World Journal of Nuclear Science and Technology
Vol.06 No.02(2016), Article ID:65604,7 pages
10.4236/wjnst.2016.62010
Anomalous Rutherford Scattering Solved Magnetically
Bernard Schaeffer
7 Rue de l’Ambroisie, Paris, France

Copyright © 2016 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 22 February 2016; accepted 16 April 2016; published 19 April 2016
ABSTRACT
After one century of nuclear physics, the anomalous Rutherford scattering remains a puzzle: its underlying fundamental laws are still missing. The only presently recognized electromagnetic interaction in a nucleus is the so-called Coulomb electric force, in 1/r, only positive thus repulsive in official nuclear physics, explaining the Rutherford scattering at low kinetic energy of the impacting alpha particles. At high kinetic energy the Rutherford scattering formula doesn’t work, thus called “anomalous scattering”. I have discovered that, to solve the problem, it needs only to replace, at high kinetic energy, the Coulomb repulsive electric potential in 1/r, by the also repulsive magnetic Poisson potential in 1/r3. In log-log coordinates, one observes two straight lines of slopes, respectively −2 and −6. They correspond with the −1 and −3 exponents of the only repulsive electric and magnetic interactions, multiplied by 2 due to the cross-sections. Both Rutherford (normal and anomalous) scattering have been calculated electromagnetically. No attractive force needed.
Keywords:
Strong Force, Nuclear Physics, Rutherford Scattering, Electric Scattering, Magnetic Scattering, Anomalous Scattering, Nuclear Scattering

1. Introduction
Rutherford explained the low energy scattering, electric. At high energies, a singularity appears, followed by a steeper slope, called anomalous Rutherford scattering. During one century, it remained a puzzle due to the wrong assumption of inadequacy of classical forces [1] . The first empirical theory was Yukawa’s [2] . According to Mott [3] , “it is natural to assume that for smaller distances, the force becomes attractive”, wrong. At high kinetic energies, Bieler [4] had almost solved the so-called anomalous scattering, unfortunately with a wrong attractive magnetic force. I solved the problem by just changing the sign, with a repulsive force. As the electric force, the magnetic force is fundamental: no need of a hypothetical strong force.
2. Rutherford Scattering
Alpha particles, from a radioactive source, striking a thin gold foil produce a tiny, but visible flash of light when they strike a fluorescent screen (Figure 1). Surprisingly, α particles were found at large deflection angles and, unexpectedly, some of the particles were backscattered [5] .
Rutherford explained why some alpha particles projected on an atom were reflected by a small nucleus: “Assuming classical trajectories for the scattered alpha particles, Coulomb’s law was found to hold for encounters between alpha particles and nuclei” [6] (N. B. In this paper, Coulomb’s and Poisson’s laws are repulsive except signalled to be wrong). The first evidence of departures from repulsive Coulomb’s law [7] other than those in α scattering by H and He was observed by Bieler [4] . The discontinuity, appearing near 22 MeV, lower than the total absolute value of the α particle binding energy, −28 MeV, is called Rutherford singularity. For kinetic energies larger than 28 MeV [9] , the relative cross section decreases anomalously faster than predicted by the electric Rutherford formula (Figure 2). “The energy dependence of the cross section in the falling region is given quite accurately by an exponential” [9] becoming a straight line in log-log coordinates (Figure 3). Magnetic interpretations have been tempted without success [4] [10] , due to the wrong sign chosen for the magnetic moment. The make-believe strong force cannot be used: its fundamental laws are unknown.
The purpose of this paper is to solve the unsolved problem of the so-called anomalous scattering of α particles.
2.1. Nuclear Potentials
Rutherford discovered that the impacting electrically charged α particles are deviated by the repulsive electrostatic force of the impacted nuclei. The origin of the concept of strong force comes from the observation of the discrepancy between Rutherford theory and experiment at high kinetic energies (Figure 2). Up to now no theory with only fundamental laws and constants was able to explain quantitatively the nuclear scattering at kinetic energies larger than 28 MeV.
At high kinetic energies, Geiger observed that the deviation was larger than predicted by the electric force, repulsive [5] . Chadwick and Bieler [8] [11] determined that forces of very great intensity hold the nucleus together, a force distinct from the electromagnetism [8] [12] [13] . The repulsive electric potential was assumed to be equilibrated by a “new type of force”, attractive [8] , wrong:
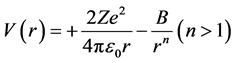 (1)
(1)
 is the electric potential function of r, the separation distance between the impacting α particle with 2
is the electric potential function of r, the separation distance between the impacting α particle with 2
protons and an atomic nucleus with a number Z of protons, e is the elementary electric charge, 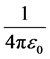 is Cou-
is Cou-
lomb’s constant. The first term of the Equation (1) corresponds to the “normal” electric Rutherford scattering
Figure 1. Rutherford experiment. The α particles are emitted by radium, impact- ing thin gold, lead or other metal foils. The number of deflected α particles depends upon the scattering angle and the metal foils defining their velocity. The experiment consists to count the number of flashes viewed through the microscope during a given time and for a fixed solid angle. The α particles are scattered all around, even backwards, astonishing Rutherford [4] - [6] [8] .
Figure 2. Rutherford scattering at low and high kinetic energies. This figure shows the experimental points [9] with calculated curves, electrically (dashed curve) and magnetically (continuous curve). The relative differential cross section 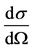 is a targeted area per solid angle per unit time. The α particles are projected on Ta foils at a fixed scattering angle θ = 60˚ with initial kinetic energies varying between 13 and 42 MeV [9] . The α particles are repulsed and deviated by the Ta nucleus electric force in the direction of the particle exit trajectory (Figure 1). The inverse electric potential energy coincides with the experimental points of the original figure, used as background. The Rutherford singularity appears for a kinetic energy of 25 MeV. At higher kinetic energies, the curve deviates, due to a wrong attractive “strong force” [8] . Bieler [4] assumed a wrong attractive magnetic force opposite to the electric repulsion. In this paper, it is discovered, for scattering, that the Poisson magnetic force is repulsive as the electric force. The Rutherford formula works fine, even for the not so anomalous scattering, provided that the electric −2 be replaced by the magnetic −6 better seen on Figure 3.
is a targeted area per solid angle per unit time. The α particles are projected on Ta foils at a fixed scattering angle θ = 60˚ with initial kinetic energies varying between 13 and 42 MeV [9] . The α particles are repulsed and deviated by the Ta nucleus electric force in the direction of the particle exit trajectory (Figure 1). The inverse electric potential energy coincides with the experimental points of the original figure, used as background. The Rutherford singularity appears for a kinetic energy of 25 MeV. At higher kinetic energies, the curve deviates, due to a wrong attractive “strong force” [8] . Bieler [4] assumed a wrong attractive magnetic force opposite to the electric repulsion. In this paper, it is discovered, for scattering, that the Poisson magnetic force is repulsive as the electric force. The Rutherford formula works fine, even for the not so anomalous scattering, provided that the electric −2 be replaced by the magnetic −6 better seen on Figure 3.
and the second term to the “anomalous” scattering, magnetic if n = 3. The sign of B [14] , not specified, seems to be positive. Bieler hypothesized the existence of attractive magnetic moments, wrong, thus with n = 3 for the potential [4] :
 (2)
(2)
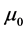 is the magnetic constant,
is the magnetic constant, 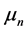 and
and 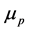 are the magnetic moments of the neutron and of the proton. This formula was rejected, replaced by the following, also wrong [8] :
are the magnetic moments of the neutron and of the proton. This formula was rejected, replaced by the following, also wrong [8] :
 (3)
(3)
The sign of a, not specified, seems to be positive. The α particles having no magnetic moment, no magnetic interaction between them seems should be possible. Nevertheless, when the kinetic energy becomes larger than the binding energy (absolute value), the freed nucleons get kinetic energy and magnetic moments. Nuclear scattering became thus entirely and only electromagnetic. Unfortunately, with the wrong attractive negative sign of the magnetic potential, Bieler was unable to solve the problem although the solution was simply to replace the wrong attractive negative sign of Equation (2) by a repulsive positive sign:
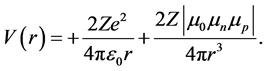 (4)
(4)
This electromagnetic formula works fine, as shown on Figure 2 and Figure 3. At low kinetic energies, r being large, we have the electric Rutherford formula with an electric −1/r potential. At high kinetic energies, the α particles approach more to the impacted nucleus. r becomes small, the magnetic 1/r3 potential increases and dominates the electric potential, becoming the main component of the potential. Sexl [15] and Houtermans [16] discussed, for the potential formula, different exponents n, from 2 to 4.
Figure 3. Electric Coulomb potential, [7] and Poisson magnetic potential, [17] , both positive, thus repulsive with slopes negative. In contrast with semi-logarith- mic coordinates (Figure 2), the log-log coordinates give straight lines with slopes −2 and −6 corresponding to the electric 1/r and magnetic 1/r3 potentials, crossing at the Rutherford singularity, coinciding approximately with the total α particle binding energy, 28 MeV, in absolute value. Ce and Cm are constants adjusted to the singularity. At low kinetic energies the α particles are deviated electrically by the heavy nucleus. At high kinetic energies, the α particles approach more to the nucleus. For kinetic energies larger than 28 MeV, the α particles, having a binding energy of −28 MeV, are destroyed. The magnetic potential, in 1/r3, of the protons and the neutrons, overcomes the electric potential, in 1/r, explaining the slope change from −2 to −6.
2.2. Differential Cross-Section
The differential cross-section 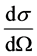 is defined as the ratio of the number of particles scattered into a constant direction θ, per unit time and per unit solid angle dΩ. Squaring the initial kinetic energy of the α particle,
is defined as the ratio of the number of particles scattered into a constant direction θ, per unit time and per unit solid angle dΩ. Squaring the initial kinetic energy of the α particle, 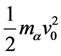 , one obtains the so-called differential cross-section
, one obtains the so-called differential cross-section

The Rutherford formula may be simplified for given θ, z and Z:

The exponent −2, due to the electrostatic interaction cross-section, becomes, logarithmically, the coefficient −2:

where Ce is to be adjusted to the singularity, near to the α particle binding energy, 

The variables are the differential cross section 
adjusted to make coincide the intersection between the electric and magnetic straight lines with the Rutherford singularity. At the singularity, the initial kinetic energy is more or less lower than the absolute value of the α particle total binding energy, 
The kinetic energy at the Rutherford singularity is a little less than the experimental value of the total binding energy of the α particle, 
2.3. Discussion
According to [8] [11] , Bieler’s hypothesis [4] of an additional magnetic potential in the law of force is an assumption solely made to account for the anomalous scattering. As said above, only the sign [4] was wrong. By changing it, it works fine (Figure 3), with an electromagnetic Rutherford scattering. The Bieler’s magnetic hypothesis is the solution, except that the sign was wrong.
The magnetic interaction between nucleons is ignored in “conventional” nuclear physics. The 4He also called α particle has no apparent magnetic moment. When the kinetic energy is high enough to destroy the 4He, the magnetic moments of protons and of neutrons reappear. The magnetic potential being in 1/r3, the slope of the curve (Figure 3) changes from −2, electric, to −6 magnetic. As far as I know, nobody tried a magnetic repulsive force: during one century of nuclear physics nobody tried to change the sign of Bieler’s magnetic term [4] .
At short r, the repulsive magnetic potential in r−3 overcomes the also repulsive electric potential in r−1. No need of an attractive wrong “strong force”. No need also of quantum mechanics and/or relativity, at least for kinetic energies between 10 and 50 MeV.
3. Conclusions on Nuclear Scattering
One century ago, Rutherford discovered the electric part of the nuclear scattering. In order to explain the discrepancy at high energy, Chadwick hypothesized a new type of force, strong and attractive. Bieler assumed a magnetic strong force, also attractive, with exponent +6 instead of −6, thus missing the discovery [4] . Indeed, in log-log coordinates, it suffices to replace the −2 of the electric repulsive formula by the −6, magnetic formula, to obtain two straight lines coinciding with the experimental points (Figure 3).
The Rutherford scattering is electric at low kinetic energy and magnetic at high kinetic energy of the impacting α particles. Nuclear scattering and binding energy [18] [19] have been now both proved to be electromagnetic: the hypothetical strong force will disappear.
Acknowledgements
Thanks to persons at Dubna for their interest to my electromagnetic theory of the nuclear energy. The first question was about scattering. I said I don’t know. Now I know: the anomalous Rutherford scattering is magnetic. The second question was: “The strong force doesn’t exist?” and a third one about orbiting nucleons.
Cite this paper
Bernard Schaeffer, (2016) Anomalous Rutherford Scattering Solved Magnetically. World Journal of Nuclear Science and Technology,06,96-102. doi: 10.4236/wjnst.2016.62010
References
- 1. Evans, R.D.E. (1969) The Atomic Nucleus. McGraw-Hill Book Co.
- 2. Yukawa, H. (1935) On the Interaction of Elementary Particles. Proc. Phys. Math. Soc. Jap, 17, 48.
- 3. Rutherford, L., Chadwick, J., Ellis, C.D., Fowler, R.H., McLennan, J.C., Lindemann, F.A. and Mott, N.F. (1932) Discussion on the Structure of Atomic Nuclei. Proceedings of the Royal Society A, 735.
- 4. Bieler, E.S. (1924) Large-Angle Scattering of Alpha-Particles by Light Nuclei. Proceedings of the Royal Society of London A, 105, 434-450.
http://dx.doi.org/10.1098/rspa.1924.0029 - 5. Geiger, H. and Marsden, E. (1909) On a Diffuse Reflection of the α-Particles. Proceedings of the Royal Society, 82, 495.
http://dx.doi.org/10.1098/rspa.1909.0054 - 6. Rutherford, E. (1911) The Scattering of α and β Particles by Matter and the Structure of the Atom. Philosophical Magazine, 21, 669-688.
http://dx.doi.org/10.1080/14786440508637080 - 7. Coulomb (1785) Second Mémoire sur l’électricité et le magnétisme.
- 8. Rutherford, E., Chadwick, J. and Ellis, C.D. (1951) Radiations from Radioactive Substances. Cambridge University Press, Cambridge.
- 9. Eisberg, R.M. and Porter, C.E. (1961) Scattering of Alpha Particles. Reviews of Modern Physics, 33, 190-230.
http://dx.doi.org/10.1103/RevModPhys.33.190 - 10. Paetz, H. (2014) Nuclear Reactions: An Introduction. Springer, Berlin.
http://dx.doi.org/10.1007/978-3-642-53986-2 - 11. Chadwick, J. and Bieler, E.S. (1921) The Collisions of Alpha Particles with Hydrogen Nuclei. Philosophical Magazine, 42, 923.
http://dx.doi.org/10.1080/14786442108633834 - 12. Burkhardt, C.E. and Leventhal, J.J. (2008) Foundations of Quantum Physics. Springer Science.
http://dx.doi.org/10.1007/978-0-387-77652-1 - 13. Farwell, G. and Wegner, H.E. (1954) Elastic Scattering of Intermediate Energy Alpha Particles by Heavy Nuclei. Physical Review, 95, 1212-1217.
http://dx.doi.org/10.1103/PhysRev.95.1212 - 14. Gamow, G. and Critchfield, C.L. (1949) Theory of Atomic Nucleus and Nuclear Energy-Sources. Oxford at the Clarendon Press, 9.
- 15. Sexl, T. (1931) Bemerkungen zur Theorie der anomalen Streuung von α-Teilchen durch leichte Kerne. Zeitschrift für Physik, 67, 766-779.
http://dx.doi.org/10.1007/BF01390757 - 16. Houtermans, F.G. (1939) Neuere Arbeiten über Quantentheorie des Atomkerns. Naturwissenschaten, Neunter band, 124-184.
- 17. Poisson (1824) Théorie du magnétisme, Mémoires de l’Académie Royale des Sciences.
- 18. Schaeffer, B. (2011) Electromagnetic Theory of the Binding Energy of the Hydrogen Isotopes. Journal of Fusion Energy, 30, 377-381.
http://dx.doi.org/10.1007/s10894-010-9365-0 - 19. Schaeffer, B. (2013) Electric and Magnetic Coulomb Potentials in the Deuteron. Advanced Electromagnetics, 2, 69-72.
http://dx.doi.org/10.7716/aem.v2i1.218




