Open Journal of Inorganic Chemistry
Vol.08 No.01(2018), Article ID:81027,7 pages
10.4236/ojic.2018.81002
Preparation of Single Substituted Phenyl Porphyrins Form Meso-Tetraphenyl Porphyrin-Synthetic Example from Symmetric Porphyrin into Asymmetric Porphyrins
Hui Lin, Xiaoting Chen, Yahong Wu, Shanling Tong, Sheng Hu, Jian Yu, Yan Yan*
College of Chemical Engineering & Light Industry, Guangdong University of Technology, Guangzhou, China
Copyright © 2018 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: October 26, 2017; Accepted: December 10, 2017; Published: December 13, 2017
ABSTRACT
Two asymmetric porphyrins, 5-(4-chloromethylphenyl)-10, 15, 20-triphenyl porphyrin and 5-(4-formylphenyl)-10, 15, 20-triphenyl porphyrin, were successfully prepared by the symmetric meso-tetraphenyl porphyrin and relative molecular configurations and properties were characterized by spectral determinations. This work presented an example for synthesis of asymmetric porphyrin derivatives from the symmetric porphyrin. Both asymmetric porphyrins are reactive in molecular assembly, the concerned reactions including alkylation with Grignard reagents, etherification with alcohols, aldol condensation and Mannich reaction for modification and enhancing their functionality. In this work, the reaction conditions were improved, synthetic strategy and route were confirmed.
Keywords:
Asymmetric Porphyrin, Spectral Characterization, Redox, Chloromethylation, Formylation

1. Introduction
Matured technique for preparation of single substituted porphyrin was based on the Adler’s method by using condensation of pyrrole with different ratio of substituted benzaldehydes. The resulted asymmetric porphyrins were excellent candidates in assembly of molecular devices, such as molecular wires [1] [2] , sensitive reagent in photodynamic therapy (PDT) for tumors and cancers [3] [4] , dyes in dye sensitized solar cells [5] [6] . In practice, the porphyrin reagents must be water-soluble and compatible with organisms, and therefore the asymmetric porphyrins must be modified to satisfy the condition in PDT. For overcoming the low yield limitation in synthesis of asymmetric porphyrins, a symmetric meso-tetraphenyl porphyrin (TPP) was selected as initiator, and an asymmetric porphyrin was successfully prepared by inducing a single substituent in para-position of one phenyl in the symmetric meso-tetraphenyl porphyrin. The resulted asymmetric product was 5-(4-chloromethylphenyl)-10, 15, 20-triphenyl porphyrin (ClMPTPP) with an active chlorine, and series of single substituted porphyrins can be derived from this asymmetric product, such as its derivative 5-(4-formylphenyl)-10, 15, 20-triphenyl porphyrin (FPTPP). According to reference method [7] , the improved synthesis route was described in Scheme 1.
2. Experimental
Pyrrole was distilled and the clear single molecule distillation at 130˚C - 131˚C was collected before using. Other reagents were purchased and used directly without further purification. IR spectra were recorded by Avatar 370 FT-IR spectrometer (Nicolet); A Bruker AVANCE III 400 MHz magnetic resonance spectrometer was employed for 1H NMR determinations; electronic spectra was performed by UV 2450 spectrophotometer (Shimadzu). Fluorescence spectra were recorded by a Fluoro Max-4 fluorescence spectrometer (HORIBA Jobin Yvon); a Bruker A200-9.5/12 Electron Paramagnetic Resonance spectrometer was selected to determine the paramagnetic properties. CV curves were recorded by A CHI660C electrochemical analyzer (Shanghai).
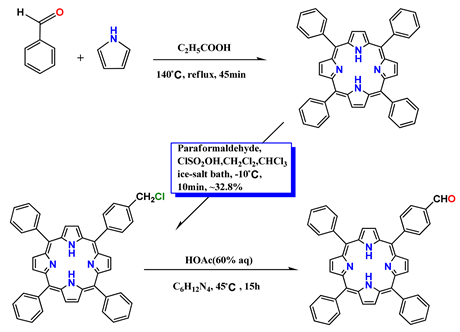
Scheme 1. The improved synthesis route of 5-(4-chloromethylphenyl)-10, 15, 20-triphenyl porphyrin (ClPTPP) and its derivative 5-(4-formylphenyl)-10, 15, 20-triphenyl porphyrin (FPTPP).
Preparation of 5-(4-chloromethyl phenyl)-10, 15, 20-triphenyl porphyrin (ClMPTPP): 2.4 g paraformaldehyde (82.8 mmol), NaCl (0.3 g, 5.1 mmol), 0.29 g ZnCl2 (2.1 mmol) and 10 mL dried CH2Cl2 were mixed well in a flask. The mixture was controlled at −10˚C by an ice-salt bath. Then 4.5 mL ClSO3H (68.19 mmol) was added and the mixture was well dissolved by stirring. 5 mL dried CHCl3 with TPP [8] (0.3 g, 0.489 mmol) was slowly dripped into the reaction system under stirring within 5 min. The reaction was kept for another 10 min and terminated by adding a large amount of ice-water. The resulted mixture was extracted by CHCl3 for 3 times, washed by water and then dried with Na2SO
Preparation of 5-(4-formylphenyl)-10, 15, 20-triphenyl porphyrin (FPTPP): 0.6 g (0.9 mmol) 5-(4-chloromethylphenyl)-10, 15, 20-triphenyl porphyrin (ClMPTPP), 1.26 g (9 mmol) hexamethylene-tetramine and 35 mL chloroform was mixed in a flask, after complete dissolution the solution was stirred continuously at 45˚C for 15 h. Then the solvent was removed by rotary vacuum evaporation, followed by adding 30 mL HOAc (60%) and keeping in reflux for 10 h. After cooling down and diluting with water until complete precipitation, the produced rough product in purple was separated by filtration and dried in a vacuum oven. Furthermore purification was carried out by chromatography as above description with a mix eluent of chloroform/mineral ether (v/v = 3/1), the second purple band was collected and after vacuum evaporation pure 5-(4-formylphenyl)-10, 15, 20-triphenyl porphyrin (FPTPP) in purple color was obtained in yield of 89% (0.5 g, 0.8 mmol). 1H-NMR (400 MHz, DMSO-d6) d/ppm: −2.77 (2H N-H in pyrrole); 6.82 - 7.77 (11H, para and meta H in phenyl); 8.02-8.63 (8H, ortho H in phenyl); 8.88 (8H, β-H in pyrrole); 10.34 (1H, single -PhCHO). The IR spectrum/cm−1: 3438.44 (nN-H in pyrrole); 994.08 (dN-H in pyrrole); 3053.09 (nC-H in pyrrole); 3003.08 and 1440.38 (nC-H in phenyl); 1597.34 (nC=C in phenyl); and 1738.05 (nC=O in PhCHO).
3. Results and Discussions
Figure 1 was the compared UV-Vis spectra for FPTPP, ClMPTPP and TPP. All porphyrins presented one Soret band and four Q bands. Since only a hydrogen atom in para position of one peripheral phenyl of TPP was replaced by chloromethyl to form ClMPTPP, their UV-Vis spectra displayed high similarity in
Figure 1. The UV-Vis spectra for 5-(4-formylphenyl)-10, 15, 20-triphenyl porphyrin (FPTPP, short dash), 5-(4-chloromethylphenyl)-10, 15, 20-triphenyl porphyrin (ClMPTPP, dash) and meso-tetraphenyl porphyrin (TPP, solid) in CH2Cl2 solution. For FPTPP: 421nm (Soret), 511, 568, 611 and 652nm (Q). For ClMPTPP: 412 nm (Soret), 512, 545, 587 and 644 nm (Q). For TPP: 413 nm (Soret), 511, 545, 587 and 644 nm (Q).
shapes and positions. But owing to formyl replacement, the spectrum for FPTPP displayed much differently both in shape and positions. The Soret band became more broadly, and all absorption peaks appeared with clear red-shift, excepting Q band at 511 nm, the Q band at 652 nm nearly disappeared. This broadly spectral change can enhance light absorption of porphyrin derivatives, and in assembly of photosensitized molecular devices, the formyl substituted porphyrin should be considered preferentially. The influences of substituent replacement were more effectively reflected in photoluminescence. After replacement of one H atom in TPP by chloromethyl and formyl to form ClMPTPP and FPTPP, the emission spectra for the latter two were quenched in large scale (Figure 2), although this H atom was just at the para position of one peripheral phenyl! In the inner figure, both emission spectra for ClMPTPP and FPTPP were enlarged nearly 100 times. The reason for fluorescence quenching is arranged to be explored.
The compared EPR spectra for 5-(4-chloromethylphenyl)-10, 15, 20-triphenyl porphyrin (ClMPTPP, broad line) and meso-tetraphenyl porphyrin (TPP, thin line) in solid state were showed in Figure 3. Both TPP and ClMPTPP displayed a strong radical signal around 0.35 T orientated from the unpaired π electron which was stabled by conjugated porphyrin ring; zero field splitting in porphyrin molecule resulted the asymmetric EPR signal. After substitution by chloromethyl, the EPR peak was furthermore split by H atoms in methylene (-CH2-Cl), the coupling result between single π electron and protons.
Figure 2. Emission spectra for for 5-(4-formylphenyl)-10, 15, 20-triphenyl porphyrin (FPTPP, λex = 454 nm, short dash), 5-(4-chloromethylphenyl)-10, 15, 20-triphenyl porphyrin (ClMPTPP, λex = 431 nm, dash) and meso-tetraphenyl porphyrin (TPP, λex = 436 nm, solid) in CH2Cl2 solution. Emission peaks: For FPTPP: 685 nm (strong), without the weak peak. For ClMPTPP: 653 (strong) and 716 nm (weak). For TPP: 657 nm (strong) and 717 nm (weak).
Figure 3. The compared EPR spectra in solid state for ClMPTPP (broad line) and TPP (thin line).
Figure 4 showed the cyclic voltammetric curves at 0.05 - 0.5 V/s. The curves clearly indicated that ClMPTPP gave three reductive peak around −1.4 V, −0.75 V and −0.25 V. These redox potentials were associated with the following reactions:
Por+ + e− = Por0 + e− = Por− + e− = Por2−
Figure 4. Cyclic voltammetric curves at different scan speeds for 5-(4-chloromethylphenyl)-10, 15, 20-triphenyl porphyrin in CH2Cl2 with TBAP (0.1 mol/L) vs. Ag+/Ag in MeCN.
4. Conclusion
Two single substituted porphyrins, the asymmetric 5-(4-chloromethylphenyl)-10, 15, 20-triphenyl porphyrin (ClMPTPP) and 5-(4-formylphenyl)-10, 15, 20-triphenyl porphyrin (FPTPP) were successfully prepared from the symmetric meso-tetraphenyl porphyrin (TPP). The resulted asymmetric porphyrins were well characterized by spectral method. Photoluminescence experiment indicated that after chloromethyl and formyl replacement, the emission spectra were quenched in large scale for both asymmetric porphyrins. These asymmetric porphyrins can be selected as intermediates to assemble molecular blocks, such as porphyrin dimmers with bridge group of glycols, Grignard reagents, polyaldehydes, polyketones, and even polyamines. The porphyrin dendrimer can also be derived from these asymmetric porphyrins with bridging molecules possessing multiple side groups. These products would be potential molecule in photoelectric applications.
Acknowledgements
This work was supported by the Natural Science Fund in Guangdong Province (Grant No. 2017A030313071), the Excellent Young Teacher Development Project of Universities in Guangdong Province (Grant No. 261532106) and the Guangzhou Science and Technology Program (Grant No. 502150105).
Cite this paper
Lin, H., Chen, X.T., Wu, Y.H., Tong, S.L., Hu, S., Yu, J. and Yan, Y. (2018) Preparation of Single Substituted Phenyl Porphyrins Form Meso-Tetraphenyl Porphyrin-Synthetic Example from Symmetric Porphyrin into Asymmetric Porphyrins. Open Journal of Inorganic Chemistry, 8, 21-27. https://doi.org/10.4236/ojic.2018.81002
References
- 1. Maier, S., Fendt, L.-A., Zimmerli, L., Glatzel, T., Pfeiffer, O., Diederich, F. and Meyer, E. (2008) Nanoscale Engineering of Molecular Porphyrin Wires on Insulating Surfaces. Small, 4, 1115-1118. https://doi.org/10.1002/smll.200701259
- 2. Vail, S.A., Krawczuk, P.J., Guldi, D.M., Palkar, A., Echegoyen, L., Tom, J.P.C., Fazio, M.A. and Schuster, D.I. (2005) Energy and Electron Transfer in Polyacetylene-Linked Zinc-Porphyrin-Fullerene Molecular Wires. Chemistry—A European Journal, 11, 3375-3388. https://doi.org/10.1002/chem.200401348
- 3. Bakar, M.B., Oelgemller, M. and Senge, M.O. (2009) Lead Structures for Applications in Photodynamic Therapy. Part 2: Synthetic Studies for Photo-Triggered Release Systems of Bioconjugate Porphyrin Photosensitizers. Tetrahedron, 65, 7064-7078. https://doi.org/10.1016/j.tet.2009.06.037
- 4. Liang, X., Li, X., Yue, X. and Dai, Z. (2011) Conjugation of Porphyrin to Nanohybrid Cerasomes for Photodynamic Diagnosis and Therapy of Cancer. Angewandte Chemie International Edition, 50, 11622-11627. https://doi.org/10.1002/anie.201103557
- 5. Son, H.-J., Jin, S., Patwardhan, S., Wezenberg, S.J., Jeong, N.C., So, M., Wilmer C.E., Sarjeant, A.A., Schatz, G.C., Snurr, R.Q., Farha, O.K., Wiederrecht, G.P. and Hupp, J.T. (2013) Light-Harvesting and Ultrafast Energy Migration in Porphyrin-Based Metal-Organic Frameworks. Journal of the American Chemical Society, 135, 862-869. https://doi.org/10.1021/ja310596a
- 6. Zervaki, G.E., Tsaka, V., Vatikioti, A., Georgakaki, I., Nikolaou, V., Sharma, G.D. and Coutsolelos, A.G. (2015) A Triazine Di(Carboxy)Porphyrin Dyad versus a Triazine Di(Carboxy)Porphyrin Triad for Sensitizers in DSSCs. Dalton Transactions, 44, 13550-13564. https://doi.org/10.1039/C5DT01141H
- 7. Jia, X.L. (2006) Synthesis and Property Research of Novel Porphyrin. Master Thesis, Zhejiang University, Hangzhou, 43-47.
- 8. Lindsey, J.S., Schreiman, I.C., Hsu, H.C., Kearney, P.C. and Marguerettaz, A.M. (1987) Rothemund and Adler-Longo Reactions Revisited: Synthesis of Tetraphenylporphyrins under Equilibrium Conditions. The Journal of Organic Chemistry, 52, 827-836. https://doi.org/10.1021/jo00381a022





