Open Journal of Pediatrics
Vol.4 No.1(2014), Article ID:43497,9 pages DOI:10.4236/ojped.2014.41002
The Expression of Surfactant Proteins A and D in the Intestines and Pancreas of Murine Fetuses
Ryuta Saka1,2, Hiroomi Okuyama1*, Kaoru Uchida3, Kumiko Nakahira3, Takashi Sasaki1, Satoko Nose1, Masahiro Nakayama4, Masahiro Fukuzawa2, Itaru Yanagihara3
1Department of Pediatric Surgery, Hyogo College of Medicine, Nishinomiya, Japan
2Department of Pediatric Surgery, Osaka University Graduate School of Medicine, Suita, Japan
3Department of Developmental Medicine, Osaka Medical Center and Research Institute for Maternal and Child Health, Izumi, Japan
4Pathology and Laboratory Medicine, Osaka Medical Center and Research Institute for Maternal and Child Health, Izumi, Japan
Email: *okuyama@hyo-med.ac.jp
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 28 December 2013; revised 25 January 2014; accepted 2 February 2014
ABSTRACT
Purpose: Surfactant proteins exist in the digestive tract and may play an important role in the host defense. However, the expression of surfactant proteins in the premature digestive system remains unclear. The aim of this study was to investigate the expression of surfactant proteins in the intestines and pancreas of murine fetuses. Methods: Immunostaining for SP-A and SP-D was assessed in the small intestine and pancreas of ICR murine fetuses on days 15, 16, 17 and 18 of gestation (normal duration of pregnancy: 19 - 21 days). RT-PCR was performed to detect the expression of spa and spd mRNA in the small intestine and pancreas on day 16, 17 and 18 of gestation. Results: Immunoreactivity for SP-A and SP-D in the acinar cells of pancreas and intestinal mucosal surface were positive on day 16 of gestation onward. RT-PCR revealed that the expression of spa and spd mRNA was significant in the pancreas but weak in the small intestine. Conclusions: Our data revealed that surfactant proteins are present in the fetal intestines and pancreas and that a significant expression of spa and spd mRNA is detected in the fetal pancreas. Pancreas may be a possible organ involved in the synthesis and secretion of surfactant proteins into the intestinal lumen.
Keywords:Surfactant Protein; Fetus; Intestine; Pancreas

1. Introduction
Pulmonary surfactant is a mixture of lipids and proteins that contains four specific surfactant proteins named SP-A, SP-B, SP-C and SP-D. Recent studies have revealed that these surfactant proteins are involved in the host defense, immunomodulation and production of surfactant lipids [1] [2] .
SP-A and SP-D are hydrophilic and belong to the collectin subgroup of C-type lectins, playing an important role in innate immunity [3] . Although most studies regarding the function of SP-A and SP-D have focused on their potential roles in the lungs, spa and spd mRNA has been detected in human extrapulmonary tissues [4] [5] .
Recently, it has been reported that surfactant proteins exist in the gastrointestinal mucosal surface [6] . Considering their surface activity and immunomodulatory effects in the lungs, surfactant proteins in the digestive tract may also play an important role in the host defense, especially in neonates. However, the expression of surfactant proteins in the fetal digestive system has not yet been described.
In this study, in order to clarify the development of innate immunity in the digestive system, possibly supported by natural detergents, the localization and expression levels of surfactant proteins were investigated in murine fetuses.
2. Materials and Methods
2.1. Ethics Statement
This study was approved by the institutional committee of animal care and research at Hyogo College of Medicine. All procedures were carried out in accordance with the Hyogo College of Medicine animal care policy.
2.2. Animals
Timed-pregnant ICR mice were used. The animals were maintained at room temperature in a humidity-controlled room on a 12-hour light/12-hour dark cycle and given sterilized solid food and water ad libitum. The day of vaginal plug detection was designated as day 0 of gestation. On days 15, 16, 17 and 18 of gestation (normal duration of gestation: 19 - 21 days), the pregnant dams were anesthetized using pentobarbital (0.75 mg/10g body weight) intraperitoneal injections and sacrificed by cutting the inferior vena cava. Fetuses were taken from the uterus, and the fetal lungs, small intestines and pancreas were collected. The organs of the three fetuses and the adult mouse were fixed in zinc for immunohistochemistry, snap-frozen in liquid nitrogen and stored at −80˚C for the molecular analyses.
2.3. Immunohistochemistry
Immunostaining for SP-A and SP-D was assessed in the small intestine and pancreas on day 15, 16, 17 and 18 of gestation. All samples were fixed with IHC Zinc Fixative (BD, NJ, USA), embedded in paraffin and cut into 3-μm sections. Following deparaffinization in xylene and rehydration in a graded series of ethanol, the sections were incubated with 0.3% H2O2 in methanol for 30 minutes to block the endogenous peroxidase activity. After three washes with PBS, the sections were incubated with Protein Block, Serum-Free (Dako, Glostrup, Denmark) to inhibit nonspecific staining for 30 minutes and washed with PBS. Then, the sections were incubated overnight at 4˚C with either anti-SP-A polyclonal Abs (1:500, Chemicon, Temecula, CA, #AB3420) or anti-SP-D polyclonal Abs (1:1000, Chemicon #AB3434). After three washes with PBS, the sections were incubated with EnVisionTM + Rabbit/HRP (Dako) for one hour at room temperature. The tissue sections were then incubated with DAB substrate (Nichirei Biosciences, Tokyo, Japan) for chromogen reaction and counterstained with hematoxylin. The sections were mounted on slides with Malinol (Muto Pure Chemicals, Tokyo, Japan) and visualized using light microscopy.
2.4. Quantitative Real-Time PCR Analysis
Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed on a panel of RNAs obtained from the small intestine and pancreatic tissues of three murine fetuses to detect the spa and spd mRNA expression on day 16, 17 and 18 of gestation. Tissues obtained from day 15 fetuses were not examined, because they were too small to perform RT-PCR. Total RNA was extracted from the frozen fetal tissues using ISOGEN® (Nippon Gene, Tokyo, Japan) reagent according to the manufacturer’s protocol. The cDNA was reverse transcribed from total RNA (2 μg per sample) with random primers using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) with RNase Inhibitor (Takara Bio, Otsu, Japan). Quantitative real-time PCR was performed to evaluate the gene expression of spa and spd mRNA using the TaqMan® Gene Expression Master Mix and 20x TaqMan® Gene Expression Assays (Applied Biosystems). The TaqMan® Gene Expression Assays IDs are SP-A: Mm00499170_m1 and SP-D: Mm00486060_m1, and the housekeeping gene 18S that was used as an internal standard was Hs99999901_s1, which can be used for eukaryotic cells. The detection and analyses were performed on the ABI 7500 Fast Real-Time PCR System using the ABI 7500 System Sequence Detection Software program Version 1.3.1. Real-time PCR was performed under the following thermal cycling conditions: 2 min at 50˚C, 10 min at 95˚C and 40 cycles of 15 sec at 95˚C for denaturation and 1 min at 60˚C for transcription. All samples were normalized with 18S.
2.5. Statistics
The results of the quantitative RT-PCR analysis are expressed as the mean ± SD. The mRNA values were statistically analyzed using the Wilcoxon rank-sum test. All statistical analyses were performed using the JMP®8 software package (SAS Institute Inc., Cary, NC). A P-value of <0.05 was considered to be significant.
3. Results
3.1. Immunohistochemical Analysis of SP-A and SP-D
The lungs, pancreas and small intestines of the adult mice were examined with respective antibodies. The epithelial cells of lungs and the acinar cells of pancreas exhibited positive immunoreactivity for SP-A and SP-D. The mucosal surface of small intestine was only slightly immunostained for SP-A and SP-D (Figures 1, 2).
The pancreas and small intestines of murine fetuses (days 15, 16, 17 and 18 of gestation) were also examined with respective antibodies. Reactivity for SP-A and SP-D in the acinar cells of the fetal pancreas was negative on day 15 of gestation but positive on days 16, 17 and 18 of gestation (Figures 3, 4). Different from the reactivity observed in the pancreas, the mucosal surface of the fetal intestines was positively immunostained for both SP-A and SP-D starting on day 15 of gestation, then the mucosal cells exhibited intracellular weak reactivity for both SP-A and SP-D on day 18 of gestation (Figures 5, 6). The negative controls are shown in Figure 7.
3.2. Expression of spa and spd mRNA Analyzed with RT-PCR
A significant expression of spa mRNA was detected in the fetal pancreas on day 16, 17 and 18 of gestation, and
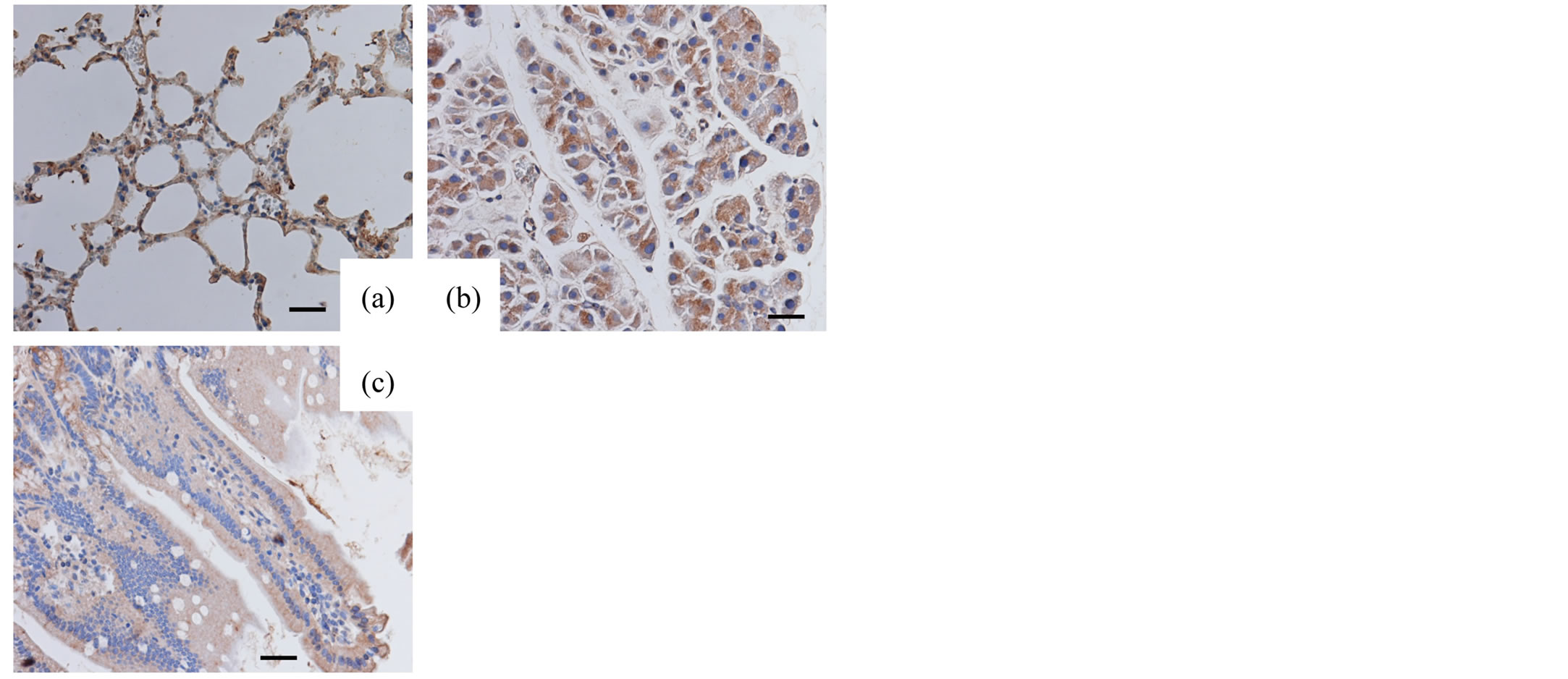
Figure 1. Immunohistochemical analysis (adult tissues, SPA) Adult lungs (a), pancreas (b) and small intestine (c) immunostained for SP-A. Original magnification: 400×. Bar = 20 μm.
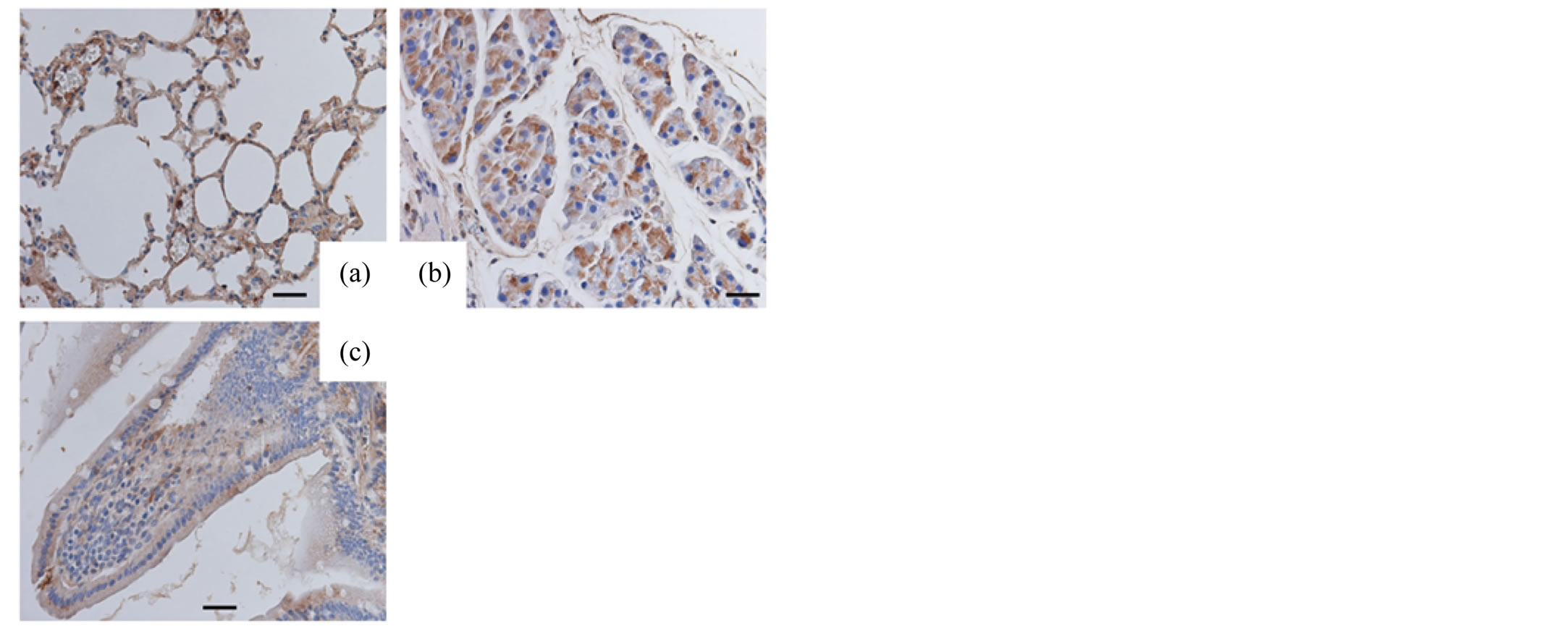
Figure 2. Immunohistochemical analysis (adult tissues, SP-D). Adult lungs (a), pancreas (b) and small intestine (c) immunostained for SP-D. Original magnification: 400×. Bar = 20 μm.
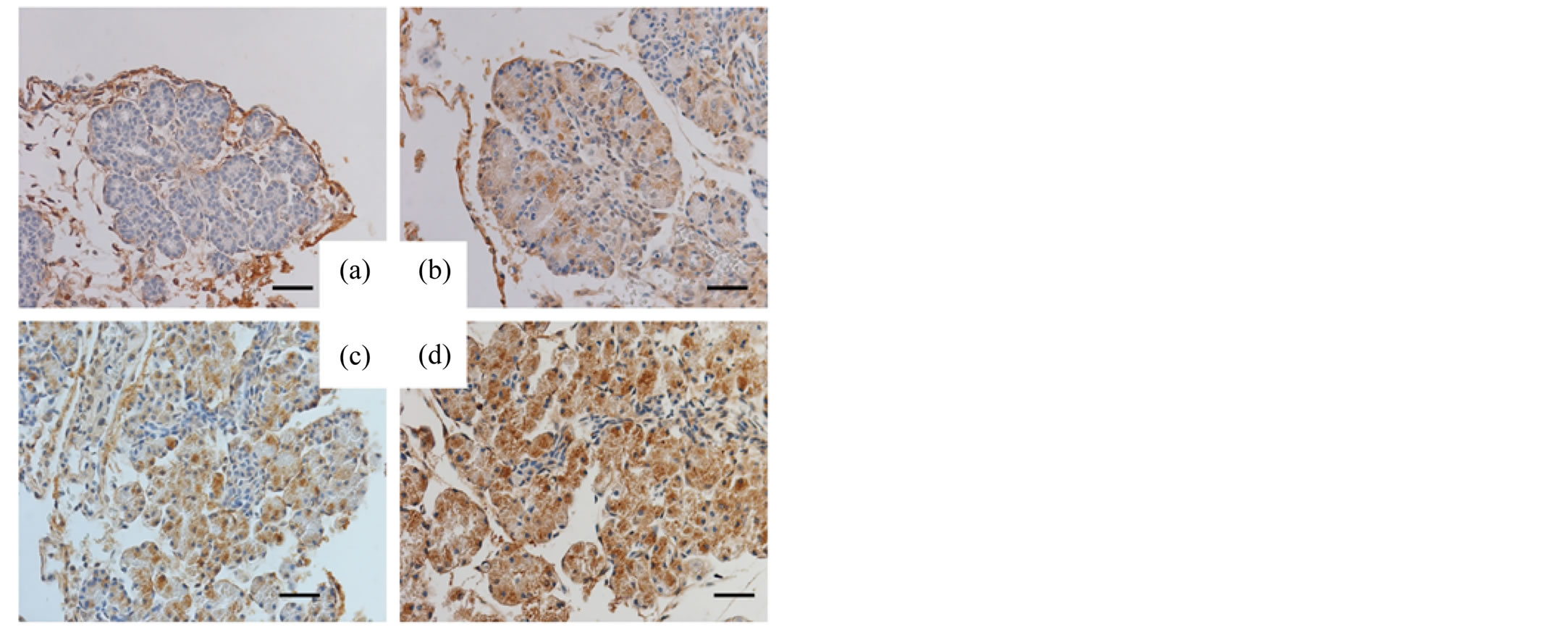
Figure 3. Immunohistochemical analysis (fetal pancreas, SP-A). The pancreatic tissues of the murine fetuses on day 15 (a), 16 (b), 17 (c) and 18 (d) of gestation immunostained for SP-A. Original magnification: 400×. Bar = 20 μm.
the peak was found on day 17 of gestation. The spa/18S ratio of the pancreas was 10.6 ± 5.3 on day16, 31.1 ± 7.7 on day 17 and 5.7 ± 3.1 on day 18 of gestation, respectively. Compared to the pancreas, the expression in the intestine was significantly lower from day 16 to 18 of gestation (Figure 8(a)).
The expression of spd mRNA in the pancreas gradually increased from day 16 to 18 of gestation. The spd/18S ratio was 0.11 ± 0.19 on day16, 1.33 ± 0.65 on day 17 and 3.86 ± 1.47 on day 18 of gestation, respectively. Finally, the expression of spd mRNA in the pancreas was significantly higher than that observed in the small intestine on day 18 of gestation (p = 0.04) (Figure 8(b)).
4. Discussion
Pulmonary surfactant contains four characteristic apoproteins, including surfactant proteins SP-A, SP-B, SP-C and SP-D. Although they account for only approximately 5% - 10% (by weight) of pulmonary surfactant, these proteins are functionally crucial components of the lungs [7] . Among them, SP-B is essential for reducing surface tension, and SP-B-deficient mice exhibit fatal respiratory failure [8] . SP-C plays an important role in the
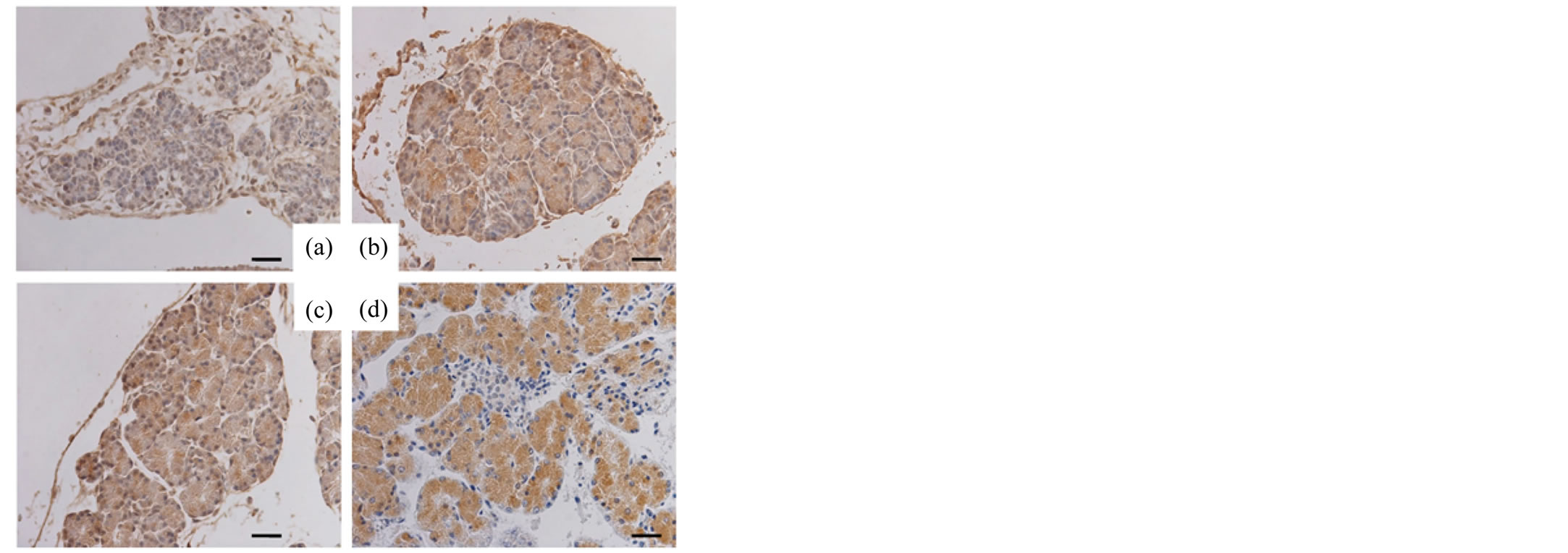
Figure 4. Immunohistochemical analysis (fetal pancreas, SP-D). The pancreatic tissues of the murine fetuses on day 15 (a), 16 (b), 17 (c) and 18 (d) of gestation immunostained for SP-D. Original magnification: 400×. Bar = 20 μm.
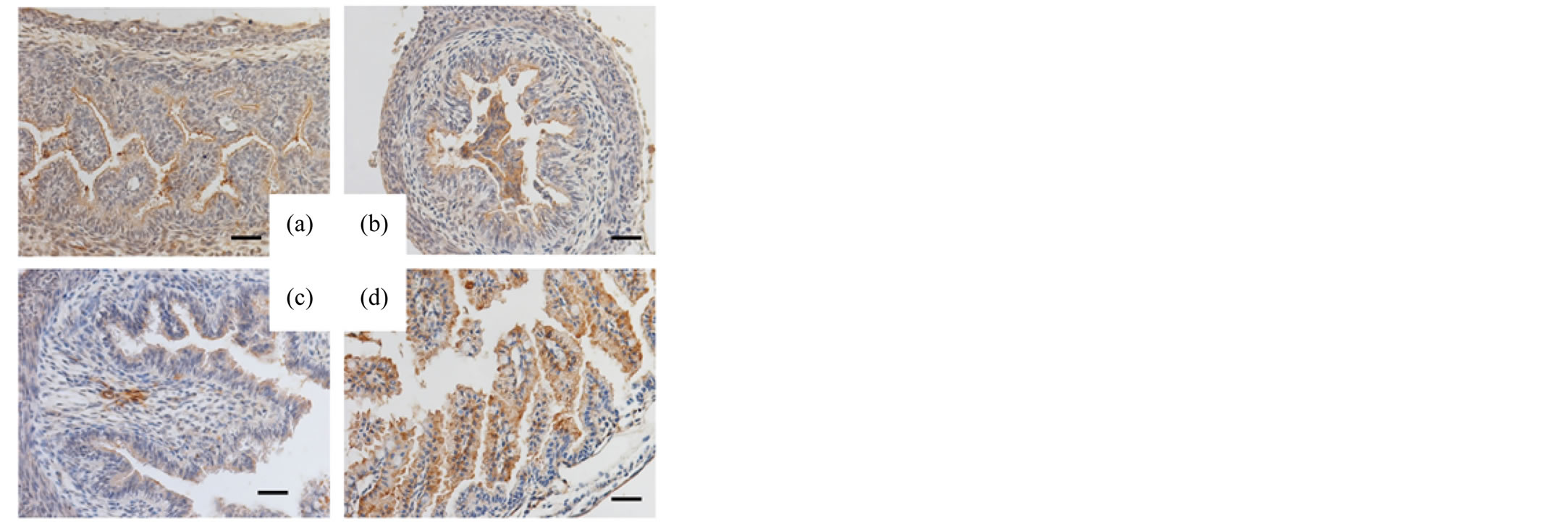
Figure 5. Immunohistochemical analysis (fetal intestine, SP-A) The small intestines of the murine fetuses on day 15 (a), 16 (b), 17 (c) and 18 (d) of gestation immunostained for SP-A. Original magnification: 400×. Bar = 20 μm.
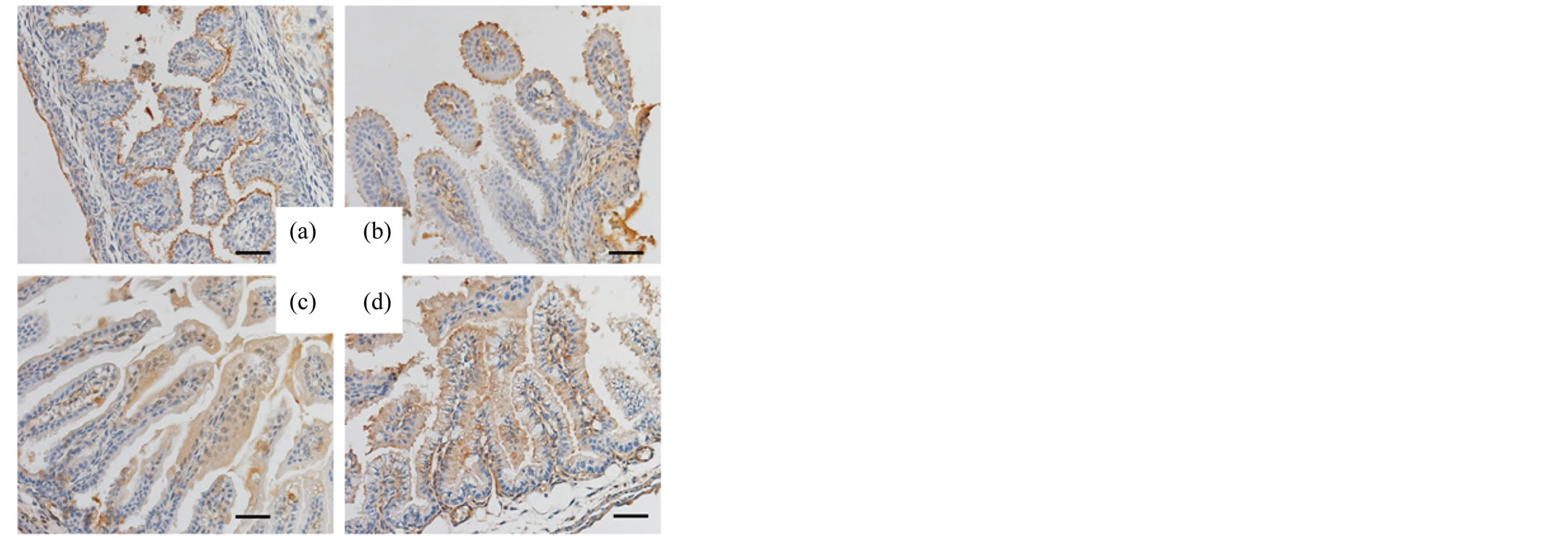
Figure 6. Immunohistochemical analysis (fetal intestine, SP-D). The small intestines of the murine fetuses on day 15 (a), 16 (b), 17 (c) and 18 (d) of gestation immunostained for SP-D. Original magnification: 400×. Bar = 20 μm.

Figure 7. Negative control. The lungs (a), pancreas (b) and small intestines (c) of the murine fetuses on day 18 of gestation were stained without antibodies for SP-A and SP-D.
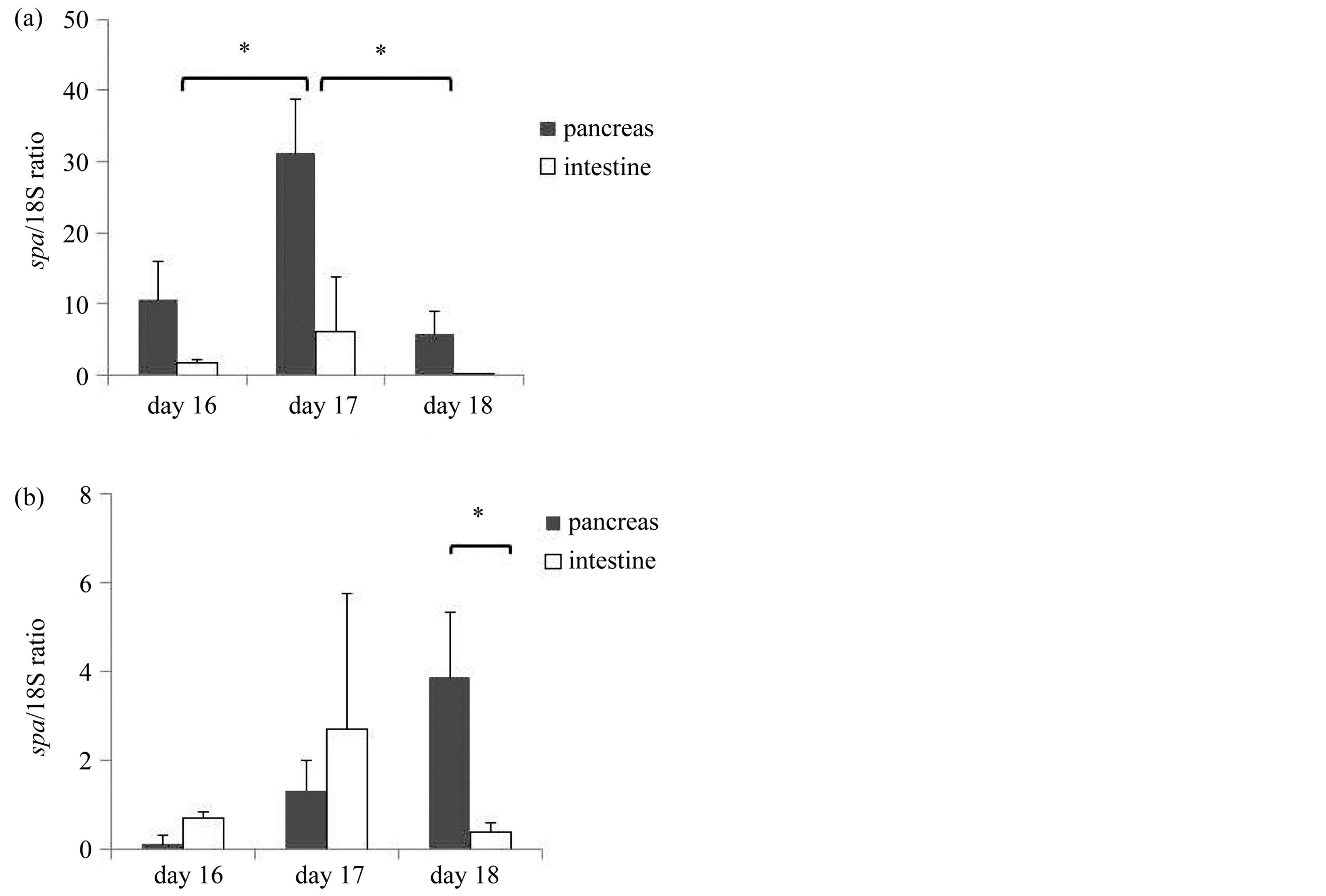
Figure 8. The expression of spa and spd mRNA in the pancreas and the intestine on day 16, 17 and 18 of gestation (RT-PCR). (a) Expression of spa mRNA. (n = 3) The data was expressed as spa/18S ratio. The expression of spa mRNA in the pancreas was significant from day 16 to day 18 of gestation, and had its peak on day17. The expression in the intestine was lower than that in the pancreas. (b) Expression of spd mRNA. (n = 3) The data was expressed as spd/18S ratio. The expression of spd mRNA gradually increased from day 16 to day18 of gestation. *Indicates statistical significance at p < 0.05.
formation and maintenance of the layer of surfactant, and its genetic mutations cause severe interstitial lung disorders [9] .
SP-A and SP-D belong to the collectin family of C-type lectins, which contribute to the host defense functions. SP-A is known to be the most abundant surfactant protein [7] .
Although the expression of spa mRNA is readily found in the lungs, trachea, prostate, pancreas and thymus, immunoreactivity for SP-A is restricted to the respiratory system in humans [5] . SP-A null mice demonstrate enhanced susceptibility to respiratory infections associated with enhanced inflammation and production of proinflammatory cytokines [10] . The expression of spd mRNA is readily amplified in various tissues, including the pancreas and small intestines, in humans [11] . SP-D immunostaining is observed in duct cells in various organs, including the pancreas in humans, and the wide distribution of SP-D is distinct from that of SP-A.
SP-A and SP-D enhance microbial phagocytosis by opsonizing and aggregating bacteria and viruses, acting as activation ligands and upregulating the expressions of immune cell surface receptors. In addition, these proteins alter the production of cytokines and free radicals [12] . There is significant interspecies variation in the distribution and abundance of SP-A and SP-D [13] . Although immunolocalization of SP-D in human fetuses and newborns has been previously reported [4] , there are no reports regarding the expression of spa and spd mRNA in the fetal gastrointestinal system.
In this study, we used zinc fixative, which has been reported to be effective for preserving fixation-sensitive antigens in paraffin-embedded tissues [14] . The immunohistochemical analysis revealed positive reactivity for SP-A and SP-D in the acinar cells of the fetal pancreas. Our results are consistent with the findings of previous reports showing positive immunoreactivity for SP-D in the beta cells of fetal and neonatal rats [15] . In contrast, previous reports have shown positive reactivity for SP-D localized in the intercalated duct, not in islets, in humans [4] [11] . Madsen et al. showed that no immunoreactivity for SP-A is found in extrapulmonary tissues, including the pancreas and small intestines, in humans [5] . We assume that the extrapulmonary tissue distribution and abundance of SP-A and SP-D differ according to the species and age.
Our data also showed that a significant expression of spa mRNA was detected in the fetal pancreas, but a low expression in the fetal intestines. The fact that the expression of spa mRNA has its peak on day 17 of gestation suggests that the role of the fetal pancreas may be changed after birth. Our expression profiles of SP-A in murine tissues are consistent with those observed in human tissues, which show a positive expression of spa mRNA in the pancreas and a low expression of spa mRNA in the small intestine [5] . As far as SP-D was concerned, the expression of spd mRNA in the pancreas gradually increased before birth. In contrast, the expression of spd mRNA in the intestine was barely detected on day 18 of gestation.
These results support the hypothesis that the fetal intestinal lumen is covered with SP-A and SP-D derived from ‘upstream’ organs, such as the pancreas. Although swallowed amnion fluid may be another source of SP-A and SP-D, the fetal pancreas is an organ that possibly establishes innate immunity and produces surface activity in the fetal intestines before birth.
Interestingly, Aye et al. reported that spd mRNA is expressed in pancreatic beta cells in fetuses and newborns and is hardly detectable in adult rats [15] . Our results and the findings of previous reports suggest that the role of the fetal pancreas may differ from that of the adult pancreas.
Because the digestive system requires a firm barrier against microorganism invasion immediately after birth, SP-A and SP-D present on the fetal intestinal luminal surface may play a role in the innate immunity against pathogens at sites of entry into various organs. Our data also showed that the synthesis and secretion of SP-A and SP-D in the pancreas begin on day 16 of gestation (normal duration of gestation for mice: 19 - 21 days). Interestingly, George et al. reported that SP-A null mice exhibit significant gastrointestinal pathology, including bile-colored stomach and proximal small bowels, marked dilatation of the intestinal lumen and neutrophil accumulation in the stomach and small intestine, with little lung pathology [1] . The mortality of SP-A null mice is significantly improved with the oral administration of SP-A. This suggests that premature neonates without sufficient surfactant proteins in the digestive tract may develop life-threatening intestinal complications, such as necrotizing enterocolitis (NEC), meconium-related ileus (MRI) [16] and focal intestinal perforation (FIP) [17] , which are specific to premature infants. The fetal pancreas may contribute to the establishment of the intestinal tract defense before and immediately after birth.
5. Conclusion
The present study suggests that surfactant proteins play important roles in the neonatal digestive and respiratory systems. Further studies would allow for the development of new curative therapies, such as surfactant replacement therapy, to prevent intestinal complications in premature infants.
Acknowledgements
This work was supported in part by Grants-in-Aid from the Ministry of Health, Labour and Welfare, Japan and the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. I would like to express my gratitude to Dr. Shigeru Ariki and Prof. Kuroki Yoshio for their insightful comments and permission to use the antibodies.
References
- George, C.L., Goss, K.L., Meyerholz, D.K., Lamb, F.S. and Snyder, J.M. (2008) Surfactant-Associated Protein A Provides Critical Immunoprotection in Neonatal Mice. Infection and Immunity, 76, 380-390. http://dx.doi.org/10.1128/IAI.01043-07
- Hogenkamp, A., Herias, M.V., Tooten, P.C., Veldhuizen, E.J. and Haagsman, H.P. (2007) Effects of Surfactant Protein D on Growth, Adhesion and Epithelial Invasion of Intestinal Gram-Negative Bacteria. Molecular Immunology, 44, 3517-3527. http://dx.doi.org/10.1016/j.molimm.2007.03.013
- Wright, J.R. (1997) Immunomodulatory Functions of Surfactant. Physiological Reviews, 77, 931-962.
- Stahlman, M.T., Gray, M.E., Hull, W.M., Whitsett, J.A. (2002) Immunolocalization of Surfactant Protein-D (SP-D) in Human Fetal, Newborn, and Adult Tissues. Journal of Histochemistry & Cytochemistry, 50, 651-660. http://dx.doi.org/10.1177/002215540205000506
- Madsen, J., Tornoe, I., Nielsen, O., Koch, C., Steinhilber, W., et al. (2003) Expression and Localization of Lung Surfactant Protein A in Human Tissues. American Journal of Respiratory Cell and Molecular Biology, 29, 591-597. http://dx.doi.org/10.1165/rcmb.2002-0274OC
- Bourbon, J.R. and Chailley-Heu, B. (2001) Surfactant Proteins in the Digestive Tract, Mesentery, and Other Organs: Evolutionary Significance. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 129, 151-161. http://dx.doi.org/10.1016/S1095-6433(01)00312-9
- Kishore, U., Greenhough, T.J., Waters, P., Shrive, A.K., Ghai, R., et al. (2006) Surfactant Proteins SP-A and SP-D: Structure, Function and Receptors. Molecular Immunology, 43, 1293-1315. http://dx.doi.org/10.1016/j.molimm.2005.08.004
- Clark, J.C., Wert, S.E., Bachurski, C.J., Stahlman, M.T., Stripp, B.R., et al. (1995) Targeted Disruption of the Surfactant Protein B Gene Disrupts Surfactant Homeostasis, Causing Respiratory Failure in Newborn Mice. Proceedings of the National Academy of Sciences of the United States of America, 92, 7794-7798. http://dx.doi.org/10.1073/pnas.92.17.7794
- Glasser, S.W., Detmer, E.A., Ikegami, M., Na, C.L., Stahlman, M.T., et al. (2003) Pneumonitis and Emphysema in sp-C Gene Targeted Mice. The Journal of Biological Chemistry, 278, 14291-14298. http://dx.doi.org/10.1074/jbc.M210909200
- LeVine, A.M., Whitsett, J.A., Gwozdz, J.A., Richardson, T.R., Fisher, J.H., et al. (2000) Distinct Effects of Surfactant Protein A or D Deficiency during Bacterial Infection on the Lung. The Journal of Immunology, 165, 3934-3940.
- Madsen, J., Kliem, A., Tornoe, I., Skjodt, K., Koch, C., et al. (2000) Localization of Lung Surfactant Protein D on Mucosal Surfaces in Human Tissues. The Journal of Immunology, 164, 5866-5870.
- Pastva, A.M., Wright, J.R. and Williams, K.L. (2007) Immunomodulatory Roles of Surfactant Proteins A and D: Implications in Lung Disease. Proceedings of the American Thoracic Society, 4, 252-257. http://dx.doi.org/10.1513/pats.200701-018AW
- Akiyama, J., Hoffman, A., Brown, C., Allen, L., Edmondson, J., et al. (2002) Tissue Distribution of Surfactant Proteins A and D in the Mouse. Journal of Histochemistry & Cytochemistry, 50, 993-996. http://dx.doi.org/10.1177/002215540205000713
- Beckstead, J.H. (1994) A Simple Technique for Preservation of Fixation-Sensitive Antigens in Paraffin-Embedded Tissues. Journal of Histochemistry & Cytochemistry, 42, 1127-1134. http://dx.doi.org/10.1177/42.8.8027531
- Aye, T., Toschi, E., Sharma, A., Sgroi, D. and Bonner-Weir, S. (2010) Identification of Markers for Newly Formed Beta-Cells in the Perinatal period: A Time of Recognized Beta-Cell Immaturity. Journal of Histochemistry & Cytochemistry, 58, 369-376.http://dx.doi.org/10.1369/jhc.2009.954909
- Kubota, A., Shiraishi, J., Kawahara, H., Okuyama, H., Yoneda, A., et al. (2011) Meconium-Related Ileus in Extremely Low-Birthweight Neonates: Etiological Considerations from Histology and Radiology. Pediatrics International, 53, 887-891. http://dx.doi.org/10.1111/j.1442-200X.2011.03381.x
NOTES
*Corresponding author.

