Advances in Biological Chemistry
Vol. 2 No. 4 (2012) , Article ID: 24536 , 6 pages DOI:10.4236/abc.2012.24041
Study of glyceraldehyde-3-phosphate dehydrogenase expression in the tumor process of: Breast, cervix and prostate cancers
![]()
1Laboratoire de Physiologie et Génétique Moléculaire, Département de Biologie, Faculté des Sciences, Université Hassan II, Casablanca, Morrocco
2Laboratoire d’Anatomo-Cyto-Pathologie, Institut Pasteur du Maroc, Casablanca, Morrocco
Email: a_soukri@hotmail.com
Received 31 July 2012; revised 3 September 2012; accepted 15 September 2012
Keywords: Cancer; GAPDH; Immunohistochemistry; Expression; Isoforms
ABSTRACT
Tumor proliferation of cancer cells requires a high intake of oxygen by angiogenesis. Deep cancer cells suffer from asphyxia and meet their energy needs through the enzymes of glycolysis. The anti-angiogenesis approach has been recognized for therapeutic purposes, but the deep cancers, difficult to reach by this therapy, could be targeted by inhibiting an enzyme of the glycolytic cycle. Our work focused on the study of the expression of GAPDH, a key enzyme of glycolysis, in cervix, breast and prostate tumors, for two approaches: Fundamental and targeted therapeutics. 60 samples, taken at the Anatomopathology laboratory of the Pasteur Institute of Morocco, were examined histologically and immunohistochemically, demonstrating the expression and cellular localization of GAPDH. The three organs have shown an overexpression of GAPDH in tumor tissues. At the cellular level, the localization of GAPDH in cancer tissue is diffuse but mostly nuclear whereas it remains focused at the membrane and/or the cytoplasm in benign tumor tissues. From these results we could assume that GAPDH is involved in the cancer process and draws attention to a possible new nuclear role that could be either specific to one form or different isoforms of GAPDH enzyme.
1. INTRODUCTION
According to WHO, cancer is a disease that affects over 10 million people worldwide. Due to its potential severity, the disease affects the quality of the patient’s life. It seems to affect people at random and the treatment remains heavy expensive.
Usually, Cancer is presented as a tumor mass which is the culmination of a series of transformations that can occur over a period of several years. The understanding of cancer natural history remains unclear/uneasy because of its frequency, complexity, malignity and diversity of signaling pathways and therefore, more difficult to develop new therapeutic strategies.
While the Anatomopathological analysis seems to be the most reliable technique for both diagnosis and prognosis, researchers are trying to find new tumor markers and prognostic indicators.
Oxygen plays a key role in the functioning of healthy and cancer cells. Some studies estimate that the tumor proliferation of cancer cells requires a high intake of oxygen via angiogenesis. In the case of less advanced cancers, some antiangiogenic therapies seek to cause cell degeneration by preventing the proliferation of blood vessels that feed oxygen. However, deep cancer cells escape this therapy, because they suffer from asphyxia and instead rely on the conversion of glucose to energy needs through the enzymes of glycolysis [1]. This is in accordance with the Warburg studies; which demonstrated that tumor cells have an increased rate of glycollysis[2].
The glyceraldehyde-3-phosphate dehydrogenase (GAPDH), is described as a key enzyme of glycolysis. It is considered one of the best characterized glycolytic enzymes at the biochemical and structural level [3,4].
It catalyzes the oxidative phosphorylation of D-glyceraldehyde-3-phosphate (G3P) to 1.3 diphospho-glyceric acid (1.3 DPG).
In addition to glycolysis, this enzyme is also implicated in the Krebs cycle and pentose phosphate pathway, being in this way involved in all three lanes of central carbon metabolism [5]. GAPDH is a ubiquitous enzyme known among all living beings including the three major evolutionary lineages; Archaebacteria, Eubacteria and Eukaryotes. It is considered among the best conserved proteins [5,6].
The native protein is a tetramer with a molecular mass of 140 kDa and 220 kDa depending on the type of GAPDH. Each monomer consists of approximately 330 - 498 amino acids and has a molecular weight between 35 and 50 kDa. [7].
Human GAPDH gene organization was examined using the selective loss of human chromosomes in human-rodent somatic cell hybrids [8]. The GAPDH gene was localized to chromosome 12 based on its concordant expression with lactate dehydrogenase B (LDH-B), with triosephosphate isomerase (TPI) as well as the lack of such associations with 28 other human enzymes [9].
Apart from its glycolytic function, phosphorylating GAPDH presents a variety of activities depending on its membrane, cytoplasmic or nuclear localization [10]. Having in addition to its catalytic function other roles in physiological processes, a series of studies has shown the involvement of GAPDH in initiating the cascade of hepatocyte apoptosis [11].
Further investigations have shown that cancers have a metabolism based on glycolysis, comprising the conversion of glucose into pyruvate and in the case of oxidative stress; the accumulation of errors in the pot-translational structure of GAPDH increases the aging process [12].
Recently different teams have examined the expression analysis of GAPDH in tumors and human cancer cell lines. Thereby in Ovarian cancer, GAPDH expression increases mRNA stability of CSF, an important cytokine in tumor progression [13]. In Thyroid cancer, GAPDH undergoes S-nitosylation to facilitate its translocation to the nucleus in order to activate the TRAIL (Tumor Necrosis Factor Related Apoptosis Inducing Ligand) [14].
What about the expression of GAPDH in breast and gynecological cancers, representing an estimated 25% of the cancer deaths in women [15]. And in prostate cancer, which is one of the most common male cancers in the world [16].
In this study we have attempted to show the involvement of GAPDH in breast, cervix and Prostate cancers.
2. PATIENTS AND METHODS
2.1. Ethics Statement
This study was performed in accordance with Helsinki principles.
2.2. Patients
60 samples including 20 of the cervix, 20 breast and 20 prostate, were recruited at the Anatomopathology laboratory of the Pasteur Institute of Morocco (IPM) from patients for diagnosing, were studied histologically and immunohistochemically.
For each tissue, we studied the expression of GAPDH in both malignant and benign lesions.
2.3. Methods
2.3.1. Histological Study
The Histological study was performed on biopsy or surgical specimens, collected by the specialist, fixed in formalin, and then routed to the Laboratory of Anatomy-pathology where they are listed.
The samples were then subjected to a macroscopic study and were described by the pathologist. Then they were passed through a series of intermediate liquids in a circulation automaton before being embedded in paraffin to obtain blocks ready to be cut by microtome in order to obtain transparent ultrathin sections with a thickness of about 4 to 5 micrometers. The biofilms were delicately placed on slides previously treated with distilled water and a drop of glycerine albumin that allows the bonding of biofilm on the slide to be stained with hematoxylin eosin (HE).
2.3.2. Immunohistochemical Study
Sections of 5 μm were made from paraffin blocks used for diagnosis.
These sections were collected on silanized slides and were dried overnight in a stove at 37˚C. They were then deparaffinized in three toluene baths: 5 min (×3), Rehydrated in decreasing degree alcohol baths: Absolute ethanol: 5 min, Absolute ethanol: 5 min, 96˚ Ethanol: 5 min, 80˚ Ethanol: 5 min, 70˚ Ethanol: 5 min. Washed with distilled water: 10 min. Placed in 10% citrate buffer in a water bath preheated to 75˚C for 20 minutes to unmask specific antigenic sites.
The sections were then left to cool for 15 minutes in the same buffer at room temperature and washed with PBS (Phosphate Baffer Salin) for 5minutes.
The outline of each cut was dried with filter paper and circled with Pap Pen. The endogenous peroxidase activeity was inhibited by incubating the sections for 5 minutes in 3% hydrogen peroxide followed by rinsing with two PBS baths for 5 min (×2).
Tissue sections were incubated in the presence of skim milk for 5 min then for 45 minutes at room temperature with primary antibody polyclonal anti human GAPDH [17] diluted to 1:50, 1:80, 1:100, 1:500, 1:800, 1:1000 and 1:1500 in PBS buffer. After rinsing with PBS, sections were incubated for 30 minutes at room temperature in a humid chamber with secondary antibody linked to peroxidase diluted to 1:1000 in PBS and then rinsed with PBS.
Before adding the substrate/chromogenic solution, the slides were rinsed with distilled water and stained with hematoxylin of Mayer.
Negative controls were obtained by replacing the primary antibody with buffer. The immunostaining was performed through slides observation at optical microscope in white light using the objectives (×10) and (×40).
3. RESULTS AND DISCUSSION
The development of technical conditions is a key step in each experiment: it can determine its optimal conditions. The adjusted parameter for this technique was the dilution factor that has never been clarified before (Table 1).
Several dilutions were performed. The threshold dilutions whose labeling intensity remained stable, was observed for dilutions ranging from 1/50 to 1/1000. This last dilution was chosen because of its specificity and its economic purpose.
It should be noted that the quality of immunostaining by large dilutions is likely due to the decrease in crossreactions, knowing that the more the antibody is diluted, the more its specificity increases.
For many years, various studies have shown increased glycolytic activity in tumor tissues [18]. Therefore, we planned to study the expression of GAPDH, a key enzyme of glycolysis, in 3 tissue types (lesions of the breast, cervix and prostate).
To date, all work performed on the expression of GAPDH consisted on direct quantification of specific messenger RNA levels of GAPDH in human tumor tissues or in human cell lines, this work was done by RT-PCR and Northen Blot.
Thus, in prostate cancer, it was demonstrated that the expression of GAPDH was dependent on the stage of the disease so that it increased significantly in the most advanced stages (Table 2). Another study showed that there
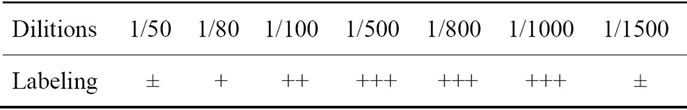
Table 1. Results of the labeling intensity according to dilutions of the primary antibody (± More or less intense; + Less intense; ++ intense; +++ very intense).
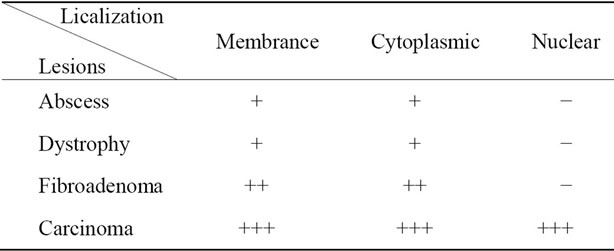
Table 2. Identification of the immuno-localization of GAPDH in breast lesions by immunohistochemical study (− Absent; + Scarce; ++ Abundant; +++ Excessive).
are different isoforms of GAPDH in prostate cancer [19]. According to Jin, gene expression of GAPDH increases in Cervix cancer tissue [20].
The immunohistochemical results were used to estimate the expression level of GAPDH and its immunolocalization in different lesions of breast, cervix and prostate.
We found that the expression level of GAPDH became more intense and diffuse in tumor tissues compared with benign tissue. This applies to the three tissue types (breast, cervix and prostate) (Figures 1-3). These results complement and are consistent with those of KIM et al. for cervix cancer [20,21], those of Aparecida Corrêa et al. for breast cancer [22] and those of Daniel Epner et al., for prostate [23]. This result seems to prove the involvement of GAPDH in the tumor process. It is also clear that the localization of GAPDH in the three organs vary
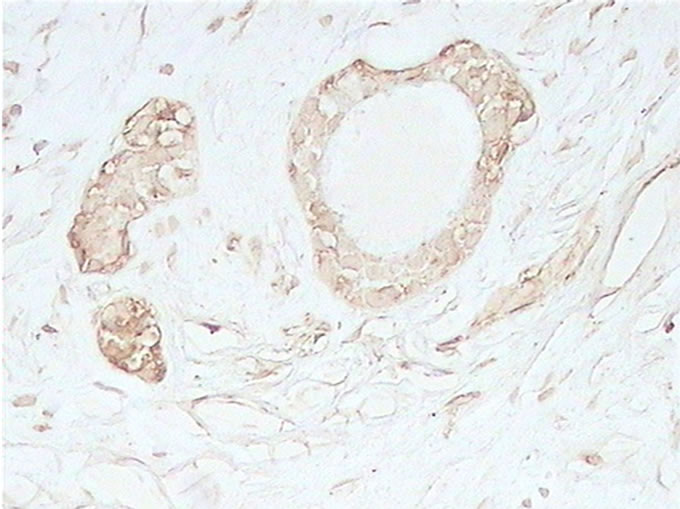 (a)
(a)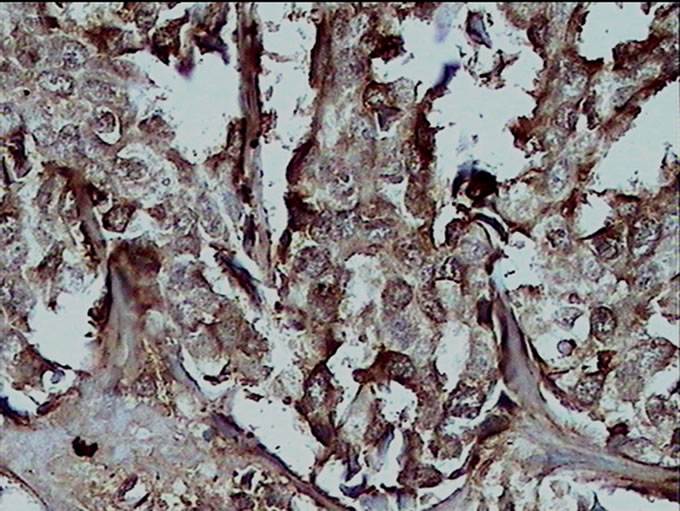 (b)
(b)
Figure 1. (a) Detection of GAPDH expression in benign breast lesion by immunohistochemical analysis with the polyclonal anti human GAPDH (×40); (b) Detection of GAPDH expression in malignant breast lesion by immunohistochemical analysis with the polyclonal anti human GAPDH (×40).
 (a)
(a)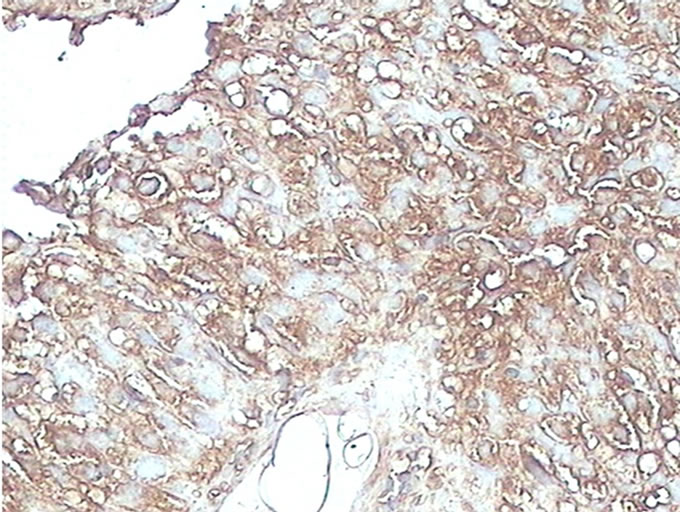 (b)
(b)
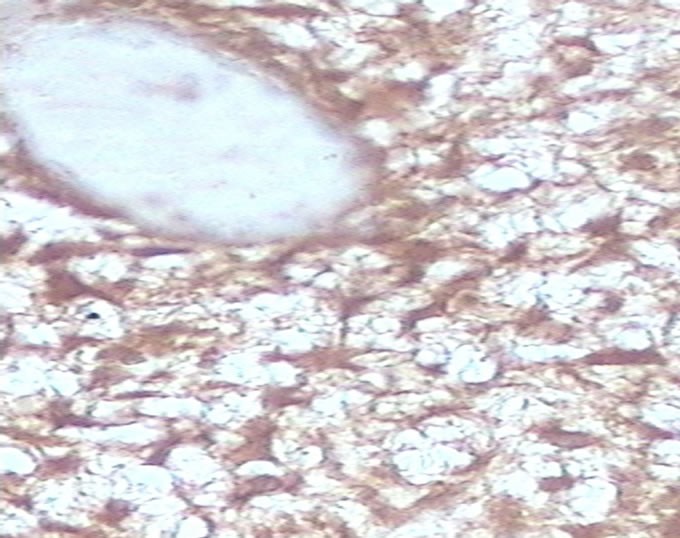
Figure 2. (a) Detection of GAPDH expression in benign cervix lesion by immunohistochemical analysis with the polyclonal anti human GAPDH (×40); (b) Detection of GAPDH expression in malignant cervix lesion by immunohistochemical analysis with the polyclonal anti human GAPDH (×40).
with the degree of tumor differentiation (Tables 2-4). It should be noted that it has cytoplasmic or membrane localization in breast, cervix and prostate benign lesions. Whereas the membrane localization is probably related to its role as a carrier and its ability to bind to the membrane, as described by Sirover [9], the cytoplasmic localization can be related to its pivotal role as glycolytic enzyme [10,11]. Or it may be related to its role as kinase capable of phosphorylating cytoplasmic proteins [9].
In the three cancer cases, we have found a membrane, cytoplasmic and particularly a nuclear overexpression. This overexpression suggests either its involvement in the cancer process, and therefore, draws attention to a new possible role of GAPDH in carcinogenesis which can be added to those already described or it suggests
 (a)
(a) (b)
(b)
Figure 3. (a) Detection of GAPDH expression in benign prostate lesion by immunohistochemical analysis with the polyclonal anti human GAPDH (×40); (b) Detection of GAPDH expression in malignant prostate lesion by immunohistochemical analysis with the polyclonal anti human GAPDH (×40).
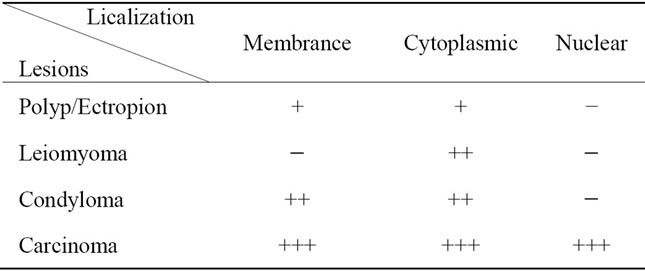
Table 3. Identification of the immuno-localization of GAPDH in cervix lesions by immunohistochemical study (− Absent; + Scarce; ++ Abundant; +++ Excessive).
that GAPDH undergoes regulation in tumor tissue, in relation to carcinogenesis, being involved in other roles independently of its classical glycolytic role [24]. Although GAPDH seems involved in malignant tumor
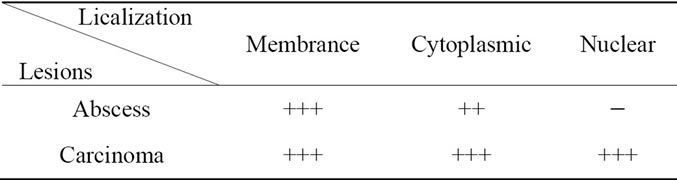
Table 4. Identification of the immuno-localization of GAPDH in Prostate lesions by immunohistochemical study (− Absent; ++ Abundant; +++ Excessive).
process, until now, there is no certainty about this likely role.
Various questions arise: how GAPDH perform its role in carcinogenesis? How is it induced? And how is it regulated? Is it carried out by a single form of GAPDH or it results from the induction of different isoforms?
This could help to develop a gene therapy with antisense oligodeoxynucleotides directed against the mRNA isoform (s) in cancer cases involving overexpression of GAPDH.
4. CONCLUSIONS
Our study has shown that in the three organs studied: Cervix, Breast and Prostate, GAPDH showed an overexpression in malignant tumor tissues. It has been shown that labeling was essentially nuclear in malignant lesions.
These results suggest GAPDH involvement in cancer process and draw attention to a probable new nuclear role. In addition to other functions already described such as its implication in glycolysis, apoptosis or oxidative stress, GAPDH may be implicated in DNA replication or repair.
Although GAPDH seems to be involved in malignant tumor process, there is no certainty about its specific role in the studied pathologies. Therefore it would be very interesting to evaluate the expression of the gene encoding this enzyme.
Moreover, it seems important to demonstrate whether the new role of GAPDH remains specific to a single form, or it is related to different isoforms of the enzyme, which can help to develop a gene therapy with antisense oligodeoxynucleotides directed against isoform(s) mRNA(s) in cases of cancer involving overexpression of GAPDH.
![]()
![]()
REFERENCES
- Sygusch, J., Azema, L. and Dax, C. (2006) A first weapon against cancer metastases. Univalor Journal, 1, 1-2.
- Warburg, O.H. (1956) On the origin of cancer cells. Science, 123, 309-314. doi:10.1126/science.123.3191.309
- Soukri, A., Valverde, F., Hafid, N., Elkebbaj, M.S. and Serrano, A. (1996) Occurrence of a differential expression of the glyceraldehyde-3-phosphate dehydrogenase gene in muscle and liver from euthermic and induced hibernating jerboa (Jaculus orientalis). Gene Journal, 181, 139-145. doi:10.1016/S0378-1119(96)00494-5
- Iddar, A., Valverde, F., Serrano, A. and Soukri, A. (2002) Expression, purification and characterization of recombinant non-phosphorylating NADP-dependent glyceraldehyde-3-phosphate dehydrogenase from Clostridium acetobutylicum. Journal of Protein Expression and Purification, 25, 519-526. doi:10.1016/S1046-5928(02)00032-3
- Fothergill-Gilmore, L.A. and Pam, M. (1993) Evolution of Glycolysis. Journal of Progress in Biophysics and Molecular Biology, 95, 105-135. doi:10.1016/0079-6107(93)90001-Z
- Figge, R.M., Schubert, M., Brinkman, H. and Cerff, R. (1999) Glyceraldehyde phosphate dehydrogenase gene diversity in eubacteria an eukaryotes: Evidence for intraand inter-kingdom gene transfer. Journal of Molecular Biology and Evolution, 16, 429-440. doi:10.1093/oxfordjournals.molbev.a026125
- Habenicht, A. (1997) The non-phosphorylating glyceroldehyde-3-phosphate dehydrogenase: Biochemistry, structure, occurrence and evolution. Journal of Biological Chemistry, 378, 1413-1419.
- Bruns, G.A.P. and Gerald, P.S. (1976) Human glyceroldehyde-3-phosphate dehydrogenase in man-rodent somatic cell hybrids. Science, 192, 54-56. doi:10.1126/science.176725
- Sirover, M.A. (1999) New insights into an old protein: The functional diversity of mammalian glyceraldehyde- 3-phosphate dehydrogenase. Journal of Biochimica and Biophysica Acta, 1432, 159-184. doi:10.1016/S0167-4838(99)00119-3
- Barbini, L., Rodríguez, J., Dominguez, F. and Vega, F. (2007) Glyceraldehyde-3-phosphate dehydrogenase exerts different biologic activities in apoptotic and proliferating hepatocytes according to its subcellular localization. Journal of Molecular and Cellular Biochemistry, 300, 19- 28. doi:10.1007/s11010-006-9341-1
- Sirover, M.A. (2005) New nuclear functions of the glycolytic protein, glyceraldehyde-3-phosphate dehydrogenase, in mammalian cells. Journal of Cellular Biochemistry, 95, 45-52. doi:10.1002/jcb.20399
- Pitot, H.C. (1986) The biochemistry of neoplasia in vivo. Fundamentals of Oncology, Marcel Dekker, New York, 323-346.
- Zhou, Y.Y.X, Stoffer, B.J., Bonafe. N., Gilmore-Hebert, M., McAlpine, M. and Chambers, S.K. (2008) The multifunctional protein glyceraldehyde-3-phosphate dehydrogenase is both regulated and controls colony-stimulating factor-1 messenger RNA Stability in Ovarian Cancer. Journal of Molecular Cancer Research, 6, 1375- 1384. doi:10.1158/1541-7786.MCR-07-2170
- Du, Z.-X., Wang, H.Q., Zhang, H.Y. and Gao, D.X. (2007) Involvement of glyceraldehyde-3-phosphate dehydrogenase in tumor necrosis factor-related apoptosisinducing ligand-mediated death of thyroid cancer cells. Journal of endocrinology, 148, 4352. doi:10.1210/en.2006-1511
- Laffargue, F., Dargent, D. and Piana, L. (2002) Contribution des Sociétés françaises de chirurgie d’organe. Bulletin du Cancer, 89, 52-54.
- Parkin, D.M., Bray, F.I. and Devesa, S.S. (2001) Cancer burden in the year 2000 the global picture. Journal of European Journal of Cancer, 3, 4-66.
- Mountassif, D., Baibai, T., Fourrat, F., Moutaouakkil, A., Iddar, A., El Kebbaj, M.S. and Soukri, A. (2009) Immunoaffinity purification and characterization of Glyceraldehyde-3-phosphate dehydrogenase from human erythrocytes. Journal of Acta Biochimica & Biophysica Sinica, 41, 309-406.
- Pederson, P.L. (1978). Tumor mitochondria and the bioenergetics of cancer cells. Journal of Progress in Experimental Tumor Research, 22, 198-274.
- Epner, D.E., Partin, A.W., Schalken, J.A., Isaacs, J.T. and Coffey, D.S. (1993) Association of glyceraldehyde-3- phosphate dehydrogenase expression with cell motility and metastatic potential of rat prostatic adenocarcinoma. Journal of Cancer Research, 53, 1995-1997.
- Kim, J.W., Kim, S.J., Han, S.M., Paik, S.Y., Hur, S.Y., Kim, Y.W., Lee, J.M. and Namkoong, S.E. (1998) Increased glyceraldehyde-3-phosphate dehydrogenase gene expression in human cervical cancers. Journal of Gynecologic Oncology, 71, 266-269. doi:10.1006/gyno.1998.5195
- Correa, C.R., Bertollo, C.M., Zouain, C.S. and Goes A.M. (2010) Glyceraldehyde-3-phosphate dehydrogenase as an associated antigen on human breast cancer cell lines MACL-1 and MGSO-3. Journal of Oncology Reports, 24, 677-685.
- Kim, J.W., Kim, T.E., Kim, Y.K., Kim, Y.W., Kim, S.J., Lee, J.M., Kim, I.K. and Namkoong, S.E. (1999) Antisense oligodeoxynucleotide of glyceraldehyde-3-phosphate dehydrogenase gene inhibits cell proliferation and induces apoptosis in human cervical carcinoma cell lines. Journal of Antisense & Nucleic Acid Drug Development, 9, 507-513. doi:10.1089/oli.1.1999.9.507
- Shan, L., Xiang, G., Hoestje, S. and Epner, D.E. (2002) Identification of an additional hypoxia responsive element in the glyceraldehyde-3-phosphate dehydrogenase gene promoter. Journal of Biochimica and Biophysica Acta (BBA)—Gene Structure and Expression, 1574, 2152- 2156.
- Sirover, M.A. (1990) Cell cycle regulation of DNA repair enzymes and pathways. In: Milo, G.E. and Casto, B.C., Eds., Transformation of Human Diploid Fibroblasts, CRC Press, Boca Raton, 29-55.

