Advances in Entomology
Vol.03 No.03(2015), Article ID:57065,7 pages
10.4236/ae.2015.33010
Galls as a Disputed Resource for Female Parasitoid Wasps Contests
Denise Dalbosco Dell’Aglio1, Milton de Souza Mendonça Jr.2
1Department of Zoology, University of Cambridge, Cambridge, UK
2Departamento de Ecologia, Instituto de Biociências, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Brazil
Email: ddd23@cam.ac.uk
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution-NonCommercial International License (CC BY-NC).
http://creativecommons.org/licenses/by-nc/4.0/



Received 2 April 2015; accepted 8 June 2015; published 11 June 2015
ABSTRACT
We investigated how the parasitoid Torymus sp. (Hymenoptera: Torymidae) attacking galls of Schismatodiplosis lantanae (Diptera: Cecidomyiidae) on Lantana camara (Verbenaceae) behaves in the presence of a conspecific female competitor in the patch. Presence of a competitor greatly changed resident exploitation behavior. Wasps alone spent more time in gall exploitation behaviors (walk-antennate and probe) and in post-oviposition behaviors (stationary and groom), and when intruders were present they spent more time walking. The attack strategy was through threatening: raising wings and pointing the antennae towards the opponent. Different from theoretical expectations, residents were not always the attacker. Number of galls in the patch and female wasp size did not affect contest outcomes, although gall exploitation time and time on leaf were significant factors for the probability of having attacks. Overall, the study highlights the fact that intruder interest in the host was the main cause of contests and also this is a unique report both in terms of the target species and the nature of the disputed resource, a gall-inducer inside a singular spatial unit, the gall.
Keywords:
Gall, Parasitoid Wasp, Female Contests, Agonistic Behavior, Patch Defense

1. Introduction
Direct competition occurs when individuals interact while exploiting the same resource. In parasitoid wasps, adult females can compete for hosts against conspecifics engaging in aggressive contests for patch ownership [1] . These contests represent the means by which individuals exclude others from resources [2] . Theoretical studies using game theory and looking for evolutionary stable strategies [3] [4] suggest that there are distinct factors influencing the results of such conflicts. First, there might be differences in competitive abilities of the contestants, known as resource-holding power (RHP) [5] such as body size, strength or fighting ability that could provide a physical benefit for the territory holder. Prior ownership might influence contest outcomes, having other advantages than physiological such as positional effects or familiarity with the local environment [6] . Second, the importance that the individual gives to the resource being defended, known as resource value (RV) [5] [7] such as resource size, number and quality. Thus, competitors with asymmetries in RHP and RV could have aggressiveness and contest outcome predicted, although different results have been found in natural settings [6] [8] [9] .
These theoretical studies on resource competition have been applied to several animal species; however, for insect parasitoids this aspect has not been fully studied. Adult parasitoid females can compete for hosts from which their offspring develop and the consequences of this competition have a direct influence on their fitness [10] . A problem faced by them is after attacking the host, it becomes vulnerable to an attack by another individual of the same or different species of parasitoid, which does not guarantee that the first parasitoid offspring will survive [11] . Conspecific females frequently exploit simultaneously the same host patch [12] . Thus, eggs can be deposited in already parasitized hosts [13] [14] , and females can show a form of protection toward the oviposition site, in a form of maternal care behavior [15] [16] . Therefore, some parasitoid females can compete by defending their hosts, or its location, from conspecific competitors, sometimes engaging in aggressive contests for possession of the patch. These contests may consist of repeated interactions between the same individuals, where female wasps interfere with each other indirectly by modifying their host exploitation strategies [17] [18] .
In animal contests, many researchers reported aggressive behavior from a variety of parasitoid species, which use insect eggs masses, larvae or pupa as oviposition resource. These studies had explored specific aspects of ownership asymmetry on contestant’s aggressiveness and contest outcome [1] [19] - [21] . Galling insects, when hosting parasitic wasps, are also a target for competition between ovipositing females [12] , and agonistic conflicts can arise with single galls or whole plant organs acting as resource patches. So, the aim of the present study was to investigate female behavior and how this can influence contest outcome in a parasitoid wasp that uses galls as oviposition resource.
A solitary parasitoid wasp Torymus sp. (Hymenoptera: Torymidae) of a gall-forming cecidomyiid Schismatodiplosis lantanae (Diptera: Cecidomyiidae) was used as a model. Foraging gall strategies of Torymuscapite wasp were described [22] , but no record of female contests. This is a unique report both in terms of the target species and the nature of the disputed resource, a gall-inducer inside a singular spatial unit, the gall. The purpose was, first, to describe how they behave in the absence and in the presence of a conspecific female competitor in the patch and agonistic interactions displayed during contests, and, second, to test contest theory predictions about the effect of ownership status, number of hosts in patch, galls size, female size and behavior lengths in contests occurrences and outcomes.
2. Material and Methods
2.1. Study System
Torymussp. (Hymenoptera: Torymidae) is a metallic green small wasp with females approximately 2.7 mm (±0.1 mm) long without the ovipositor, and although it was not described yet, wasp identification was provided by Dr. Valmir Antonio Costa (voucher 432, deposited in Entomophagous Insects Oscar Monte Collection, IB- CBE, Brazil).
This solitary parasitoid wasp attacks a gall-forming midge, Schismatodiplosis lantanae Rübsaamen, 1916 (Diptera: Cecidomyiidae). The galler induces green round monothalamous gall on leaves of Lantana camara L. (Verbenaceae) [23] , a common and native shrub in south Brazil. Galls diameter is about 7.4 mm (± 0.3 mm) when fully developed [24] . The wasp uses its long and thin ovipositor to lay one egg inside each gall, reaching the solitary insect chamber in both larvae and pupae state. Oviposition process leaves a small round scar on the gall, which is identifiable under a stereomicroscope. A single parasitoid larva develops per host, consuming it around 15 to 20 days in laboratory conditions.
Lantanacamara shrubs are patchily distributed in forest borders and open spaces, and were collected in trails located in Campus do Vale of the Federal University of Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil (30˚04'S 51˚07'W). The parasitism rate was 43% from 1869 galls collected, which fluctuated during the year (Dell’Aglio D D, unpublished data).
2.2. Experimental Procedures
Galled leaves collected were taken to the laboratory until the adult parasitoid wasp emergence. Emergent wasps were isolated in glass containers with a honey and distilled water solution (1:3) droplet for feeding. Both female wasps used in the experiments were of the same age (emerged on the same day), unmated and had no oviposition experience (parthenogenetic parasitoid wasps). Behavior of 20 pairs of females was observed, each was used only once in the experiment.
Behaviors were observed in laboratory by releasing females in a Petri dish (9 cm) containing one leaf of L. camara with non-parasitized galls as an arena. The wasps were able to walk freely in the arena, leaving and returning to the leaf, but not leaving the Petri dish. Before the experiments, galls on freshly collected leaves were observed under a stereomicroscope and checked for parasitoid wasp oviposition marks to ensure galls were not previously parasitized. The experiments were performed under laboratory ambient temperature (25˚C). Wasp behavior was recorded using a digital camera (JVC camcorder) for later detailed observation. To explore the mechanisms underlying contest resolution, we varied the number of galls on the leaf (1 to 8), size of the galls (2.5 to 12 mm diameter) and size of the female wasps (1.55 to 2.60 mm wing length).
Experiments were divided in three steps. First, behavior of a single wasp on a galled leaf was observed for 30 min, in order to reveal the standard behavioral responses. Second, after 24 h of foraging, the first wasp is considered resident; a second wasp was then introduced and both observed for 60 min. Third, the resident wasp was removed, leaving the intruder alone with the gall parasitized but in the absence of a resident, in order to observe the behavior of the intruder for 30 min. Recordings started at the moment wasps were released in the arena. The use of a Petri dish as an arena apparently did not restricted any behavior. After the experiments, females had the first pair of wings measured since Torymus sp. raises their wings in conflicts (Table 1).
2.3. Data Analysis
The digital videos were analyzed using event-recording open software CowLog and its R-package [25] for the continuous time records of each behavioral category. Behavioral categories were composed of more than one behavioral element, not distinguished for practical observations. In probe behavior, direct observation of egg deposition was impossible because the gall is concealed. Behavior categories were further grouped in two different groups: gall exploitation (walk-antennate and probe) and patch exploitation (walking, stationary and groom) (Table 1). The leaf was considered the “patch” for the wasps. The threat and retreat behaviors were reported by frequency since they have very short durations (Table 1). All analyses were carried out in the statistical software R [26] . Nonparametric tests (Mann-Whitney and Wilcoxon) were used to analyze the behavioral categories comparing duration times between wasp alone, resident, intruder and intruder alone behaviors.
We also analyzed the effect of behavior duration, female size, gall size and number on the probability of a contest occurrence. The explanatory variables were differences in wings size (length in mm) between contestants,
Table 1. Behavioral categories and definitions of Torymus sp. (Hymenoptera: Torymidae), based on Field (1998).
galls size (mm), number of galls per leaf and time spent in gall and patch exploitation (min). Logistic regression analyses were employed, as they are commonly used to test influences of factors and duration in behaviors on contests outcomes [27] . Two tailed t-test was used to compare significant differences in wings size in terms of attack and chi-square test to relate patch ownership and occurrence of threat behavior to previous occurrence of probe behavior.
3. Results
3.1. Behavioral Categories
Detailed behavioral categories were established for the particular species used in this study (Table 1). Although sequences and respective durations of observed behaviors vary among individuals, general behavior patterns are similar (Table 2). The stationary behavior normally occurs subsequent to gall exploitation, when females usually walk away from the host and rest, and by this time, all the hosts were usually already probed. Wasps may remain motionless for extensive periods of time, moving their antennae and grooming (Table 2).
Resident and intruder wasps did not differ significantly in behavior durations; however, wasps alone differed from wasps with conspecifics in the patch (Table 3). Wasps alone spent significantly more time in stationary, groom, walk-antennate and probe behaviors than when with the intruder (Table 3). They also remained on or near the host throughout the experiment, with short periods of absence, thus they spent more time in gall exploitation behaviors and on the leaf. Most of the behavioral patterns of the intruder with the resident did not differ from the intruder alone, however stationary and groom behaviors differ significantly (Table 3).
3.2. Resident-Intruder Contests
The agonistic behavior exhibited between the females was threats and retreats, which consisted of an individual attacking and other retreating without retaliation. The attacker raised its wings and pointed its antennae in the direction of the opponent. Since the opponent suffered no apparent injuries this can be classified as “non- aggressive” attack or “ritualized” attack, with extreme close proximity and not resulting in fights or chases. The retreater wasp temporarily flees away from the patch and begins the stationary behavior, then later returns and attempts to continue searching. Meanwhile, as there were no retaliations, the attacker could remain on the gall, walking and/or probing and was considered the winner. Residents did not attack more than intruders: 11 threat events were by residents and 9 by intruders (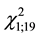 = 0.2, p = 0.65). The attack frequency was from 1 to 3 per observation and it might have been influenced by the possibility of returning to the patch after flying away.
= 0.2, p = 0.65). The attack frequency was from 1 to 3 per observation and it might have been influenced by the possibility of returning to the patch after flying away.
Table 2. Percentage of time spent in each behavior for wasps released alone in the patch, with two conspecifics in the patch (resident and intruder) and for intruder alone. Behaviors are presented individually, grouped in patch and gall exploitation categories and in terms of wasp position relative to the patch (on or off the leaf).
Table 3. Analysis results comparing the duration times of wasp alone, resident, intruder and intruder alone behaviors. Behaviors are presented individually, grouped in patch and gall exploitation categories and in terms of wasp position relative to the patch (on or off the leaf). Boldface values significant at p < 0.05.
Most of the time, both females tolerated one another’s presence and continued exploiting the patch. Some wasps left the patch without probing: 7/20 residents and 10/20 intruder wasps. Torymus sp. wasps emerged from all probed galls after the experiment, showing that those galls were in condition to be parasitized. Probability of a contest occurrence was not determined by differences in size between residents and intruders (G1 = 2.12, N = 20, p = 0.16), size of the galls (G1 = 1.53, N = 60, p = 0.21) and number of galls in the leaf (G1 = 0.11, N = 20, p = 0.75). Differences in body size between the attacker and retreater wasp were also not significant (t = 1.27, d.f. = 14.4, p = 0.22), or any interaction between the variables.
Probability of a contest occurrence showed a positive relationship with time spent by the attacking female in gall exploitation especially for intruders (G1 = 4.65, N = 40, p = 0.04, Figure 1) and time on the leaf (G1 = 8.25, N = 40, p = 0.01). Threat occurrence due the presence of probe behavior was statistically significant for the intruder (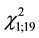 = 13.3, p < 0.001) but not for the resident (
= 13.3, p < 0.001) but not for the resident (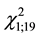 = 0.20, p = 0.65).
= 0.20, p = 0.65).
4. Discussion
In this study, we reported for the first time Torymus sp. females displaying agonistic behaviors towards conspecific competitors. The experiments allowed us to identify differences in behavior duration when females co- occur with conspecifics in a resource patch. Behavioral categories found for this particular species were similar to those reported by previous authors that described aspects of host and patch exploitation behaviors for different parasitoid wasp species [28] [29] and agonistic behaviors in other parasitoid wasp conflicts [1] [18] [21] .
Presence of a competitor changed both resident and intruder exploitation behaviors. Residents alone spend more time in gall exploitation and, after detecting conspecific females, they clearly changed their behavior sequences spending less time in the host. Probably females are avoiding losing time in an explored patch and investing time searching for a better patch. Same patterns in gall exploitation behavior were observed in another Torymus wasp, which fails to parasitize all hosts in the patch [22] . The possible explanations for this was not due to limited egg supply, mutual interference or patch depletion, but an apparently “fixed-time” foraging strategy by the wasp. Tolerance of intruders by residents reinforces the potential resource denial of an oviposited host and also the avoidance of contests by leaving the patch early [18] . It also could be explained if the resident’s first egg in the gall has an advantage in superparasitism, in which females successfully protected their host against intruders during host exploitation behavior and not after oviposition [16] . Tolerating some intrusion while exploiting a patch might be relevant in the field, where it is possible that more than a single intruder may arrive on a patch [29] .
Agonistic behaviors were linked to gall exploitation behaviors, which highlight the trade-off wasps experience
Figure 1. Predicted probability of conflicts occurring (1) or not occurring (0) in relation to gall exploitation behavior duration (min.) (p = 0.04, fitted curve: p = 0.02, R2 = 0.12). Resident wasps: open squares. Intruder wasps: filled squares.
between continuing to explore the patch and excluding their opponent from the patch [19] . Residents tend to take care of the gall and threaten the intruder only when the intruder appears to positively evaluate the gall by trying to probe it. However, Torymus female wasps did not show complete respect for ownership, as it would be expected from previous work [19] [30] . Intruders were not totally blocked from probing galls by residents and were able to initiate conflicts as well. The temporary absence from the patch and the subsequent return to continue searching by the retreater wasps has been described as a “waiting game” [29] . This behavior leads to a decision making on how often to return to the patch and check whether it was unoccupied and suitable for oviposition.
In general, residents are expected to have a better estimate of RV than intruders, which would lead to patch defense and would affect female aggressiveness [6] [9] [31] [32] . However, number and size of galls did not affect contests occurrences and did not appear to be an important factor for residents. The type of resource used did not enable us to distinguish RV asymmetry in terms quality and development stage. The host is enclosed inside the gall making it difficult to measure and galls would have to be damaged to observe the insect inside.
Asymmetries in wing size did not confer a better contest ability. Other studies on parasitoid wasp contests, female size influenced the conflicts outcome for host resources, since being larger was an important feature and conferred a competitive advantage [1] . Although there are several differences in resident behavior, contest occurrence was elicited only by intruder interest in the host, and not just patch invasion. Apparently the intruder strategy was similar to Eupelmus vuilleti (Craw) (Hymenoptera: Eupelmidae), in which intruders perceived the stage reached by the residents in their oviposition sequence and adapted their agonistic behavior accordingly, fighting or waiting [33] .
Although the best strategy for short-lived insects may be not wasting time in conflicts but searching for more resources, the evolution of such agonistic behavior can be justified because levels of competition and superparasitism in the field can be high [13] [14] [34] . There are more species of parasitoids wasps that use S. lantanae as host (Dell’Aglio D D, unpublished data) and the competition shown here may be important for the dynamics of host-parasitoid communities. Thus, other parasitoid wasp species that share the same host might have different competitive strategies to allow their coexistence [35] . Also, in order to protect their offspring from other parasitoids, these species might have developed other forms of defense such as larval competition inside the gall [36] .
The current theories on contest resolution may not be applicable when finding other patches is not a limiting factor or female-female contests are costly in time and energy [18] . Further research in this system, employing more complex arenas, comparing different parasitoid species attacking the same galling host and outcomes of superparasitism, can help to refine our knowledge of the species involved. Wasp behavior responses and the fact that a gall holds a host inside but is a motionless, plant-based structure, occurring on a plant organ, are important to further understanding of parasitoid wasp behavioral ecology and its evolution.
Acknowledgements
We thank all anonymous referees, Marlène Goubault, Paula Beatriz de Araujo, Geraldo LuizGonçalvesSoares and Luiz Ernesto Costa Schmidt for suggestions and comments. Simone MundstockJahnke and Valmir Antonio Costa for helping in wasp identification. This study was supported by CAPES.
References
- Petersen, G. and Hardy, I.C.W. (1996) The Importance of Being Larger: Parasitoid Intruder-Owner Contests and Their Implications for Clutch Size. Animal Behaviour, 51, 1363-1373. http://dx.doi.org/10.1006/anbe.1996.0139
- Riechert, S.E. (1998) Game Theory and Animal Contests. In: Game Theory and Animal Behavior, Oxford University Press, Oxford, 64-93.
- Maynard-Smith, J. (1982) Evolution and the Theory of Games. Cambridge University Press, Cambridge. http://dx.doi.org/10.1017/CBO9780511806292
- Hammerstein, P. (1998) What Is Evolutionary Game Theory? In: Game Theory and Animal Behavior, Oxford University Press, Oxford, 3-15.
- Maynard-Smith, J. and Parker, G.A. (1976) The Logic of Asymmetric Contests. Animal Behaviour, 24, 159-175. http://dx.doi.org/10.1016/S0003-3472(76)80110-8
- Hardy, I.C.W., Goubault, M. and Batchelor, T.P. (2013) Hymenopteran Contests and Agonistic Behaviour. In: Animal Contests, Cambridge University Press, Cambridge, 147-177. http://dx.doi.org/10.1017/cbo9781139051248.010
- Hammerstein, P. (1981) The Role of Asymmetries in Animal Contests. Animal Behaviour, 29, 193-205. http://dx.doi.org/10.1016/S0003-3472(81)80166-2
- Alcock, J. (2009) Animal Behavior: An Evolutionary Approach. Sinauer Associates Publishers, Sunderland, MA.
- Hurd, P.L. (2006) Resource Holding Potential, Subjective Resource Value, and Game Theoretical Models of Aggressiveness Signalling. Journal of Theoretical Biology, 241, 639-648. http://dx.doi.org/10.1016/j.jtbi.2006.01.001
- Van Baalen, M. and Hemerik, L. (2008) Parasitoid Fitness: From a Simple Idea to an Intricate Concept. In: Behavioral Ecology of Insect Parasitoids: From Theoretical Approaches to Field Applications, Blackwell Publishing Ltd., Oxford, UK, 31-50. http://dx.doi.org/10.1002/9780470696200.ch2
- Van Alphen, J.J.M., Bernstein, C. and Driessen, G. (2003) Information Acquisition and Time Allocation in Insect Para- sitoids. Evolution, 18, 81-87.
- Godfray, H.C.J. (1994) Parasitoids: Behavioral and Evolutionary Ecology. Princeton University Press Books, Princeton.
- Van Alphen, J.J.M. and Visser, M.E. (1990) Superparasitism as an Adaptive Strategy for Insect Parasitoids. Annual Re- view of Entomology, 35, 59-79. http://dx.doi.org/10.1146/annurev.en.35.010190.000423
- Field, S.A., Keller, M.A. and Calbert, G. (1997) The Pay-Off from Superparasitism in the Egg Parasitoid Trissolcus basalis, in Relation to Patch Defence. Ecological Entomology, 22, 142-149. http://dx.doi.org/10.1046/j.1365-2311.1997.t01-1-00057.x
- Hardy, I.C.W. and Blackburn, T.M. (1991) Brood Guarding in a Bethylid Wasp. Ecological Entomology, 16, 55-62. http://dx.doi.org/10.1111/j.1365-2311.1991.tb00192.x
- Goubault, M., Scott, D. and Hardy, I.C.W. (2007) The Importance of Offspring Value: Maternal Defence in Parasitoid Contests. Animal behaviour, 74, 437-446. http://dx.doi.org/10.1016/j.anbehav.2006.11.029
- Visser, M.E., Van Alphen, J.J.M. and Hemerik, L. (1992) Adaptive Superparasitism and Patch Time Allocation in So- litary Parasitoids: An ESS Model. Journal of Animal Ecology, 61, 93-101. http://dx.doi.org/10.2307/5512
- Goubault, M., Outreman, Y., Poinsot, D. and Cortesero, A.M. (2005) Patch Exploitation Strategies of Parasitic Wasps under Intraspecific Competition. Behavioral Ecology, 16, 693-701. http://dx.doi.org/10.1093/beheco/ari043
- Field, S.A. and Calbert, G. (1999) Don’t Count Your Eggs before They’re Parasitized : Contest Resolution and the Tra- de-Offs during Patch Defense in a Parasitoid Wasp. Behavioral Ecology, 10, 122-127. http://dx.doi.org/10.1093/beheco/10.2.122
- Humphries, E.L., Hebblethwaite, A.J., Batchelor, T.P. and Hardy, I.C.W. (2006) The Importance of Valuing Resources: Host Weight and Contender Age as Determinants of Parasitoid Wasp Contest Outcomes. Animal behaviour, 72, 891- 898. http://dx.doi.org/10.1016/j.anbehav.2006.02.015
- Stokkebo, S. and Hardy, I.C.W. (2000) The Importance of Being Gravid: Egg Load and Contest Outcome in a Parasitoid Wasp. Animal behaviour, 59, 1111-1118. http://dx.doi.org/10.1006/anbe.2000.1407
- Weis, A.E. (1983) Patterns of Parasitism by Torymus capite on Hosts Distributed in Small Patches. Journal of Animal Ecology, 52, 867-877. http://dx.doi.org/10.2307/4460
- Gagné, R.J. (1994) The Gall Midges of the Neotropical Region. Cornell University Press, Ithaca.
- Moura, M.Z.D., Soares, G.L.G. and dos Santos Isaias, R.M. (2008) Species-Specific Changes in Tissue Morphogenesis Induced by Two Arthropod Leaf Gallers in Lantana camara L. (Verbenaceae). Australian Journal of Botany, 56, 153- 160. http://dx.doi.org/10.1071/BT07131
- Hänninen, L. and Pastell, M. (2009) CowLog: Open Source Software for Coding Behaviors from Digital Video. Beha- vior Research Methods, 41, 472-476. http://dx.doi.org/10.3758/BRM.41.2.472
- R Core Team (2013) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
- Hardy, I.C.W. and Field, S.A. (1998) Logistic Analysis of Animal Contests. Animal Behaviour, 58, 787-792. http://dx.doi.org/10.1006/anbe.1998.0833
- Field, S.A. (1998) Patch Exploitation, Patch-Leaving and Pre-Emptive Patch Defence in the Parasitoid Wasp Trissolcus basalis (Insecta: Scelionidae). Ethology, 104, 323-338. http://dx.doi.org/10.1111/j.1439-0310.1998.tb00072.x
- Field, S.A., Calbert, G. and Keller, M.A. (1998) Patch Defence in the Parasitoid Wasp Trissolcus basalis (Insecta: Sce- lionidae): The Time Structure of Pairwise Contests, and the “Waiting Game”. Ethology, 104, 821-840. http://dx.doi.org/10.1111/j.1439-0310.1998.tb00034.x
- Bentley, T., Hull, T.T., Hardy, I.C.W. and Goubault, M. (2009) The Elusive Paradox: Owner-Intruder Roles, Strategies, and Outcomes in Parasitoid Contests. Behavioral Ecology, 20, 296-304. http://dx.doi.org/10.1093/beheco/arp007
- Leimar, O. and Enquist, M. (1984) Effects of Asymmetries in Owner-Intruder Conflicts. Journal of theoretical Biology, 111, 475-491. http://dx.doi.org/10.1016/S0022-5193(84)80235-0
- Mohamad, R., Monge, J. and Goubault, M. (2010) Can Subjective Resource Value Affect Aggressiveness and Contest Outcome in Parasitoid Wasps? Animal Behaviour, 80, 629-636. http://dx.doi.org/10.1016/j.anbehav.2010.06.022
- Mohamad, R., Monge, J.-P. and Goubault, M. (2012) Wait or Fight? Ownership Asymmetry Affects Contest Behaviors in a Parasitoid Wasp. Behavioral Ecology, 23, 1330-1337. http://dx.doi.org/10.1093/beheco/ars125
- Plantegenest, M., Outreman, Y., Goubault, M. and Wajnberg, E. (2004) Parasitoids Flip a Coin before Deciding to Superparasitize. Journal of Animal Ecology, 73, 802-806. http://dx.doi.org/10.1111/j.0021-8790.2004.00844.x
- Mohamad, R., Monge, J. and Goubault, M. (2011) Agonistic Interactions and Their Implications for Parasitoid Species Coexistence. Behavioral Ecology, 22, 1114-1122. http://dx.doi.org/10.1093/beheco/arr098
- Le Lann, C., Outreman, Y., van Alphen, J.J.M., Krespi, L., Pierre, J. and van Baaren, J. (2008) Do Past Experience and Competitive Ability Influence Foraging Strategies of Parasitoids under Interspecific Competition? Ecological Entomology, 33, 691-700. http://dx.doi.org/10.1111/j.1365-2311.2008.01017.x



