World Journal of Nuclear Science and Technology
Vol.05 No.03(2015), Article ID:57819,7 pages
10.4236/wjnst.2015.53015
Reduction Kinetics of Uranium Trioxide to Uranium Dioxide Using Hydrogen
Pedro Orrego Alfaro1, José Hernández Torres1, Fernando Puchi Thiele2
1Sección de Geología y Minería, Departamento de Materiales Nucleares, Comisión Chilena de Energía Nuclear, Santiago, Chile
2Ingeniería Civil Metalurgia, Universidad Andrés Bello, Santiago, Chile
Email: porrego@cchen.cl, jjhernandez@cchen.cl, fernando.puchi@unab.cl
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 24 April 2015; accepted 6 July 2015; published 9 July 2015
ABSTRACT
This article presents a study on the kinetics of the uranium conversion process, consisting in the reduction of uranium trioxide to uranium dioxide using hydrogen gas at temperatures of 500˚C, 600˚C and 700˚C. Hydrogen concentrations used in the flow were 0.25 M, 0.50 M and 0.75 M. The mechanism established for the study of the kinetics of reduction of uranium trioxide was through the formation of an intermediate compound, U3O8. For this reason, these tests were divided into 2 stages: the first one the reduction from UO3 to U3O8, and second one from U3O8 to UO2. The results of each test were quantified by the release of H2O(g) produced by both reactions. Tests showed that the ideal working conditions are for hydrogen concentration flows of 0.75 M and temperatures in the range of 500˚C - 600˚C, with the intent to decrease the occurrence of side reactions that interfere with the process.
Keywords:
Conversion, Uranium Oxides, Nuclear Fuel Cycle

1. Introduction
One of the most important energy sources for the development of the worldwide industry is from nuclear fuels. Uranium is used in power reactors as natural uranium for the development of other fuel elements alloys or as UO2 pellets. The most important condition for its use in these applications is the impurity level, especially boron and cadmium, which cannot be more than 0.2 to 300 µg/g respectively, because they decrease fuel efficiency. For this purpose, uranium concentrate must be purified for the manufacture of the fuel element [1] .
The purification process for uranium concentrate is shown in Figure 1.
Figure 1. Purification process of uranium concentrates [2] .
The purification process in Figure 1 consists in the oxidizing dissolution of uranium concentrate, which may be ammonium diuranate (ADU) or ammonium uranyl carbonate (AUC), by using nitric acid, to ensure that all uranium is dissolved. Subsequently, the uranyl nitrate solution is treated by using solvent extraction techniques. At this stage, the latter compound is selectively extracted by the organic reagent tributyl phosphate (TBP), and subsequently discharged onto a water flow at 60˚C, to precipitate it as ADU or AUC. Finally, the precipitate must be calcined to produce UO3 [2] [3] .
The conversion process from Figure 2 consists in the reduction of UO3 to UO2 using hydrogen gas at high temperatures. Finally, in order to obtain metallic uranium, the UO2 compound goes to hydrofluorination stage, where it contacts hydrogen fluoride to produce UF4 and obtain fuel elements [2] .
From Figure 1 and Figure 2, most of the sub stages have well-defined reaction mechanisms. From these, the stage that causes most controversy in these terms is the conversion of uranium trioxide (UO3) to uranium dioxide (UO2). The reason for this problem is the large amount of uranium compounds formed during UO3 reduction using hydrogen gas at temperatures between 500˚C and 700˚C. The most known oxidized uranium compounds that may formare U3O8, U3O7 and U4O9, which have different oxidation states at their nets, and they are able to affect in several ways the conversion process [4] .
It is for these reasons that this article will study the kinetics of reduction from uranium trioxide to uranium dioxide with hydrogen, together with the influence of each parameter on the overall process.
The conversion process consists in the reduction reaction of uranium trioxide to uranium dioxide according to Equation (1): [5] [6]
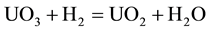 (1)
(1)
Figure 3 shows the uranium Pourbaix diagram:
According to Figure 3, before the uranium trioxide is reduced to uranium dioxide, it will show up a third oxide species, U3O8. This sub process is shown in reaction (2): [5] [6]
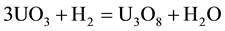 (2)
(2)
Once formed this compound, it is from U3O8 that uranium dioxide is formed by hydrogen reduction, according to reaction (3): [5] [6]
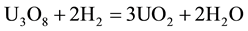 (3)
(3)
This process is normally carried out at temperatures above 500˚C, since, as shown in Figure 3, it is possible to obtain the UO2 using manganese oxides [7] or reducing agents such as iron and bacterial enzymes [8] . However, according to the Pourbaix diagram, UO2 precipitates at wide pH ranges. At room temperature, impure products can precipitate, because besides uranium precipitation, other oxides of many elements, especially iron, would precipitate. Temperatures over 500˚C during the process will prevent the occurrence of parasitic reactions that could contaminate the final UO2 concentrate.
The importance of this work arises from the need of knowledge of the reaction mechanism for the develop-
Figure 2. UF4 production from UO3 by hydrofluorination [2] .
Figure 3. Uranium Pourbaixdiagram [4] .
ment of a new reactor, capable of the calcination of different uranium concentrates and reducing them directly to uranium dioxide.
Thus, the study of this process was performed for the study of reactions (2) and (3) in separate ways.
2. Experimental Development
The experimental setup is shown in Figure 4.
The dimensions of the conversion reactor are 1.66 m long and 4.5 cm. radius. It contains a vessel of 100 mm long and 20 mm wide, with 5 g. of UO3 inside. From the right end, it will enter a mixture of H2/N2gases with a flow of 2.5 L/min, where hydrogen concentrations are of 0.25 M, 0.5 M and 0.75 M. Hydrogen is stored in a special containment room and its feed rate is controlled with a pressure gauge. The H2/N2 gas mixture is feeded through the upper section, and its composition is controlled with the valves shown in Figure 4. In the right section of the conversion reactor, the UO3 reduction was carried out. The temperature range will be set at 500˚C, 600˚C and 700˚C. The middle section of the reactor was insulated to prevent heat loss from the system. The left section, where calcinations take place, was provided with a resistance, which remains at a constant drying temperature of 400˚C. In the left end, two P2O5 columns capture the water steam generated due to reactions (2) and (3). The results obtained for the reduction kinetics will be determined based on the difference in weight of the columns at the beginning and the end of each experiment. Once each experience was finished, in order to prevent the escape of the residual hydrogen, it is transported outside the P2O5 columns and slowly burned under a special bell, outside the reactor, to prevent accidents during the experience.
Figure 4. Reactor used for work experience.
3. Results and Discussions
The main results for each experiment are shown in Figure 5 and Figure 6.
From Figure 5, it can be seen the influence of temperature on the reduction of UO3 to UO2. Initially, it is expected that the overall kinetic process improve at higher temperatures. However, that is not the case for this work system. The reason for the occurrence of this phenomenon is based on the several uranium oxide compounds formed as intermediate stages and their oxidation states for each one of them.
According to other authors [9] , the first compound of uranium oxide formed from the hydrogen reduction of UO3, U3O8, consists of uranium atoms with different oxidation states: one having the value (+4), while the other two (+6). This means that hydrogen, by reducing the uranium trioxide, form networks of uranium dioxide and uranium trioxide. In the case of U3O8:
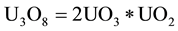 (4)
(4)
As the process go through, the U3O8 reduction continues to form the uranium oxide U3O7. This latter compound has oxidation state (+5) in its structure [10] , which is unstable under these temperature conditions. According to papers concerning touranium conversion [1] , this oxide, at temperatures above 250˚C, undergoes a dismutation reaction, where this compound is oxidized and reduced at the same time, according to Equation (5):
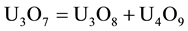 (5)
(5)
For U4O9 compounds, other studies [11] indicate it has 2 uranium ions in oxidation state (+4) and the other 2 in oxidation state (+5). Consequently, because of its instability in these conditions, it will be quickly reduced to UO2. However, oxidation of U3O7 to U3O8 at high temperatures (600˚C - 700˚C), decreases the performance of the overall reaction and forces the system to run for longer times.
In consequence, if the temperature of the system is very high, the oxide U3O7 can again become U3O8, which gradually decrease the rate of conversion of UO3. Therefore, for this process the preferred temperatures are 500˚C - 600˚C.
With respect to the hydrogen concentration in the feed flow of Figure 6, it is possible to conclude that the rate-controlling step is the hydrogen diffusion into the gas-uranium trioxide concentrate interface, because at concentrations of 0.25 M H2, the transformed fraction of UO3 to UO2 reach a value of 80% in about 15 minutes. However, at hydrogen pressures of 0.5 M to 0.75 M, after 5 min of starting the process, the transformed fraction reaches values above 90%.
Considering this issue, it will set the influence of both variables in the kinetics of reduction of UO3 to UO2, along with the unconverted UO3 solid. For this study, based on the results of Figure 5 and Figure 6, the kinetics of reduction of UO3 is determined by Equation (6):
Figure 5. Transformed fraction for reduction from UO3 to UO2.
Figure 6. Influence of the hydrogen pressure in the reduction kinetics of uranium trioxide.
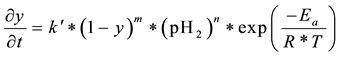 (6)
(6)
where:
 Conversion rate of moles of UO3;
Conversion rate of moles of UO3;
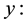 Conversion of UO3;
Conversion of UO3;
m: reaction order regarding to unconverted UO3 fraction;
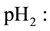 Hydrogen partial pressure;
Hydrogen partial pressure;
n: reaction order regarding to hydrogen partial pressure;
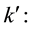 Proportionality constant reaction kinetics;
Proportionality constant reaction kinetics;



The results for the tests of reaction rate studies for UO3 concentrates are shown in Table 1.
4. Results First Stage: Reduction of UO3 to U3O8
According to Table 1, the reaction order with respect to hydrogen is constant, because of the absence of intermediate compounds in the formation of U3O8.
Form Table 2, the value of the activation energy for the first stage is 96,799 kcal/mol. This value shows that the process is highly dependent on temperature.
From Table 3, it is possible to observe that the reaction order for this parameter is negative or very close to zero. Consequently, the reaction kinetics is independent of the fraction of unconverted solid. This is because UO3 concentrate has a high surface area per volume unit, and under these working conditions, these kinds of processes must always be controlled by the diffusion of hydrogen from the boundary layer to the surface of the uranium trioxide.
Accordingly, the equation modeling the reduction kinetics of UO3 to U3O8 for the ideal working range (500˚C - 600˚C) is:

5. Results Second Stage: Reduction of U3O8 to UO2
From Table 4, the value of the activation energy in accordance with this data is 203,693 kcal/mol, indicating that the second stage reduction is much more sensitive to temperature than the first stage.
From Table 5 and Figure 7, it is possible to observe that the reaction order is variable for the hydrogen pressure and the unconverted solid in the working system. This is because, unlike the case of the first stage, the surface available for reaction is variable, because of the presence of the side reaction (5). At greater presence of
Table 1. Reaction order with respect to hydrogen.
Table 2. Values for the determination of the activation energy of the reduction reaction.
Table 3. Reaction order with respect to the unconverted solid.
Figure 7. Reaction order with respect to hydrogen.
Table 4. Activation energy of the reduction reaction.
Table 5. Reaction order with respect to the unconverted solid, second stage.
U3O8, the greater is the dependency on reaction kinetics respect to both parameters.
From the graphs above, we can conclude that the equation that models the rate of reaction of U3O8 for the ideal working range (500˚C - 600˚C) is:

6. Conclusions
・ Reduction of uranium trioxide to uranium dioxide occurs in two stages: in the first one, the UO3 is reduced to U3O8 directly, whereas in the second stage, the U3O8 is reduced to UO2, through various intermediate reactions.
・ At temperatures over 600˚C, the intermediate reaction of dismutation of the U3O7 decreases the overall performance of the global process, which triggers the loss of effective superficial area of reaction. Therefore, the main reaction mechanism is the diffusion of the hydrogen inside of the uranium trioxide.
・ According to the reaction kinetics for the UO3 reduction (Equation (1)), it mainly depends of hydrogen pressure and working temperature. The effect of both variables benefits the diffusion of hydrogen, stimulating the reaction in the surface of the oxide.
・ The rate controlling step for the reduction of U3O8 to UO2 (Equation (8)), depends besides of the unconverted solid, caused by a decrease in active sites, affecting the chemisorption of hydrogen.
・ The optimum operating range of the conversion process is in the range of 500˚C - 600˚C and hydrogen concentrations in gaseous solution of 0.75 M. These parameters allow reaching higher transformed fraction values in less time.
References
- Cordfunke, E.H.P. (1969) The Chemistry of Uranium. Elsevier Publishing Company, Amsterdam.
- Radiochemistry Group of the Royal Science of Chemistry, the Nuclear Fuel Cycle. http://www.rsc.org/images/essay7_tcm18-17769.pdf
- Bonini, A., Cabrejas, J., De Lio, L., Dell’Occhio, L., Devida, C. Dupetit, G., Falcón, M., Gauna, A., Gil, D., Guzmán, G., Neuringer, P., Pascale, A. and Stankevicius, A. (1998) Nuclear Fuel Cycle Head-Enriched Uranium Purification and Conversion into Metal. International Reduced Enrichment for Test Reactor Conference, Sao Paulo.
- Pourbaix, M. (1974) Atlas of Electrochemical Equilibrium in Aqueous Solutions. National Association of Corrosion Engineers.
- Thein, S.M. and Bereolos, P.J. (2000) Thermal Stabilization of 233UO2, 233UO3, and 233U3O8.
- Valdivieso, F., Pijolat, M., Soustelle, M. and Jourde, J. (2000) Reduction of Uranium Oxide U3O8 into Uranium Dioxide UO2 by Ammonia. In: Solymosi, F. and Rask, O., Eds., 14th International Symposium on the Reactivity of Solids, August 2000, Budapest, Elsevier, 117-122, 141-142.
- Fredrickson, J.K., Zachara, J.M., Kennedy, D.W., Liu, C.X., Duff, M.C., Hunter, D.B. and Dohnalkova, A. (2002) Influence of Mn Oxides on the Reduction of Uranium(VI) by the Metal-Reducing Bacterium Shewanella putrefaciens. Geochimica et Cosmochimica Acta, 66, 3247-3262. http://dx.doi.org/10.1016/S0016-7037(02)00928-6
- Duff, M.C., Coughlin, J.U. and Hunter, D.B. (2002) Uranium Co-Precipitation with Iron Oxide Minerals. Geochimica et Cosmochimica Acta, 66, 3533-3547.
- Zhang, F.X., Lang, M., Wang, J.W., Li, W.X., Sun, K., Prakapenka, V. and Erwing, R.C. (2014) High-pressure U3O8 with the Fluorite-Type Structure. Journal of Solid State Chemistry, 213, 110-115.
- Salbu, B., Janssensb, K., Linda, O.C., Proost, K., Gijsels, L. and Danesi, P.R. (2005) Oxidation States of Uranium in Depleted Uranium Particles from Kuwait. Journal of Environmental Radioactivity, 78, 125-135. http://dx.doi.org/10.1016/j.jenvrad.2004.04.001
- Kvashnina, K.O., Butorin, S.M., Martin, P. and Glatzel, P. (2014) The Chemical State of Complex Uranium Oxides. European Synchrotron Radiation Facility, Grenoble.









