Open Journal of Physical Chemistry
Vol.4 No.1(2014), Article ID:42599,9 pages DOI:10.4236/ojpc.2014.41002
DFT Calculations for Corrosion Inhibition of Ferrous Alloys by Pyrazolopyrimidine Derivatives
1Chemistry Department, Faculty of Science, King Abdul-Aziz University, Jeddah, KSA
2Institute of Graduate Studies and Research, University of Alexandria, Alexandria, Egypt
Email: *nwazzan@kau.edu.sa
Received December 25, 2013; revised January 18, 2014; accepted January 25, 2014
ABSTRACT
The inhibition performance of 5-tolyl-2-phenylpyrazolo[1,5-c] pyrimidine-7(6H)thione (Tolyl), 5-tolyl-2-pheenylpyrazolo [1,5-c]pyrimidine-7(6H)one (Inon) was investigated as corrosion inhibitors using density functional theory (DFT) at the B3LYP/6-31 + G(d,p) level of theory. The calculated quantum chemical parameters correlated to the inhibition efficiency are: the highest occupied molecular orbital energy (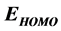 ), the lowest unoccupied molecular orbital energy (
), the lowest unoccupied molecular orbital energy (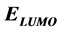 ), the energy gap (
), the energy gap (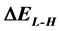 ), dipole moment (
), dipole moment (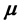 ), ionization energy (
), ionization energy (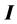 ), electron affinity
), electron affinity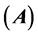 , absolute electronegativity (
, absolute electronegativity (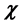 ), absolute hardness (
), absolute hardness (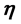 ), absolute softness (
), absolute softness (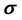 ), the fraction of electron transferred (
), the fraction of electron transferred (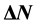 ), and the total energy (
), and the total energy (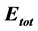 ) which were calculated. The local reactivity has been analyzed through the Fukui function and local softness indices in order to compare the possible sites for nucleophilic and electrophilic attacks. The success of DFT calculations in predicting the inhibition efficiency was assessed.
) which were calculated. The local reactivity has been analyzed through the Fukui function and local softness indices in order to compare the possible sites for nucleophilic and electrophilic attacks. The success of DFT calculations in predicting the inhibition efficiency was assessed.
Keywords:Pyrazolopyrimidine Derivative; Corrosion Inhibitors; Adsorption; DFT Calculations; Fukui Function
1. Introduction
Corrosion of metals is a major industrial problem that has attracted many investigation and researches. The use of inhibitors is one of the most practical methods to protect metals against corrosion. Most efficient inhibitors are organic compounds containing electronegative functional groups and π-electrons in triple or conjugated double bonds. Researchers conclude that the adsorption on the metal surface depends mainly on the physicochemical properties of the inhibitor group, such as the functional group, molecular electronic structure, electronic density at the donor atom, π-orbital character and the molecular size [1-]">3]. A number of heterocyclic compounds containing nitrogen, oxygen, and sulphur either in the aromatic or long chain carbon system have been reported to be effective inhibitors [4,]">5] . The inhibition efficiency has been closely related to the inhibitor adsorption abilities and the molecular properties for different kinds of organic compounds [6,]">7] . Organic compounds, which can donate electrons to unoccupied d-orbital of metal surface to form coordinate covalent bonds and can also accept free electrons from the metal surface by using their anti-bonding orbital to form feedback bonds, constitute excellent corrosion inhibitors [8,]">9].
A lot of work on heterocyclic inhibitors has been studied experimentally [10-]"> 14]. On the other hand, many reported theoretical studies in order to correlate between structural and electronic parameters and the inhibition efficiency [6,8,15-1]">9 ]. The results from these researches have been used to interpret very well many experimental phenomena.
Density functional theory (DFT) [20,]">21] following the approach of Kohn and Sham has proven to be an important tool in modern quantum chemistry because of its ability to include some effects of electron correlation at a greatly reduced computational cost [22-]"> 24]. Recently, DFT methods have been used to analyze the characteristics of the inhibitor/surface mechanism and to describe the structural nature of the inhibitor in corrosion process [6,25-]">28].
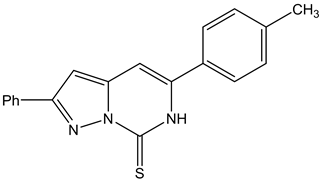
The object of the present paper is to carry out a DFT calculations on the electronic parameters of two pyrazolo[1,5-c]pyrimidine derivatives and they are:
5-tolyl-2-phenylpyrazolo[1,5-c]pyrimidine-7(6H)thione (Tolyl)
5-tolyl-2-phenylpyrazolo[1,5-c]pyrimidine-7(6H)one (Inon)
used as inhibitors, and to determine a relationship between some quantum chemical parameters obtained from the structure of the compounds and the inhibition efficiencies of corrosion of ferrous alloys obtained experimentally by Mahgoub et al. [12]. The chemical structures of the studied inhibitors are shown in Figure 1.
Computational calculations were obtained by means of DFT/B3LYP/6-31 + G(d,p) level of theory. Parameters such as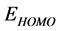 ,
, 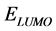 , energy gap (
, energy gap (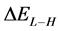 ), dipole moment (
), dipole moment (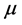 ), ionization energy (
), ionization energy (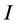 ), electron affinity
), electron affinity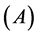 , absolute electronegativity (
, absolute electronegativity (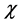 ), absolute hardness (
), absolute hardness (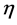 ), absolute softness (
), absolute softness (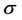 ), the fraction of electron transferred (
), the fraction of electron transferred (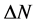 ), and the total energy (
), and the total energy (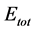 ) were calculated. The local reactivity has been analyzed by means of the Fukui indices, since they indicate the reactive region, in the form of the nucleophilic and electrophilic behavior of each atom in the molecule.
) were calculated. The local reactivity has been analyzed by means of the Fukui indices, since they indicate the reactive region, in the form of the nucleophilic and electrophilic behavior of each atom in the molecule.
2. DFT Calculations
The present calculations were performed using Gaussian
 (a)
(a)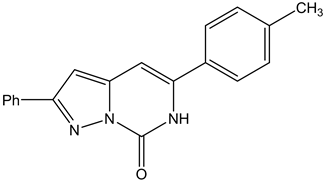 (b)
(b)
Figure 1. The molecular structures of the investigated inhibitors: (a) 5-tolyl-2-phenylpyrazolo [1,5-c] pyrimidine- 7(6H) thione (Tolyl), (b) 5-tolyl-2-phenylpyrazolo [1,5-c] pyrimidine-7(6H)one (Inon).
09 program package [29]. Geometry optimizations were conducted by DFT using Becke’s three parameter exchange functional (B3) [30], and includes a mixture of HF with DFT exchange terms associated with the gradient corrected correlation functional of Lee, Yang, and Parr (LYP) [31] and the 6-31 + G(d,p) basis set.
In geometry optimizations every bond length, bond angle and dihedral angle was allowed to relax, free of constraints. The nature of the stationary points was confirmed by vibrational frequency analysis, to verify that only real frequencies values (i.e. no imaginary frequency) were obtained for all geometries. In order to calculate the Fukui functions, the electron populations for neutral Tolyl, Tolyl cation, Tolyl anion, neutral Inon, Inon cation, and Inon anion species were calculated (using: the keyword Pop = Full). For the ionic forms, the calculations were performed at the optimized geometries of the neutral forms (using: single point energy calculation; charge = ±1; and multiplicity = Doublet). This avoids the problem of trying to do a geometry optimization on a species that may not be a stationary point on the potential energy surface.
Frontier molecular orbitals; highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) was used to predict the adsorption centers of the inhibitor molecule. For the simplest transfer of electrons, adsorption should occur at the part of the molecule where the softness, 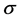 , which is a local property, has the highest value. According to Koopman’s theorem [32], the energies of the HOMO and the LUMO orbitals of the inhibitor molecule are related to the ionization potential,
, which is a local property, has the highest value. According to Koopman’s theorem [32], the energies of the HOMO and the LUMO orbitals of the inhibitor molecule are related to the ionization potential, 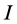 , and the electron affinity,
, and the electron affinity, 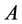 , respectively, by the following relationships:
, respectively, by the following relationships:
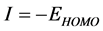 (1)
(1)
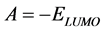 (2)
(2)
Absolute electronegativity, c, and absolute hardness, h, of the inhibitor molecule are given [33]:
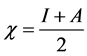 (3)
(3)
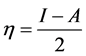 (4)
(4)
The softness is the inverse of the hardness [33]:
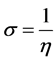 (5)
(5)
Electronegativity, hardness, and softness have proved to be very useful quantities in chemical reactivity theory. When two systems, Fe and inhibitor, are brought together, electrons will flow from lower 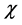 (inhibitor) to higher
(inhibitor) to higher 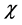 (Fe), until the chemical potentials become equal.
(Fe), until the chemical potentials become equal.
The number of transferred electrons (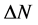 ) was also calculated by using the equation below [32]:
) was also calculated by using the equation below [32]:
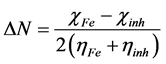 (6)
(6)
where 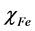 and
and 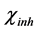 denote the absolute electronegativity of iron and inhibitor molecule, respectively,
denote the absolute electronegativity of iron and inhibitor molecule, respectively, 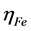 and
and 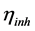 denote the absolute hardness of iron and the inhibitor molecule, respectively.
denote the absolute hardness of iron and the inhibitor molecule, respectively.
In this study, we use the theoretical value of 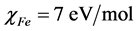 and
and 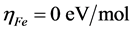 for the computation of number of transferred electrons [32]. The absolute electrophilicity index is given by [34]:
for the computation of number of transferred electrons [32]. The absolute electrophilicity index is given by [34]:
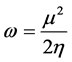 (7)
(7)
According to the definition, this index measures the tendency of chemical species to accept electrons. More reactive nucleophile is characterized by lower value of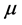 , and
, and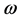 ; and conversely more reactive electrophile is characterized by a higher value of
; and conversely more reactive electrophile is characterized by a higher value of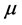 , and
, and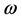 . This new reactivity index measures the stabilization in energy when the system acquires an additional electronic charge
. This new reactivity index measures the stabilization in energy when the system acquires an additional electronic charge 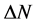 from the environment.
from the environment.
3. Results and Discussion
3.1. Experimental Findings [12]
F. M. Mahgoub et al. investigated experimentally the inhibition of corrosion of ferrous alloys using a group of pyrazolo [1,5-c] pyrimidine as corrosion inhibitors. Using electrochemical measurements and corrosion tests such as Tafel extrapolation and linear polarization resistance techniques they indicated clearly a decrease in the corrosion rate in the presence of Tolyl and Inon inhibitors. The inhibition efficiencies of the Tolyl compound are markedly higher than that of the Inon compound (Table 1).
3.2. Theoretical Findings
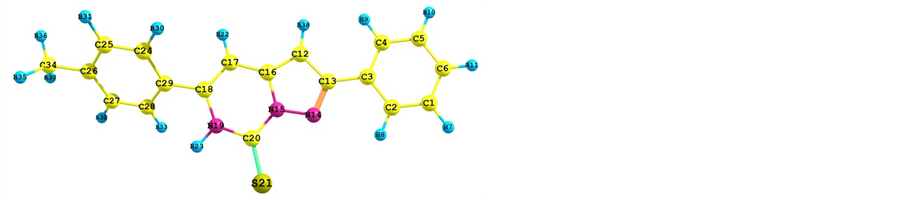
Figure 2 shows the optimized structures of Tolyl and Inon inhibitors along with atomic numbering. For Tolyl and Inon molecules, the computed quantum chemical properties such as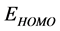 ,
, 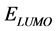 ,
, 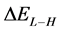 , and dipole moment (
, and dipole moment (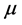 ) are given in Table2
) are given in Table2
Frontier molecular orbital (FMO): The interaction between the inhibitor and the metal is through the donation of the electrons from the inhibitor occupied orbitals (mainly from the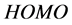 ) to the d-orbital of the metal [27], and also through the acceptance of the electrons from the d-orbital of the metal to the unoccupied orbitals (mainly to the
) to the d-orbital of the metal [27], and also through the acceptance of the electrons from the d-orbital of the metal to the unoccupied orbitals (mainly to the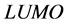 ) of the inhibitor. Thus,
) of the inhibitor. Thus, 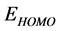 measures the tendency of donating electron by a molecule [35]. Therefore, higher value of
measures the tendency of donating electron by a molecule [35]. Therefore, higher value of 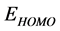 indicates better tendency of donating electron, and enhancing the
indicates better tendency of donating electron, and enhancing the
Table 1 . Corrosion rate and the protection efficiency (%P) of 0.01 M of pyrazolo [1,5-c] pyrimidine inhibitors from Tafel and linear polarization plots for carbon steel in stagnant cooling water (200 ppm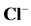 ) at 25˚C [12].
) at 25˚C [12].
 (a)
(a) (b)
(b)
Figure 2. Optimized geometries of (a) Tolyl and (b) Inon molecules along with atomic numbering calculated at B3LYP/6-31 + G(d, p) level of theory.
Table 2. Calculated quantum chemical parameters of Tolyl and Inon molecules calculated at B3LYP/6-31 + G(d,p) level of theory.
adsorption of the inhibitor on the metal surface and therefore better inhibition efficiency. On the other hand,  indicates the ability of the molecule to accept electrons, therefore, lower value of
indicates the ability of the molecule to accept electrons, therefore, lower value of 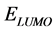 indicates better ability to accept electrons, and also this will enhance the adsorption of the inhibitor on the metal surface and therefore better inhibition efficiency. The binding ability of the inhibitor to the metal surface increases with increasing of the
indicates better ability to accept electrons, and also this will enhance the adsorption of the inhibitor on the metal surface and therefore better inhibition efficiency. The binding ability of the inhibitor to the metal surface increases with increasing of the 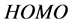 and decreasing of the
and decreasing of the 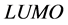 energy values. Frontier molecular orbital diagrams of Tolyl and Inon is shown in Figure 3. From Table 2, the highest value of
energy values. Frontier molecular orbital diagrams of Tolyl and Inon is shown in Figure 3. From Table 2, the highest value of 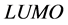 −0.21463 eV of
−0.21463 eV of
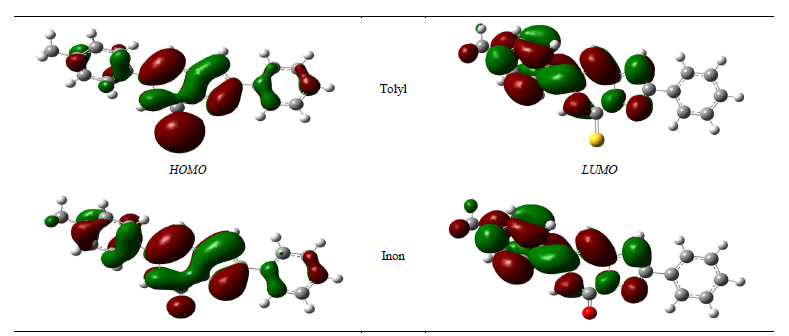
Figure 3. Frontier molecular orbitals (FMO) of Tolyl and Inon molecules calculated at B3LYP/6-31 + G(d,p) level of theory.
Tolyl compared to that of Inon −0.21943 eV, also the lowest value of  −0.06821 eV of Tolyl compared to that of Inon −0.06535eV indicates the better inhibition efficiency of Tolyl, a result which is in total a agreement with the experimental finding.
−0.06821 eV of Tolyl compared to that of Inon −0.06535eV indicates the better inhibition efficiency of Tolyl, a result which is in total a agreement with the experimental finding.
The energy gap ( ) is an important parameter as a function of reactivity of the inhibitor molecule towards the adsorption on the metal surface. As
) is an important parameter as a function of reactivity of the inhibitor molecule towards the adsorption on the metal surface. As  decreases the reactivity of the molecule increases leading to better inhibition efficiency [28]. Table 2 shows that Tolyl inhibitor has the lowest energy gap 0.14642 eV compared to of Inon inhibitor 0.15408 eV, with a difference equals 0.0077 eV; this means that Tolyl molecule could have better performance as carrion inhibitor than Inon molecule, a conclusion which is in total agreement with the experimental finding.
decreases the reactivity of the molecule increases leading to better inhibition efficiency [28]. Table 2 shows that Tolyl inhibitor has the lowest energy gap 0.14642 eV compared to of Inon inhibitor 0.15408 eV, with a difference equals 0.0077 eV; this means that Tolyl molecule could have better performance as carrion inhibitor than Inon molecule, a conclusion which is in total agreement with the experimental finding.
Dipole moment: The dipole moment (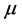 ) is another important electronic parameter that result from non-uniform distribution of charges on the various atoms in the molecule. The high value of dipole moment probably increases the adsorption between the inhibitor and the metal surface [36]. The energy of the deformability increases with the increase in
) is another important electronic parameter that result from non-uniform distribution of charges on the various atoms in the molecule. The high value of dipole moment probably increases the adsorption between the inhibitor and the metal surface [36]. The energy of the deformability increases with the increase in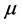 , making the molecule easier to adsorb at the ferrous surface. In addition, the volume of the inhibitor molecules also increases with the increase of
, making the molecule easier to adsorb at the ferrous surface. In addition, the volume of the inhibitor molecules also increases with the increase of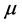 , this increase the contact area between the molecule and the surface of iron and increasing the corrosion inhibition ability of the inhibitor. In our study, the value 7.2231 Debye of Tolyl indicates its better inhibition efficiency compared to 6.8992 Debye of Inon which totally agrees with the experimental findings (Table 2).
, this increase the contact area between the molecule and the surface of iron and increasing the corrosion inhibition ability of the inhibitor. In our study, the value 7.2231 Debye of Tolyl indicates its better inhibition efficiency compared to 6.8992 Debye of Inon which totally agrees with the experimental findings (Table 2).
Other computed quantum chemical properties such as ionization energy (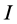 ), electron affinity
), electron affinity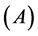 , absolute electronegativity (
, absolute electronegativity (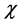 ), absolute hardness (
), absolute hardness (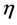 ), absolute softness (
), absolute softness (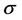 ), absolute electrophilicity (
), absolute electrophilicity (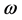 ), the fraction of electron transferred (
), the fraction of electron transferred (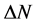 ), and the total energy (
), and the total energy (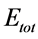 ) for Tolyl and Inon inhibitors are given in Table3
) for Tolyl and Inon inhibitors are given in Table3
Ionization energy: Ionization energy (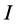 ) is a fundamental descriptor of the chemical reactivity of a toms and molecules. High ionization energy indicates high stability and chemical inertness and vice versa [37]. The low ionization energy 0.21463 eV of Tolyl indicates its high inhibition efficiency compared to 0.21943 eV of Inon which totally agrees with the experimental findings (Table 3).
) is a fundamental descriptor of the chemical reactivity of a toms and molecules. High ionization energy indicates high stability and chemical inertness and vice versa [37]. The low ionization energy 0.21463 eV of Tolyl indicates its high inhibition efficiency compared to 0.21943 eV of Inon which totally agrees with the experimental findings (Table 3).
Electronegativity: Table 3 shows the order of electronegativity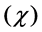 as Tolyl < Inon. Hence, an increase in the difference of electronegativity between the metal and the inhibitor is observed in the order Tolyl > Inon. According to Sanderson’s electronegativity equalization principal [38], Inon with a high electronegativity and low difference of electronegativity quickly reaches equalization and hence low reactivity is expected which in turn indicates low inhibition efficiency, also conclusion in total agreement with the experimental finding.
as Tolyl < Inon. Hence, an increase in the difference of electronegativity between the metal and the inhibitor is observed in the order Tolyl > Inon. According to Sanderson’s electronegativity equalization principal [38], Inon with a high electronegativity and low difference of electronegativity quickly reaches equalization and hence low reactivity is expected which in turn indicates low inhibition efficiency, also conclusion in total agreement with the experimental finding.
Hardness and softness: Absolute hardness 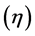 and softness
and softness 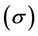 are important properties to measure the molecular stability and reactivity. It is apparent that the chemical hardness fundamentally signifies the resistance towards the deformation or polarization of the electron cloud of the atoms, ions, or molecules under small perturbation of chemical reaction. A hard molecule has a large energy gap (large
are important properties to measure the molecular stability and reactivity. It is apparent that the chemical hardness fundamentally signifies the resistance towards the deformation or polarization of the electron cloud of the atoms, ions, or molecules under small perturbation of chemical reaction. A hard molecule has a large energy gap (large 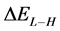 value) and a soft molecule has a small energy gap (small
value) and a soft molecule has a small energy gap (small 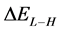 value) [1]. In this study Tolyl with low hardness value
value) [1]. In this study Tolyl with low hardness value 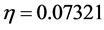 eV compared to that of the Inon
eV compared to that of the Inon 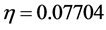 eV has a low energy gap. Normally, the inhibitor with the least value of absolute hardness (hence, the highest value of absolute softness) is expected to have the highest inhibition efficiency [39]. Tolyl with the softness value of 13.65934 eV
eV has a low energy gap. Normally, the inhibitor with the least value of absolute hardness (hence, the highest value of absolute softness) is expected to have the highest inhibition efficiency [39]. Tolyl with the softness value of 13.65934 eV
Table 3 . Calculated quantum chemical parameters of Tolyl and Inon molecules calculated at B3LYP/6-31 + G(d,p) level of theory.
has the highest inhibition efficiency compared to 12.98027 eV of Inon; this result is also in agreement with the experimental finding (Table 3)
Number of electrons transferred: The number of electrons transferred (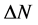 ) was also calculated and tabulated in Table3 If
) was also calculated and tabulated in Table3 If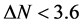 , the inhibition efficiency increases by increasing the electron-donating ability of these inhibitors to donate electrons to the metal surface [40] and it increases in the following order: Tolyl > Inon. The results indicate that
, the inhibition efficiency increases by increasing the electron-donating ability of these inhibitors to donate electrons to the metal surface [40] and it increases in the following order: Tolyl > Inon. The results indicate that 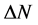 values correlate strongly with experimental inhibition efficiencies. Thus, the highest fraction of electron transferred is associated with the best inhibitor Tolyl (
values correlate strongly with experimental inhibition efficiencies. Thus, the highest fraction of electron transferred is associated with the best inhibitor Tolyl (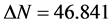 ), while, the least fraction is associated with the inhibitor that has the least inhibition efficiency Inon (
), while, the least fraction is associated with the inhibitor that has the least inhibition efficiency Inon (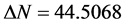 ).
).
Total energy: The total energy of Tolyl inhibitor is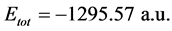 , while for Inon inhibitor it is
, while for Inon inhibitor it is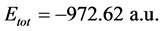 , hence, the difference in the total energies between the two inhibitors is
, hence, the difference in the total energies between the two inhibitors is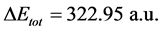 This result indicated that Tolyl inhibitor is favorably adsorbed through the active centers of adsorption on the ferrous surface (Table 3).
This result indicated that Tolyl inhibitor is favorably adsorbed through the active centers of adsorption on the ferrous surface (Table 3).
Local selectivity: The local selectivity of a corrosion inhibitor is best analyzed using the Fukui function [41, 42]. The Fukui indices permit the distinction of each part of a molecule on the basis of its chemical behavior due to different substituent functional groups. The Fukui function can be formally defined as:
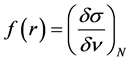 (8)
(8)
where 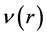 indicates that the differentiation is carried out under constant external potentials, and the functional derivative must be taken at constant number of electron,
indicates that the differentiation is carried out under constant external potentials, and the functional derivative must be taken at constant number of electron,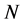 . If it is assumed that the total energy,
. If it is assumed that the total energy, 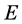 , is a function of
, is a function of 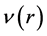 and is an exact differential, then the Maxwell relations between derivatives can be applied to derive the following equation:
and is an exact differential, then the Maxwell relations between derivatives can be applied to derive the following equation:
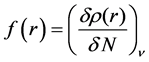 (9)
(9)
where 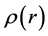 is the electron density. Equation (9) is the most standard presentation of the Fukui function. The Fukui function is provoked by the fact that if an electron
is the electron density. Equation (9) is the most standard presentation of the Fukui function. The Fukui function is provoked by the fact that if an electron  is transferred to an
is transferred to an  electron molecule, it will tend to distribute so as to minimize the energy of the resulting
electron molecule, it will tend to distribute so as to minimize the energy of the resulting 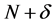 electron system. The resulting change in electron density is the nucleophilic (
electron system. The resulting change in electron density is the nucleophilic (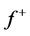 ) and electrophilic (
) and electrophilic (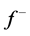 ) Fukui functions, which can be calculated using the finite difference approximation as follows:
) Fukui functions, which can be calculated using the finite difference approximation as follows:
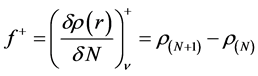 (10)
(10)
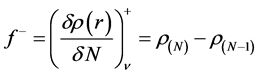 (11)
(11)
where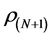 ,
, 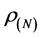 , and
, and  are the electronic densities of anionic, neutral, and cationic forms of the atom with
are the electronic densities of anionic, neutral, and cationic forms of the atom with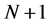 ,
, 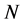 , and
, and 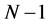 electrons.
electrons.
Calculated values of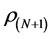 ,
, 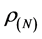 ,
, 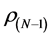 ,
,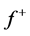 , and
, and 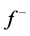 for Tolyl and Inon are presented in Tables 4 and 5. The
for Tolyl and Inon are presented in Tables 4 and 5. The 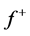 measures the changes of density when the molecule gain electron/s and it corresponds to reactivity with respect to nuecleophilic attack, thus, the site for nucleophilic attack is the site where the value of
measures the changes of density when the molecule gain electron/s and it corresponds to reactivity with respect to nuecleophilic attack, thus, the site for nucleophilic attack is the site where the value of 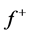 is maximum. On the other hand,
is maximum. On the other hand, 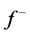 corresponds to reactivity with respect to electrophilic attack or when the molecule loss electron/s, thus, the site for electrophilic attack is the site where the value of
corresponds to reactivity with respect to electrophilic attack or when the molecule loss electron/s, thus, the site for electrophilic attack is the site where the value of 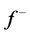 is maximum. For the Tolyl inhibitor, Table 4, it can be deduced that the sites for nucleophilic attack is in the carbon atom (C13). However, the sites for electrophilic attack are in the carbon atoms (C26 and C29). For the Inon inhibitor, Table 5, the sites for nucleophilic attack is in the carbon atom (C3). However, the sites for electrophilic attack are in the carbon atoms (C13, C18, and C24).
is maximum. For the Tolyl inhibitor, Table 4, it can be deduced that the sites for nucleophilic attack is in the carbon atom (C13). However, the sites for electrophilic attack are in the carbon atoms (C26 and C29). For the Inon inhibitor, Table 5, the sites for nucleophilic attack is in the carbon atom (C3). However, the sites for electrophilic attack are in the carbon atoms (C13, C18, and C24).
The 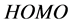 and
and 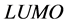 orbitals of Tolyl and Inon, Figure 2, clearly reveal the information that governs the nucleophilic and electrophilic attacks on the studied inhibitors. The information obtained from the
orbitals of Tolyl and Inon, Figure 2, clearly reveal the information that governs the nucleophilic and electrophilic attacks on the studied inhibitors. The information obtained from the 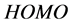 and LUMO orbitals are consistent with the findings obtained from the Fukui function. The FMO diagram of Tolyl and Inon indicates the lack of electron cloud in
and LUMO orbitals are consistent with the findings obtained from the Fukui function. The FMO diagram of Tolyl and Inon indicates the lack of electron cloud in 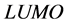 in C13 and C3 for Tolyl and Inon inhibitors, respectively, which is confirmed by the Fukui function
in C13 and C3 for Tolyl and Inon inhibitors, respectively, which is confirmed by the Fukui function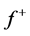 . In case of
. In case of 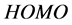 of Tolyl the dense electron cloud around (C26 and C29) indicates the site of electrophilic attack. The same is the case around (C13 and C18, and C24) in Inon as confirmed by the Fukui function
of Tolyl the dense electron cloud around (C26 and C29) indicates the site of electrophilic attack. The same is the case around (C13 and C18, and C24) in Inon as confirmed by the Fukui function 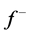 too.
too.
The local softness, 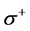 and
and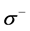 , for an atom can be expressed as the product of the Fukui function,
, for an atom can be expressed as the product of the Fukui function, 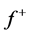 and
and
Table 4 . Electron densities (ED), Fukui (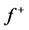 &
&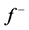 ) and local softness (
) and local softness (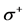 &
&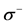 ) indices for nucleophilic and electrophilic attacks in Tolyl calculated at B3LYP/6-31 + G(d,p) level of theory.
) indices for nucleophilic and electrophilic attacks in Tolyl calculated at B3LYP/6-31 + G(d,p) level of theory.
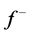 , and the global softness,
, and the global softness, 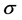 , as follows:
, as follows:
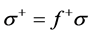 (12)
(12)
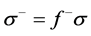 (13)
(13)
The local softness contains information similar to those obtained from Fukui function plus additional information about the molecular softness, which is related to the global reactivity with respect to a reaction partner. Calculated values of 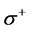 and
and 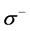 are presented in Tables 4 and 5. From the results obtained, the sites for electrophilic and nucleophilic attack in the studied molecules are not similar.
are presented in Tables 4 and 5. From the results obtained, the sites for electrophilic and nucleophilic attack in the studied molecules are not similar.
4. Conclusion
Using the DFT/B3LYP/6-31 + G(d,p) level of theory, the inhibition efficiency of two pyrazolo [1,5-c] pyrimidine
Table 5 . Electron densities (ED), Fukui (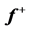 &
&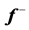 ) and local softness (
) and local softness (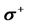 &
&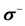 ) indices for nucleophilic and electrophilic attacks in Inon calculated at B3LYP/6-31 + G(d,p) level of theory.
) indices for nucleophilic and electrophilic attacks in Inon calculated at B3LYP/6-31 + G(d,p) level of theory.
derivatives is investigated leading to the following conclusions:
1) Through DFT calculations, a correlation between parameters related to the electronic and molecular structures of these derivatives and their ability to inhibit the corrosion process could be established.
2) The inhibition efficiency of these derivatives obtained quantum chemically increases with the increase in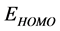 , and decrease in
, and decrease in 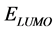 and energy gap
and energy gap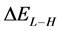 . Tolyl has the highest inhibition efficiency because it had the highest
. Tolyl has the highest inhibition efficiency because it had the highest 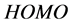 energy.
energy.
3) The parameters like hardness 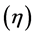 and softness
and softness 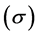 confirm the inhibition efficiency in order of Tolyl > Inon.
confirm the inhibition efficiency in order of Tolyl > Inon.
4) The total energy of the best inhibitor, Tolyl, is lower than that of the Inon inhibitor, thus, the Tolyl is favorably adsorbed through the active center/s of adsorption on the ferrous surface.
5) A direct relationship between the inhibition efficiency and the dipole movement 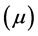 and the number of transferred electrons
and the number of transferred electrons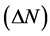 .
.
6) From the local reactivity indices, Fukui function shows the nucleophilic and electrophilic attacking sites in pyrazolo [1,5-c] pyrimidine derivatives.
Finally, this study displays a good correlation between the theoretical and experimental data which confirm the reliability of the quantum chemical methods to study the inhibition of corrosion of metal surfaces.
REFERENCES
- N. O. Obi-Egbedi, I. B. Obot, M. I. El-khaiary, S. A. Umoren and E. E. Ebenso, “Computational Simulation and Statistical Analysis on the Relationship between Corrosion Inhibition Efficiency and Molecular Structure of Some Phenanthroline Derivatives on Mild Steel Surface,” International Journal of Electrochemical Science, Vol. 6, No. 11, 2011, p. 5649.
- I. B. Obot and N. O. Obi-Egbedi, “Inhibitory Effect and Adsorption Characteristics of 2,3-Diaminonaphthalene at Aluminum/Hydrochloric Acid Interface: Experimental and Theoretical Study,” Surface Review and Letters, Vol. 15, No. 6, 2008, pp. 903-910. http://dx.doi.org/10.1142/S0218625X08012074
- H. Ju, Z.-P. Kai and Y. Li, “Aminic Nitrogen-Bearing Polydentate Schiff Base Compounds as Corrosion Inhibitors for Iron in Acidic Media: A Quantum Chemical Calculation,” Corrosion Science, Vol. 50, No. 3, 2008, pp. 865-871. http://dx.doi.org/10.1016/j.corsci.2007.10.009
- S. A. Umoren and I. B. Obot, “Polyvinylpyrollidone and Polyacrylamide as Corrosion Inhibitors for Mild Steel in Acidic Medium,” Surface Review and Letters, Vol. 15, No. 3, 2008, pp. 277-286. http://dx.doi.org/10.1142/S0218625X08011366
- S. A. Umoren, I. B. Obot, E. E. Ebenso and N. O. ObiEgbedi, “Synergistic Inhibition between Naturally Occurring Exudate Gum and Halide Ions on the Corrosion of Mild Steel in Acidic Medium,” International Journal of Electrochemical Science, Vol. 3, No. 9, 2008, pp. 1029- 1043.
- D. Wang, S. Li, Y. Ying, M. Wang, H. Xiao and Z. Chen, “Theoretical and Experimental Studies of Structure and Inhibition Efficiency of Imidazoline Derivatives,” Corrosion Science, Vol. 41, No. 10, 1999, pp. 1911-1919. http://dx.doi.org/10.1016/S0010-938X(99)00027-X
- H. Wang, X. Wang, H. Wang, L. Wang and A. Liu, “DFT Study of New Bipyrazole Derivatives and Their Potential Activity as Corrosion Inhibitors,” Journal of Molecular Modeling, Vol. 13, No. 1, 2007, pp. 147-153. http://dx.doi.org/10.1007/s00894-006-0135-x
- P. Udhayakala, T. V. Rajendiran and S. Gunasekaran, “Theoretical Approach to the Corrosion Inhibition Efficiency of Some Pyrimidine Derivatives Using DFT Method,” Journal of Computational Methods in Molecular Design, Vol. 2, No. 1, 2012, pp. 1-15.
- M. Finsgar, S. Fassbender, F. Nicolini and I. Milosev, “Polyethyleneimine as a Corrosion Inhibitor for ASTM 420 Stainless Steel in Near-Neutral Saline Media,” Corrosion Science, Vol. 51, No. 3, 2009, pp. 525-533. http://dx.doi.org/10.1016/j.corsci.2008.12.006
- S. A. Awad and K. M. Kamel, “Mechanism of CorrosionInhibition and Corrosion-Promotion of Zinc by Phosphate Ions,” Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, Vol. 24, No. 1, 1970, pp. 217- 225. http://dx.doi.org/10.1016/S0022-0728(70)80023-7
- S. A. Awad, K. M. Kamel, Z. Abd El-Hadi and H. A. Bayumi, “Mechanism of Anodic Dissolution of Copper in Aqueous Acidified Solutions of Different Anions,” Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, Vol. 199, No. 2, 1986, pp. 341-350. http://dx.doi.org/10.1016/0022-0728(86)80008-0
- F. M. Mahgoub, B. A. Abdel-Nabey and Y. A. El-Samadisy, “Adopting a Multipurpose Inhibitor to Control Corrosion of Ferrous Alloys in Cooling Water Systems,” Materials Chemistry and Physics, Vol. 120, No. 1, 2010, pp. 104-108. http://dx.doi.org/10.1016/j.matchemphys.2009.10.028
- H. Baba and T. Kodama, “Corrosion Inhibition and Characteristics of the Triazinedithiol Surface Film on Copper Under Potentiostatic Anodization,” Corrosion Science, Vol. 41, No. 10, 1999, pp. 1987-2000. http://dx.doi.org/10.1016/S0010-938X(99)00030-X
- T. Zhao and G. Mu, “The Adsorption and Corrosion Inhibition of Anion Surfactants on Aluminium Surface in Hydrochloric Acid,” Corrosion Science, Vol. 41, No. 10, 1999, pp. 1937-1944. http://dx.doi.org/10.1016/S0010-938X(99)00029-3
- M. S. Masoud, M. K. Awad, M. A. Shaker and M. M. T. El-Tahawy, “The Role of Structural Chemistry in the Inhibitive Performance of Some Aminopyrimidines on the Corrosion of Steel,” Corrosion Science, Vol. 52, No. 7, 2010, pp. 2387-2396. http://dx.doi.org/10.1016/j.corsci.2010.04.011
- M. K. Awad, F. M. Mahgoub and M. M. El-iskandarani, “Theoretical Studies of the Effect of Structural Parameters on the Inhibition Efficiencies of Mercapto-1,2,4-triazoline Derivatives,” Journal of Molecular Structure: THEOCHEM, Vol. 531, No. 1-3, 2000, pp. 105-117. http://dx.doi.org/10.1016/S0166-1280(00)00437-1
- M. K. Awad, “Semiempirical Investigation of the Inhibition Efficiency of Thiourea Derivatives as Corrosion Inhibitors,” Journal of Electroanalytical Chemistry, Vol. 567, No. 2, 2004, pp. 219-225. http://dx.doi.org/10.1016/j.jelechem.2003.12.028
- M. A. M. Ibrahim, S. S. Abd El Rehim and M. M. Hamza, “Corrosion Behavior of Some Austenitic Stainless Steels in Chloride Environments,” Materials Chemistry and Physics, Vol. 115, No. 1, 2009, pp. 80-85. http://dx.doi.org/10.1016/j.matchemphys.2008.11.016
- L. Feng, H. Yang and F. Wang, “Experimental and Theoretical Studies for Corrosion Inhibition of Carbon Steel by Imidazoline Derivative in 5% NaCl Saturated Ca(OH)2 Solution,” Electrochimica Acta, Vol. 58, 2011, pp. 427- 436. http://dx.doi.org/10.1016/j.electacta.2011.09.063
- P. Hohenberg and W. Kohn, “Inhomogeneous Electron Gas,” Physical Review, Vol. 136, No. 3, 1964, pp. B864- B871. http://dx.doi.org/10.1103/PhysRev.136.B864
- W. Kohn and L. J. Sham, “Self-Consistent Equations Including Exchange and Correlation Effects,” Physical Review, Vol. 140, No. 4, 1965, pp. A1133-A1138. http://dx.doi.org/10.1103/PhysRev.140.A1133
- R. G. Parr and W. Yang, “Density-Functional Theory of Atoms and Molecules,” Oxford University Press, Oxford, 1989.
- N. I. Levine, “Quantum Chemistry,” Prentice Hall, Englewood Cliffs, 2000.
- W. Kohn, “Nobel Lecture: Electronic Structure of Matter —Wave Functions and Density Functionals,” Reviews of Modern Physics, Vol. 71, No. 5, 1999, pp. 1253-1266. http://dx.doi.org/10.1103/RevModPhys.71.1253
- L. M. Rodríguez-Valdez, A. Martínez-Villafañe and D. Glossman-Mitnik, “CHIH-DFT Theoretical Study of Isomeric Thiatriazoles and Their Potential Activity as Corrosion Inhibitors,” Journal of Molecular Structure: THEOCHEM, Vol. 716, No. 1-3, 2005, pp. 61-65. http://dx.doi.org/10.1016/j.theochem.2004.10.082
- G. K. Gece and S. BilgiÃ, “Quantum Chemical Study of Some Cyclic Nitrogen Compounds as Corrosion Inhibitors of Steel in NaCl Media,” Corrosion Science, Vol. 51, No. 8, 2009, pp. 1876-1878. http://dx.doi.org/10.1016/j.corsci.2009.04.003
- A. Y. Musa, A. A. H. Kadhum, A. B. Mohamad, A. A. B. Rahoma and H. Mesmari, “Electrochemical and Quantum Chemical Calculations on 4,4-Dimethyloxazolidine-2-thione as Inhibitor for Mild Steel Corrosion in Hydro-chloric Acid,” Journal of Molecular Structure, Vol. 969, No. 1-3, 2010, pp. 233-237. http://dx.doi.org/10.1016/j.molstruc.2010.02.051
- R. M. Issa, M. K. Awad and F. M. Atlam, “Quantum Chemical Studies on the Inhibition of Corrosion of Copper Surface by Substituted Uracils,” Applied Surface Science, Vol. 255, No. 5, 2008, pp. 2433-2441. http://dx.doi.org/10.1016/j.apsusc.2008.07.155
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and A. D. J. Fox, Gaussian, Inc., Wallingford, 2009, p. 09.
- A. D. Becke, “Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior,” Physical Review A, Vol. 38, No. 6, 1988, pp. 3098-3100. http://dx.doi.org/10.1103/PhysRevA.38.3098
- C. Lee, W. Yang and R. G. Parr, “Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density,” Physical Review B, Vol. 37, No. 2, 1988, pp. 785-789. http://dx.doi.org/10.1103/PhysRevB.37.785
- R. G. Pearson, “Absolute Electronegativity and Hardness: Application to Inorganic Chemistry,” Inorganic Chemistry, Vol. 27, No. 4, 1988, pp. 734-740. http://dx.doi.org/10.1021/ic00277a030
- R. G. Parr and R. G. Pearson, “Absolute Hardness: Companion Parameter to Absolute Electronegativity,” Journal of the American Chemical Society, Vol. 105, No. 26, 1983, pp. 7512-7516. http://dx.doi.org/10.1021/ja00364a005
- R. G. Parr, L. V. Szentpaly and S. Liu, “Electrophilicity Index,” Journal of the American Chemical Society, Vol. 121, No. 9, 1999, pp. 1922-1924. http://dx.doi.org/10.1021/ja983494x
- E. S. H. El Ashry, A. El Nemr, S. A. Esawy and S. Ragab, “Corrosion Inhibitors: Part II: Quantum Chemical Studies on the Corrosion Inhibitions of Steel in Acidic Medium by Some Triazole, Oxadiazole and Thiadiazole Derivatives,” Electrochimica Acta, Vol. 51, No. 19, 2006, pp. 3957-3968. http://dx.doi.org/10.1016/j.electacta.2005.11.010
- X. Li, S. Deng, H. Fu and T. Li, “Adsorption and Inhibition Effect of 6-Benzylaminopurine on Cold Rolled Steel in 1.0 M HCl,” Electrochimica Acta, Vol. 54, No. 16, 2009, pp. 4089-4098. http://dx.doi.org/10.1016/j.electacta.2009.02.084
- K. R. Sandip, N. Islam and D. G. Ghosh, Journal of Quantum Information Science, Vol. 1, 2011, pp. 87-95.
- P. Geerlings and F. D. Proft, “Chemical Reactivity as Described by Quantum Chemical Methods,” International Journal of Molecular Sciences, Vol. 3, No. 4, 2002, pp. 276-309. http://dx.doi.org/10.3390/i3040276
- E. E. Ebenso, D. A. Isabirye and N. O. Eddy, “Adsorption and Quantum Chemical Studies on the Inhibition Potentials of Some Thiosemicarbazides for the Corrosion of Mild Steel in Acidic Medium,” International Journal of Molecular Sciences, Vol. 11, No. 6, 2010, pp. 2473-2498. http://dx.doi.org/10.3390/ijms11062473
- I. Lukovits, E. Kalman and F. Zucchi, “Corrosion Inhibitors—Correlation between Electronic Structure and Efficiency,” Corrosion, Vol. 57, No. 1, 2001, pp. 3-8. http://dx.doi.org/10.5006/1.3290328
- E. G. Lewars, “Computational Chemistry Introduction to the Theory and Applications of Molecular and Quantum Mechanics,” Springer, London, 2011.
- P. Fuentealba, P. Perez and R. Contreras, “On the Condensed Fukui Function,” The Journal of Chemical Physics, Vol. 113, 2000, pp. 2544-2551. http://dx.doi.org/10.1063/1.1305879
NOTES
*Corresponding author.


