Geomaterials
Vol.07 No.02(2017), Article ID:75370,13 pages
10.4236/gm.2017.72005
The Gboko Limestone, Yandev, Benue State, Nigeria: Geology, Geochemistry and Industrial Potentials
Anuba Basil Ofulume1, Sabinus Ikechukwu Ibeneme1,2*, Donatus Maduka Orazulike1, Ibrahim Vela Haruna3, Sani Aishatu4, Diugo Okechukwu Ikoro1, Stephen Ikpendu Nwankwo5, Nnaemeka Oluchukwu Ezetoha1, Joseph Azi Bulus6
1Department of Geology, Federal University of Technology, Owerri, Nigeria
2GEM Executive Education, Grenoble Ecole De Management, Rue Pierre Sẻmard Grenoble, France
3Department of Geology, Federal University of Technology, Yola, Nigeria
4Department of Geology, University of Maiduguri, Maiduguri, Nigeria
5Department of Architecture, Federal University of Technology, Owerri, Nigeria
6Department of Geology, University of Jos, Jos, Nigeria

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: November 12, 2014; Accepted: April 10, 2017; Published: April 13, 2017
ABSTRACT
The Cretaceous shallow marine Gboko limestone, Yandev, Nigeria is a component of the sedimentary fill of the 800 km NE-SW trending Benue Trough, Nigeria. The limestone is made up of thin bedded to massive limestone beds interspersed with laminated grey shale having foraminifera as the dominant fossil. The limestone has both mud supported and grain supported texture, and micrites constitute about 75% of the limestone. Bulk chemical composition analysis of the limestone reveals average CaCO3 of 92.41% and a range of 77.50% - 99.00%. Mineralogical impurities include quartz, dolomite, pyrrhotite, fluorapatite etc. Trace elements concentration analysis was carried out using Energy Dispersive X-ray (EDXRF) spectrophotometry and showed the following trace elements: Mn (841.3 ppm), Sr (444.6 ppm), Fe (470 ppm), Zn (114.6 ppm) and Pb (116.4 ppm). Calcining the limestone in a laboratory muffle furnace at 1050˚C for 90 minutes produced a compact, soft burnt porous and reactive lime that does not crumble into fines. The lime so produced neither meets the requirements of the Steel Making Shop (SMS) of the Ajaokuta Steel Plant nor could it be used in the growing sugar refining industry in Nigeria. It can however be used in the food and the food by-products industry, environmental, agricultural and petroleum industries etc. The raw stone remains a major source of raw materials for cement manufacture for the ever expanding building industry.
Keywords:
Benue Trough, Calcination, Fluxing, Geochemistry, Gboko, Limestone

1. Introduction
Nigeria in the comity of nations is rightly tagged developing country, a third world economy. The nation is definitely desirous of shedding this “developing” toga and putting on the enamored appellation of developed economy. In fact she wants to be among the top 20 developed economies by the year 2020. Attainment of this goal can only be via industrialization. Is it realizable? Yes, we can. There are abundant human and mineral resources. Many nations that do not have raw materials but have depended solely on importation have attained industrialized status. In this regard Japan and South Korea are noteworthy. But a situation where the raw materials are abundant as is the situation in Nigeria, only what is needed is the political will and focus on the part of government so as to harness the resources, exploit and utilize the raw materials to produce goods and services to uplift the standard of living and boost the Gross Domestic Product (GDP). This calls for very sound economic and political leadership to put right the priorities of the nation and acquire the needed technological expertise to utilise the abundant resources to produce goods both for export and local consumption. Nigeria is a vast market for diverse goods and services and given adequate time and focus will be satisfied and room for export created.
Limestone and its burnt derivative lime are very important raw materials for the industry. Lime on its own has more than two hundred applications and is reputed to be the largest bulk industrial chemical. Limestone is of course a sine- quanon in the making of cement―a very vital ingredient in the construction industry especially housing, bridges and factories. The task ahead is to examine the role this monomineralic rock specifically the Gboko limestone can play in enhancing the developmental economy of Nigeria.
2. Geology of Gboko Limestone, Yandev, Benue State, Nigeria
The Benue Basin is an elongate NE-SW oriented sedimentary rift that extends inland for approximately 800 km from the Nigerian coastline. The Gboko limestone (Figure 1) actually exposed at Yandev quarry is a component of the sedimentary fill of the Benue trough. The limestone is Cretacious in age and is regarded as being part of Asu River Group of [1] . The Bima Sandstone forms the basal fill of the basin and consists of the braided river, lacustrine and deltaic clastics. [2] has given a detailed description of the stratigraphic sequence of deposition at the Yandev quarry. The rock unit consists of sandstone, limestone, and shales that were laid down during the initial phase of the first tectonic cycle of [3] . The section is made up of thin bedded to massive limestone bed interspersed with laminated grey shale. Foraminifera dominate the fossils. The limestone can be divided into lower and upper units. While the lower unit is composed of limestone with minor shale parting the upper unit is made of shale/limestone alternations. [2] reported that the limestones have both mud supported and grain supported textures in a ratio 3:1 and that micrites constitute 75% of the limestone. While the lower unit is composed of limestone with mudstone-wackestone texture and oncolite and mollusk packstone the upper unit is
Figure 1. Geological map of Gboko area showing Gboko limestone outcrop at about 7 km Northwest of Yandev.
an alternation of limestone with mudstone-wackestone-packstone texure and shales. The limestone is inferred to have been deposited in a shallow coastal hypersaline lagoon. The lagoon is interpreted to have been formed from marine incursion into a localized lacustrine body during the first transgression into the Benue Trough.
3. Materials and Methods
3.1. Geochemistry
The major elements abundance in the representative samples of the limestone were determined using Atomic Absorption Spetrophotometry (AAS). After digestion of the samples the concentration of Si, Ti, Al, Fe, Mn, Mg, Ca, Na, K, P, and S in the limestone samples were measured with a single element light source. Calibration for each element was achieved using four AAS standard solutions of known concentrations. Elemental values were recalculated to oxides using appropriate conversion factors. Summary of the chemical results is presented in Table 1. Determination of trace elements concentrations in the samples was carried out using Energy Dispersive X-ray Fluorescence (EDXRF). Pellets 19 mm diameter were prepared from ground samples of less than 125 microns. Then measurements were performed using an annular 25mCi109Cd as the excitation source that assists Ag-K X-rays in which case all elements with lower characteristic excitation energies were accessible for detection in the samples. Quantitative analyses of the samples were carried out using the Emission-Transmission (E-T) method. Table 2 shows some trace elements concentrations in the Gboko limestone.
Table 1. Major oxide composition of the Gboko Limestone Samples.
Average CaCO3% = 92.41; Range CaCO3 = 77.50% - 99%; Silica is the dominant impurity followed by Magnesia.
Table 2. Some Trace Element Concentrations (ppm) in Gboko Limestone.
The low Sr content of ancient limestones like the Gboko limestone cannot be due to the variations in the mSr/mCa ratio of sea waters from which the limestones were precipitated for the mSr/mCa ratio of sea waters has remained remarkably constant throughout the Phanerozoic era ( [4] [5] ). Although ancient limestones are considerably depleted in Sr they exhibited a wide range of Sr content from a low of 50 ppm to a high of 1000 ppm [5] . The Sr in Gboko limestone has a mean value of 444.6 ppm and a range of 198 - 694 ppm. The mean value of Sr is quite close to the [6] low Sr value (less than 400 ppm) for shallow water limestone. The Mn content averages 841.3 ppm and has a range of 545 - 1760 ppm. Considering therefore the 510 ppm mean value of Mn for shallow water carbonates and 2100 ppm (mean) for deep marine limestones of [7] the Gboko limestone with the aforementioned mean values of Sr and Mn is considered a shallow marine limestone based on trace element geochemical consideration.
3.2. Mineralogy
X-ray diffraction analysis was done on pulverised (1 micron) bulk samples of the limestone using Philips P.W 1800 X-ray diffractometer. Cu-K radiation was employed. The diffraction peaks were measured and plotted by the aid of a computer. Minerals were recognized by the use of their d-values picked from X ray powder data cards. The corresponding 2theta values for d-value were traced in the diffractograms and peaks marching them were labelled (Figure 2). Mineralogical impurities associated with the limestone are dolomite, pyrrhotite, albite, fluorite and fluorapatite. Dolomite is a mineralogical impurity and source of MgO whose level is critical in the use of limestone for cement making. If a critical level is exceeded it could swell and cause disruption of structure.
Burning the Gboko limestone to produce lime is by heating the limestone either in kilns or laboratory muffle furnace. Lime production is very important
Figure 2. XRD Trace of Gboko Limestone.
because very many applications of the limestone is in the form of lime prepared from it. Lime manufacturers choose quality raw stone because quality of the lime is dictated by quality of the limestone and by its manufacturing processes. A compact highly reactive lime is desired by the industries. A general requirement for commercial lime production is a rock with minimum of 95% of CaCO3 [7] but BIS (IS: 10345-1582) prescribed CaO 52% minimum (i.e. 92.86% CaCO3) for use in lime manufacturing for fluxing in steelmaking. Gboko limestone chemically has average 92.41% CaCO3 and ranges from 77.50% - 99.00% CaCO3. This can be accepted as meeting the BIS requirement and therefore shall be calcined and the technological quality of the resultant lime tested for suitability of production of commercial lime for varied applications.
3.3. Crystal Structure
The physical characteristics of the various limestones determine the quality of the lime product. The texture of the limestone is a factor in the successful calcination of the stone into lumps of lime. Coarse grained limestones are prone to fracturing and crumbling into fines when heated. They can be disintegrated to dust by the heat of the calcination process, a situation dreaded by lime manufacturers.
On the other hand, the fine grained limestones can easily be calcined to form lumps of lime because the crystals resist the temperature stress .The Gboko limestone is fine-grained (Figure 3) and is therefore favourably disposed to calcination into lumps of lime.
3.4. Calcination
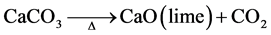
As hinted earlier, calcination is an important factor in quality lime production. The objective always is to produce a compact, softburned, highly reactive lime. To achieve this, the limestone has to be calcined in laboratory muffle furnace through a range of temperatures at varied retention times and the resultant lime then tested technologically for strength and reactivity to determine the best burning parameters. Samples of the Gboko limestone trimmed to size (2 cm
Figure 3. Representative Sample of Gboko Limestone.
diameter) were put in crucibles and then shock calcined. This implies they were introduced in the furnace only when furnance has attained and stabilized at desired calcination temperatures of 950˚C, 1000˚C, 1050˚C, and 1100˚C.
Generally larger stone sizes are more difficult to calcine uniformly and require more time. This is because dissociation always penetrates gradually from the surface to the interior of the stone [8] . To expel the CO2 from such large stone high temperatures are necessary to generate sufficient CO2 pressure in the interior of the crystal lattice for the escape of the gas. Frequently these high temperatures overburn the surface of the stone causing excessive shrinkage, which narrows and closes the pores. [9] prescribed that the ideal stone to calcine for optimum quality, uniformity and thermal efficiency would be of small size (1.25 cm) and be of uniform size and shape.
3.5. Temperature and Retention Time in the Furnance
Since the temperature and time can easily be controlled, they were varied so as to determine the best calcination parameters for the Gboko limestone by testing the quality of the lime product using technological tests such as weight loss, decrepitation, loss on ignition of lime, mechanical strength, and reactivity and by inference porosity and surface area of the lime. The calcination duration was 60, 90, and 120 minutes.
3.6. Weight Loss and Loss on Ignition of Lime
After calcining at the desired temperature and time, the lime products were allowed to cool to room temperature in a dessicator before weighing accurately to determine the weight loss during calcinations. The loss in weight represents the weight of CO2 driven off during the burning and this is expressed as percentage of the original weight. The loss on ignition of lime should not be confused with weight loss or loss in ignition of limestone. Loss on ignition is done to determine if calcination was complete. After calcining at the appropriate temperature the lime was allowed to cool in a dessicator and thereafter about two grammes weighed again accurately to four places of decimal, placed in a crucible and then calcined further at 1100˚C. It is cooled to room temperature in a dessicator and then weighed again. The difference in weight was expressed as a percentage of the original weight to give the loss in ignition of lime at the particular calcined temperature (Table 3).
3.7. Decrepitation and Mechanical Strength
The various calcines 950˚C/60minutes, 1000˚C/90minutes etc were cooled to room temperature in a desicator then poured onto 1.18 sieves and shaken gently. The quantity of minus 1.18 sieve material generated was measured and expressed as percentage of the original weight are then expressed as percent decrepitation (Table 3).
Screen analysis was carried out on the calcined stone. After shock calcining the samples at the appropriate temperature the lime products were subsequently
Table 3. Results of technological tests on the above lime.
Levels of LOI in Basic Oxygen Furnace (BOF) is generally less than 3% [11] .
Table 4. Mechanical strength screening test result.
The less than 0.6 mm sieve material for Gboko limestone is less than 1.0 grammes. This is not significant compared to 5.6 gm of Jakura marble which generates fines. The result is an indication that the lime will not generate fines in the kiln and during handling.
weighed and then poured on to a set of sieves of aperture sizes 13.20 mm, 9.50 mm, 4.75 mm, 2.36 mm, 1.18 mm and 0.60 mm and then given an extended shaking with hands for about 10 minutes. Screening the stones for 10 minutes gives an indication of the tendency of the stone to decrepitate in the kiln [9] . The result is presented in Table 4. From the test one can see that Gboko limestone does not show any tendency to decrepitate in the kiln judging from the minus 0.6 mm material.
3.8. Reactivity of the Gboko Lime
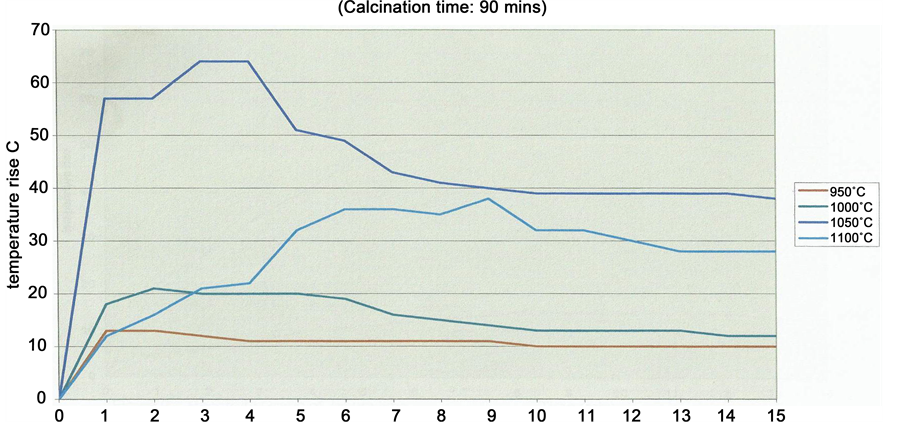
The maximum temperature reached through the exothermic reaction of quicklime with water is a good indicator of the quality of the lime in terms of the available CaO [10] . The ASTM specified the use of 100 gm of lime in 400 ml of distilled water at 25˚C. The sample was prepared to pass 2.36 sieve as quickly as possible and then left in a desicator to cool to room temperature before slaking test using a thermos flask, thermometer, and a stop clock, and a rod as stirrer, a pestle and a mortar for crushing the quicklime. Stirring was done continuously and reading of temperature taken at 30 seconds interval. Readings were recorded until less than 0.5˚C temperature change was noted in each of three consecutive readings were taken. The initial temperature was subtracted from the final temperature to obtain the total temperature rise. Also the initial temperature was subtracted from the temperature at 2 minutes to obtain temperature rise in 2 minutes (Table 5). Suitable curves showing temperature rise as the ordinate and time as the abscissa were then plotted (Figure 4). The results were reported as
Table 5. Results of the Reactivity with water (slaking test).
Optimum reactivity is defined as >40˚C rise after 2 minutes; Therefore 1050˚C/90minutes calcine yielded a highly reactive lime that is a softburned, porous lime with high surface area and that does not decrepitate.
Figure 4. Reactivity Graph of Gboko Lime Time (Min). Activity Defined by temperature rise (˚C) after 2 minutes.
temperature rise in Celsius after 2 minutes.
4. Results and Discussion
All parameters did not meet the specification except CaO (Table 6). Gboko limestone cannot be used in the growing Nigerian Sugar industry.
Table 7 presents the British Standard Institute guidelines for flux limestone for use in steel plants. The Gboko limestone can be utilized in grades 1 and II for lime manufacturing and for use in steel making. Both quicklime and hydrated lime are widely used in flotation or recovering of many non ferrous ores in particular copper ore flotation in which lime acts as a depressant (settling aid) and maintains proper alkalinity in the flotation circuit.
However, Gboko limestone does not meet the requirements for the SMS (steel making shop) limestone for the BOS (Basic Oxygen Steelmaking) shop of the Ajaokuta Steel Plant where the purity of the limestone must be greater than 95.5% and silica less than 1.0% (Table 8).
From Table 8, the sulphur content of Gboko limestone (0.20%) far exceeds the less than 0.05% required for steel making at Ajaokuta. Consequently this limestone will not be used in the Ajaokuta steel industry, Nigeria.
Table 6. (BSI(IS:3204-1978) Specification of limestone for use in sugar manufacture.
Table 7. BS1 Guideline for limestone for use as flux in steel industry.
Table 8. Average Chemical Composition of Gboko Limestone compared with Ajaokuta SMS grade lst.
5. Potential Applications
5.1. Environmental Use of Gboko Lime
Lime is useful in the sewage biosolids and sludges. Both quicklime and hydrated lime have been in use for biological organic wastes for more than 100 years. Lime neutralizes acid wastes generated in industry thereby impeding corrosion and protecting the natural environment. Lime removes silica, manganese, fluorides, iron and other impurities from water. Most importantly lime is used in the treatment of drinking water, softening, pH adjustment, coagulation. The Gboko lime can be of use in the treatment of hazardous wastes currently generated or abandoned materials for example, the hazardous waste that was imported and dumped in Koko, Delta state, Southern Nigeria.
5.2. Gboko Lime in Food Industry
In the dairy industry, lime water is often added to the cream separated from whole milk so as to reduce acidity prior to pasteuralization when butter is produced. Lime is also beneficial in preparation of the common types of baking powder, and the preservation of fruits and vegetables. The need for the setting up of dairy industry in the northern states especially Benue state that prides itself as the foods basket of the nation is highlighted with Gboko lime as adequate and available compliment for this food industry (Figure 5).
5.3. Gboko Lime for Agricultural Purposes
Gboko lime or the ground limestone can be useful in pH adjustment of agricultural soils, but the focus seems to be the use of lime in conjuction with nitrogen fertilizers because the lime allows reduced usage of fertilizers resulting in reduction of nitrogen leaching from the soils. Gboko lime can also serve useful purpose in compositing, poultry litter and pesticides and certain fertilizers (Figure 6).
5.4. Gboko Lime in Petroleum Industries
Various uses of lime in the petroleum industry are captured graphically in Figure 7. The Gboko lime will surely boost operation in the Nigerian petroleum industry.
Figure 5. Graphical presentation of uses of lime in food industry.
Figure 6. Graphical presentation of uses of lime in agriculture.
Figure 7. Graphical presentation of uses of lime in petroleum industry.
Table 9. Average Chemical Composition of Gboko Limestone.
Range CaCO3 % = 77.50 - 99.00; average CaCO3% = 92.41; LOI = 43.41; Total = 105.367.
5.5. Gboko Limestone for Cement Making
The Gboko limestone has for many years been the source of limestone for quality cement manufacture at the Benue cement factory at Yandev. The output capacity of the factory has received a tremendous boost by the acquisition of the factory by the Dangote Group. The Group presently is the greatest cement maker in the whole of Africa and owns cement factories in many African countries. Cement making by and large remains the major use of the Gboko limestone, and the diversification of its application is called for with the introduction or incoporation of lime manufacturing facilities in the factory at Yandev. Table 9 shows the average chemical composition of Gboko Limestone.
6. Conclusions
The Cretaceous Gboko limestone is made up of thin bedded to massive limestone beds interspersed with laminated grey shale. It has foraminifera as the dominant fossil. The limestone is shallow marine based on the trace elements analysis. The bulk chemical composition reveal CaCO3 range of 75.50% - 99.00% averaging 92.41%. Technological tests show that the limestone can be calcined to produce a compact soft burned, reactive lime that does not crumble into fines. The lime chemically fails to meet the requirements for fluxing application in the Steel Making Shop (SMS) of the Ajaokuta Steel Plant and for use in sugar refining industry in Nigeria.
However, it can be utilized in the food and the food by-products industry, environmental, agricultural and petroleum industries. Cement manufacturing by Dangote cement remains the main user of the raw stone.
Cite this paper
Ofulume, A.B., Ibeneme, S.I., Orazulike, D.M., Haruna, I.V., Aishatu, S., Ikoro, D.O., Nwankwo, S.I., Ezetoha, N.O. and Bulus, J.A. (2017) The Gboko Limestone, Yandev, Benue State, Nigeria: Geology, Geochemistry and Indus- trial Potentials. Geomaterials, 7, 51-63. https://doi.org/10.4236/gm.2017.72005
References
- 1. Reyment, R.A. (1965) Aspects of Geology of Nigeria. Ibadan University Press, Ibadan, 145.
- 2. Nair, K.M. and Ramanatham, R.M. (1989) Sedimentology, Stratigraphy and Paleogeographic Significance of Lower Cretaceous Gboko Limestone, Nigeria. Journal Mining and Geology, 21, 203-210.
- 3. Murat, K.C. (1972) Stratigraphy and Paleogeography of the Cretaceous and Lower Tertiary in Southern Nigeria. In: Dessauvagie, T.F.J. and Whiteman, A.J., Eds., African Geology, University of Ibadan, 251-266.
- 4. Lowestam, H.A. (1961) Mineralogy O18/O16 Ratios and Srontium and Magnessium Content of Recent and Fossil Brachiopods and Their Bearing on History of the Oceans. Journal of Geodesy, 69, 241-260.
- 5. Morrow, D.W. and Mayers, I.R. (1978) Simulation of Limestone Diagenesis—A Model Based on Strontium Depletion. Canadian Journal of Earth Sciences, 369-377.
https://doi.org/10.1139/e78-043 - 6. Bausch, W.M. (1968) Outline of Distribution of Strontium in Marine Limestone. In: Muller, G. and Friedman, G.M., Eds., Recent Developments in Carbonate Sedimentology Central Europe, Berlin-Heidelberg, Springer, New York, 106-115.
https://doi.org/10.1007/978-3-642-88052-0_13 - 7. Lutke, F. (1976) Sedmentologische and Geochemiche un arsucheungen zur zennese der flinzfazies in zur (givet and oberden) dilsch. Geol. Ges, 127, 499-508.
- 8. Ofulume, A.B. (2008) The Geology, Geochemistry and Economic Potentials of Some Nigerian Limestones and Marble Resources. Unpublished Ph.D. Thesis ATBU Bauchi, 142.
- 9. Boynton, R.S. (1980) Chemistry and Technology of Lime and Limestone. Wiley Inter Science, 482.
- 10. Ruskulis, O. (1997) Methods for Testing Lime in the Field.
http://www.itdg.org./docs/technicalinformationservice/testinglime - 11. Anderson, L.C. and Vernon, J. (1971) Quality and Production of Lime for Basic Oxygen and Steel Making. The Quarry Managers Journal Institute of Quarrying Translation, 169-175.