ESR and CRP results, CRP was elevated more often by a ratio of 1:1.5. Chart reviews, for all of the 511 instances in which ESR was elevated but CRP was not, could have been done; however, to make the process more manageable and to only address instances with a marked disparity between the ESR and CRP, the chart review was limited to patients whose ESR was more than twice the upper limit of normal with a normal CRP.
Determining the reason for an elevated ESR in patients with normal CRP was less than straightforward given that almost all patients had a long problem list and many disorders that may have inflammatory processes associated with the pathologic lesions. More than one explanation was apparent in many patients, a common scenario being diabetic foot ulcer with osteomyelitis and cellulitis. Bone pathology was also involved in patients with inflammatory arthritis, particularly in cases of postjoint replacement infections that often had joint inflammation and osteonecrosis. Inflammatory skin lesions in general and lesions with skin necrosis, in particular, were more common than expected but were not a diagnostic issue due to the obvious nature of the lesions. Colitis lesions included ulcerative colitis, Crohns disease, as well as bacterial infections with C diff and diverticulitis. It was not feasible to identify an explanation for the elevated ESR in 20 patients even though they all had multiple diagnosis but those diagnoses are almost universal in the patient population at this institution.
A relevant negative finding is the lack of instances of pneumonia, acute appendicitis, and non-osseous pyogenic lesions (with the possible exception of suspected epidural abscess in one patient) in any of the patients with elevated ESR and normal CRP.
The medical records often lacked specific comments about the role of ESR or CRP in decision making for diagnosis, prognosis or follow-up care. This is in marked contrast to the observation of the author during rounds with the medical staff. In the teaching rounds, the laboratory results play an important role in the discussions, however, these discussions are not routinely documented in the electronic medical record. A weakness of the study is the lack of longitudinal data on the affected patients. Most of the patients were admitted for short stays and even though many had ambulatory follow-up visits, these visits were often not associated with retesting for ESR and/or CRP.
Despite the fact that ESR is an ancient, manual test, without known molecular explanation, many false positives and negatives, variation in reference range by age and gender, in the age of availability of automated tests for markers of acute inflammation, it remains useful in detecting inflammatory bone lesions that fail to induce increase in CRP and hence it is recommended that ESR be maintained in the laboratory test menu [16-19]. The elevated ESR was particularly useful in patients with lower extremity cellulitis as it pointed to the presence of osteomyelitis. Both CRP and ESR could be used in the screening process and if both are elevated, the course of the disease could be followed with serial testing for CRP as CRP levels change more rapidly reflecting the changing condition and the test is automated, however, ESR remains a useful test to detect bone lesions in general and osteomyelitis in particular [20,21].
ACKNOWLEDGEMENTS
I am grateful to Mr. Vu Pham and Mr. Tom Haldiman for technical assistance.
CONFLICT OF INTEREST
The author has no disclosures or disclaimers.
REFERENCES
- Vajpayee, N., Graham, S.S. and Bem, S. (2011) Basic examination of blood and bone marrow. In: McPherson, R.A. and Pincus, M.R., Eds., Henry’s Clinical Diagnosis and Management by Laboratory Methods, Elsevier Saunders, Philadelphia, Chapter 30, 509-535. http://dx.doi.org/10.1016/B978-1-4377-0974-2.00030-0
- Bedell, S.E. and Bush, B.T. (1985) Erythrocyte sedimentation rate. From folklore to facts. American Journal of Medicine, 78, 1001-1009. http://dx.doi.org/10.1016/0002-9343(85)90224-4
- Simon, L., Gauvin, F., Amre, D.K., et al. (2004) Serum procalcitonin and C-reactive protein as markers of bacterial infection: A systematic review and meta-analysis. Clinical Infectious Diseases, 39, 206-217. http://dx.doi.org/10.1086/421997
- Woloshin, S. and Schwartz, L.M. (2006) Distribution of C-reactive protein values in the United States. The New England Journal of Medicine, 352, 1611-1613. http://dx.doi.org/10.1056/NEJM200504143521525
- “User manual for ESR STATTM PLUS—Blood analyzer,” Hema Technologies, Inc.
- Dasgupta, B., Cimmino, M.A., Maradit-Kremers, H., et al. (2012) 2012 provisional classification for polymyalgia rheumatica: A European League against Rheumatism/ American College of Rheumatology collaborative initiative. Annals of the Rheumatic Diseases, 71, 484-492. http://dx.doi.org/10.1136/annrheumdis-2011-200329
- Ridker, P.M. and Libby, P. (2007) Risk factors for atherothrombotic disease. In: Libby, P., Bonow, R.O., Mann, D.L. and Zipes, D.P., Eds., Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine, 8th Edition, Saunders Elsevier, Philadelphia, Chapter 39.
- Almirall, J., Bolibar, I., Toran, P., et al. (2004) Contribution of C-reactive protein to the diagnosis and assessment of severity of community-acquired pneumonia. Chest, 125, 1335-1342. http://dx.doi.org/10.1378/chest.125.4.1335
- Yudkin, J.S., Stehouwer, C.D., Emeis, J.J., et al. (1999) C-reactive protein in healthy subjects: Association with obesity, insulin resistance, and endothelial dysfunction: A potential role for cytokines originating from adipose tissue? Arteriosclerosis, Thrombosis, and Vascular Biology, 19, 972-978. http://dx.doi.org/10.1161/01.ATV.19.4.972
- Hasleman, B. (2000) Laboratory investigations useful in the evaluation of polymyalgia rheumatica (PMR) and giant cell arteritis (GCA). Clinical and Experimental Rheumatology, 18, S29-S31.
- Costenbader, K.H., Chibnick, L.B. and Schur, P.H. (2007) Discordance between erythrocyte sedimentation rate and C-reactive protein measurements: Clinical significance. Clinical and Experimental Rheumatology, 25, 746-749.
- Kermani, T.A., Schmidt, J., Crowson, C.S., et al. (2012) Utility of erythrocyte sedimentation rate and C-reactive protein for diagnosis of giant cell arteritis. Seminars in Arthritis and Rheumatism, 41, 866-871. http://dx.doi.org/10.1016/j.semarthrit.2011.10.005
- Walvick, M.D. and Walvick, M.P. (2011) Giant cell arteritis: Laboratory predictors of a positive temporal artery biopsy. Ophthalmology, 118, 1201-1204. http://dx.doi.org/10.1016/j.ophtha.2010.10.002
- Husain, T.M. and Kim, D.H. (2002) C-reactive protein and erythrocyte sedimentation rate in orthopedics. The University of Pennsylvania Orthopaedic Journal, 15, 13- 16.
- Remaley, A.T., Rifai, N. and Warnick, R. (2012) Lipid, lipoproteins, apolipoproteins and other cardiovascular risk factors. In: Burtis, C.A., Ashwood, E.R. and Bruns, D.E., Eds., Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 5th Edition, Elsevier, Philadelphia, Chapter 27.
- Brigden, M. (1998) The erythrocyte sedimentation rate. Still a helpful test when used judiciously. Postgraduate Medicine, 103, 257-262. http://dx.doi.org/10.3810/pgm.1998.05.493
- Katz, P.R., Karuza, J., Gutman, S.I., et al. (1990) A comparison between erythrocyte sedimentation rate (ESR) and selected acute-phase proteins in the elderly. American Journal of Clinical Pathology, 94, 637-640.
- Saadeh, C. (1998) The erythrocyte sedimentation rate: Old and new clinical applications. Southern Medical Journal, 91, 220-225. http://dx.doi.org/10.1097/00007611-199803000-00001
- Zlonis, M. (1993) The mystique of the erythrocyte sedimentation rate: A reappraisal of one of the oldest laboratory tests still in use. Clinics in Laboratory Medicine, 13, 787-800.
- Cantini, F., Salvarni, C., Oliveri, I., et al. (2000) Erythrocyte sedimentation rate and C-reactive protein in the evaluation of disease activity and severity in polymyalgia rheumatica: A prospective follow-up study. Seminars in Arthritis and Rheumatism, 30, 17-24. http://dx.doi.org/10.1097/00007611-199803000-00001
- Salvarini, C., Cantini, F., Niccoli, L., et al. (2005) Acutephase reactants and the risk of relapse/recurrence in polymyalgia rheumatica: A prospective followup study. Arthritis & Rheumatism, 53, 33-38. http://dx.doi.org/10.1002/art.20901

