Stem Cell Discovery
Vol. 2 No. 3 (2012) , Article ID: 21359 , 8 pages DOI:10.4236/scd.2012.23014
Enrichment of CD9+ spermatogonial stem cells from goat (Capra aegagrus hircus) testis using magnetic microbeads
![]()
1Biochemistry Department, National Dairy Research Institute, Karnal, India; *Corresponding Author: gkndri@gmail.com
2King Edward Memorial Hospital, University of Western Australia, Perth, Australia
Received 17 April 2012; revised 13 May 2012; accepted 15 June 2012
Keywords: SSC; MACS; Testis; CD9+
ABSTRACT
The well documented source for adult multipotent stem cells is spermatogonial stem cells (SSCs) of mammalian testis. It is foundation of spermatogenesis in the testis throughout adult life by balancing self-renewal and differentiation. SSCs isolation from mammalian testis is difficult because of their scarcity and the lack of well characterized cell surface markers. Thus, the isolation of SSCs is of great interest for exploration of spermatogonial physiology and therapeutic approaches for fertility preservation. CD9 is a surface marker expressed in mouse and rat male germline stem cells. In this study, CD9 positive SSCs were successfully isolated from the goat testis using enzymatic digestion followed by three step purification: Differential plating, percoll discontinuous density gradient followed by magnetic activated cell sorting (MACS). Percoll discontinuous density gradient showed significant differences in the percentage of CD9+ SSCs across individual fraction. The fraction 36% and 40% gave the highest percentage of CD9+ SSCs i.e. 82% ± 1.2% and 9.2% ± 1.3% respectively. Magnetic activated cell sorting of CD9+ cells in the magnetic fraction of goat testes was in the range of 15% - 18% which is upto threefolds. CD9+ SSCs were further recovered with appreciable efficiency after immunomagnetic isolation by using various bead: cells ratio in which 4:1 ratio gave the highest yield of 69.06 × 105 with 18% of CD9+ SSCs. Magnetic activated cell sorting using anti-CD9 antibodies provides an efficient and fast approach as compared to conventional approaches such as differential plating and percoll discontinuous density gradient for enrichment strategy for spermatogonial stem cells from goat testes for undertaking research on basic and applied reproductive biology.
1. INTRODUCTION
A great excitement and expectation in today’s biomedical world is the study of stem cells, owning to their ability to exist in an undifferentiated state and transforming into differing tissue types, depending on what the cells ambient are. In recent years, interest in spermatogonial stem cells (SSCs) have grown due to development of new research tools, which allow the isolation and culture of these cells. SSCs originate from the primordial germ cells (PGCs) that have ability to self-renew and differentiate into committed progenitors, thus maintaining spermatogenesis by unipotent stem cell system throughout adult life for sustained male fertility. The SSCs reside in stem cell niches located on the basement membrane of seminiferous tubules and among the basal portions of sertoli cells [1-3]. SSCs are very unique among other adult stem cells because they can transmit the parental genetic information to subsequent generation. The ability to study SSCs biology has been difficult because of their rarity in the testes and very limited availability of unique phenotypic markers. Several surface protein markers are commonly found on stem cells such as ITGA6 (also known as α6-integrin), ITGB1 (also known as β1-integrin) [4] and CD9 [5]. These markers may facilitate interactions between stem cells and their cognate niches [6]. CD9 is a type III membrane protein having four transmembrane domain and involved in many cellular process such as cell adhesion, migration, proliferation and fusion [7-11]. Flow cytometry and immunohistochemical technique revealed the expression of CD9 on mouse and rat testis cells. These cells were selected with the help of anti-CD9 antibody which resulted in an enrichment of spermatogonial stem cells from intact testis cells by 5 to 7 folds [5]. Success of SSC boilogy study depends on successful obtaining of enough amounts of these cells. The availability of isolation and culture technique will undoubtedly pave the way for innovative research into stem cell biology, leading to further breakthrough in the understanding of spermatogonial physiology and development of powerful tools. Recently magnetic micro beads connected with these markers have been used to enrich SSCs, as the method employed is rapid and effective. These techniques can facilitate the harvesting, culture, cryopreservation or transfection of SSC to preserve the male germ potential and to colonize in the testes of recipient [12,13]. These stem cells can provide a lifetime gamete production in the testis of recipient throughout adult life by balancing self-renewal and differentiation. This can be used as an invaluable tool in modifying the male germ line to generate transgenic animals, to restore male fertility for infertile man and for generation of pluripotent stem cells to differentiate into various cell lineages. Therefore, this present study was designed to enrich and sort goat spermatogonial stem cells (SSCs) by immunomagnetic microbeads, as a first step towards the manipulation and their further studies.
2. MATERIALS AND METHODS
2.1. Collection of Goat Spermatogonial Cell
Testis of normal and healthy goat (5 - 8 month) was collected from slaughter house maintained at karnal, Haryana India. Immediately after slaughter, testis was washed in PBS solution supplemented with 100 IU/ml Penicillin and 100 µg/ml streptomycin. Testis was stored in ice cold PBS and transported to the laboratory. The testis was again washed in sterile PBS containing antibiotic and dissection was done aseptically under laminar airflow hood. Spermatogonial cells (Donor cell) were collected from seminiferous tubule of the testis.
2.2. Donor Cell Preparation
Donor spermatogonial cell from goat testis was collected by following the protocol of Honaramooz et al., 2002 with some minor modifications [14]. In brief, the tunica albuginea and visible connective tissue of testis were aseptically removed. The exposed seminiferous tubules were sequentially digested. First digestion was done by incubating tissue at 30˚C in 2 mg/ml Collagenase type IV (Sigma chemical Co., USA, #D5758) in Dulbecco’s minimum essential medium (DMEM) for 30 min with intermittent agitation. Further tissues were digested with 1 mg/ml Hyaluronidase (Sigma Chemical Co., USA, #D5758) for 20 min at 37˚C followed by rinsing tissue two times in Ca2+ free Dulbecco’s phosphate buffer saline (DPBS). The tissues were further digested with trypsin at the concentration of 2.5 mg/ml in DPBS. Finally the cells were treated with 7 mg/ml DNaseI (Sigma chemical Co., USA, #D5758) followed by addition of 10% fetal bovine serum (FBS) to terminate enzyme digestion. The resulting cell suspension was filtered subsequently through two nylon mesh with pore size 80 µm and 40 µm, sequentially. The filtrate was centrifuged at 500 ×g for 15 min at 16˚C. The pellet so obtained was resuspended in small volume of DMEM. Cell viability was assessed using 0.2% trypan blue in DMEM.
2.3. Identification of SSC
In the cell suspension, SSCs were identified on the basis of their morphology in phase-contrast microscopy. SSCs are large cells with typical spherical shape and a large nucleus/cytoplasm ratio.
2.4. Enrichment of Spermatogonial Cell
The enrichment of spermatogonial cell was done to eliminate contaminating somatic cells (myoid and sertoli cells) from cell suspension. Enrichment was done by incubating the above cell suspension in DMEM containing 10% FBS for overnight at 37˚C, 5% CO2 and 85% relative humidity. During the incubation the sertoli cell and myoid cell were attached to the wall of culture plate due to its anchorage dependency. On the other hand the spermatogonial cells remain in suspension which was removed carefully using pipette. The collected cell suspension was washed in DMEM and viability assessment was done.
2.5. Enrichment of Spermatogonial Stem Cell by Discontinuous Density Gradient
The spermatogonial stem cells were enriched from above cell suspension by following the method of van Pelt et al., 1996; Morena et al., 1996 using discontinuous percoll gradient technique [15,16]. In brief, percoll was first sterilized by autoclaving and used for preparation of iso-osmotic percoll suspension. Iso-osmotic percoll was prepared by mixing 0.6% BSA, 45 µg/ml DNase in 82.2% percoll in DMEM. A discontinuous density gradient (28%, 30%, 32%, 36%, 40%, 50% and 65%) of percoll was prepared by diluting iso-osmotic percoll in diluting medium with final densities of 1.0513, 1.054, 1.056, 1.058, 1.061, 1.077 and 1.095 respectively. The gradient was made in 15 ml graduated tube by adding 1 ml each of percoll solution with different density in a sequence that highest density percoll solution comes in bottom and that of lowest in the top of the tube. The cell suspension containing 0.6% BSA, 45 µg DNase in DMEM was layered on the top of the above gradient and centrifuged at 800 ×g for 30 min at 18˚C. The cells were found in the interface between the different density percoll solution were collected and marked as fraction 1 - 8 from top to bottom.
2.6. Enrichment of SSC(s) with Magnetic Beads
Spermatogonial stem cells from above fractionated cells were done by using antibody coated magnetic beads [17]. In this method, cluster of differentiation-9 (CD-9) surface protein was used as marker of spermatogonial stem cell [5]. The paramagnetic beads (Calbiochem) were labelled with anti-CD9 antibody (Miltenyi Biotech) using following the protocol of Cristea et al., 2005 [18]. For magnetic sorting of SSC, percoll fractionated cell suspension (1 - 8) was split to two aliquot. One aliquot was mixed with anti-CD9-labelled magnetic bead in a ration of 1:6 bead per target cell and incubated for 1 hr at 38˚C. Another aliquot was used as control. The sorting was done by passing this cell suspension in the steel wool column under magnetic field. For this, the steel wool column was washed in PBS. The non-specific binding sites in the steel wool column were blocked by incubating column in 5% BSA in PBS for 60 min. The column was then flushed with ice-cold PBS containing 1% BSA. Loaded cells were applied to the column under magnetic field. The column was then rinsed three times by 500 µl of PBS containing 1% BSA, under magnetic field. CD9+ cells carry magnetic bead remain in the matrix of the column as long as it is maintained in the magnetic field. The CD9+ cells were finally eluted by removing magnetic field and by rinsing column with PBS with 1% BSA, the collected fraction was designate as sorted fraction. Mature spermatozoa was prepared by same way and used as negative control. The entire sorted fraction obtained after magnetic sorting were analysed in phase contrast microscope under 200× magnification. The number of unsorted, sorted and depleted cells was also counted.
2.7. Immunocharacterisation of SSC
For immunocharacterisation, the sorted fraction was centrifuged at 500 ×g for 15 min. The pellet containing cells were immunostained with anti-CD9 antibody conjugated with phycoerythrin for 1 hr at room temperature [19]. After incubation the cells were rinsed first in PBS with 1% BSA, then PBS only and finally PBS with 1% BSA. The slides were then analysed in a fluorescent microscope under blue filter at 400× magnifications.
2.8. Statistical Analysis
The results are presented as means ± SEM and statistical analysis was performed. Differences were considered significant when the P-value was < 0.05.
3. RESULTS
3.1. Cytological Analysis of Spermatogonial Stem Cell
The phase-contrast microscopy was used to identify spermatogonial stem cells in all the fraction on the basis of SSCs morphology. The unsorted fraction consisted of a heterogeneous single-cell fraction. The magnetic fraction (sorted fraction) consisted of relatively homogenous population of cells (Figure 1). whereas the depleted fraction was very much similar to that of the unsorted cell fraction.
3.2. Enrichment of SSCs by Differential Plating
Enzymatic digestion of testicular tissue was done with collagenase in combination with trypsin and hyaluronidase. Sequential enzymatic digestion of the decapsulated testis resulted in a single-cell suspension. After filtration of the cell suspension final cell concentration was found in the range of 5 - 8 × 106 cells/g testicular tissue and a mean of 85.5 × 106 cells were isolated from a single testis. The cell suspension was checked for CD9+ spermatogonial stem cells and found to be 5.8 ± 1.09 percent. Enrichment of cells by differential plating resulted significant (P < 0.05) increase in CD9+ cells (6.9 ± 1.2, Figure 2). Viability of the cells were observed using trypan blue and found to be 73 ± 5.1 percent.
3.3. Enrichment of Spermatogonial Stem Cell by Percoll Discontinuous Density Gradient
Second phase of enrichment of spermatogonial stem cells was done by using a gradient of percoll ranges from
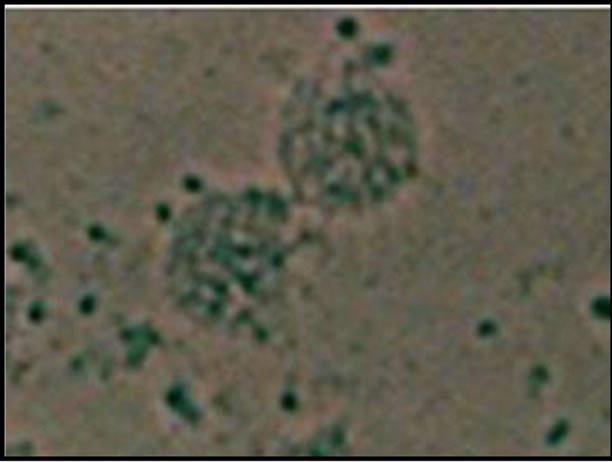
Figure 1. Phase-contrast micrograph showing a homogeneous cell population aggregated with magnetic beads forming large to small clusters in the magnetically sorted faction.

Figure 2. Enrichment of spermatogonia by differential plating method. Number of CD9+ cells slightly enhanced by differenttial plating then the control. Values reported are the mean ± SEM.
28% to 65%. For enrichment gradients were made, cells from differential plating was applied on top and centrifuged. At 28% enrichment of SSC was found to be minimum (0.8 ± 0.22) among all gradient. With increase in percentage of percoll in gradient, the number of CD9+ cells increased significantly upto 36% percoll and decreased gradually upto 65% (Figure 3). Enrichment of CD9+ cells were found to be significantly (P < 0.05) higher in 36% and 40% percoll compared to other density gradient. Statistically non significant (P > 0.05) increase in CD9+ cells were recorded at 40% (9.2 ± 1.30) compared to 36% (8.2 ± 1.2). The results indicate that significant differences in the percentage of CD9+ SSCs population in individual fraction (Figure 3). Viability of cells in all the fractions were observed and found to be almost similar (70 ± 3.1).
3.4. Enrichment of Specific Fraction of Percoll Discontinuous Density Gradient by Immunomagnetic Beads
Cell suspension of 36% and 40% fraction were further enriched by immunomagnetic beads tagged with anti-CD9 antibody, showing significantly higher percentage of CD9+ cells in sorted fraction (36%: 17.05 ± 0.5; 40%: 18.2 ± 1.2) compared to unsorted (36%: 8.2 ± 1.2; 40%: 9.2 ± 1.1) and depleted fraction (36%: 4.5 ± 1.02; 40%: 4.4 ± 1.08) in both 36% and 40% percoll gradient. Percentage of CD9+ cells in sorted fraction was statistically non significant (P > 0.05) in both 36% and 40% percoll gradient (Figure 4). Viability of cells in all the fractions were observed and found to be almost similar (68 ± 5.1).
3.5. Effect of Bead:Cell Ratio on Cell Recovery
Although SSCs represent only 4% of the cells in the testis, these were recovered with appreciable efficiency and purity using a target beads:cell ratio of 4:1 (Figure 5). Total number of cells isolated at 4:1 ratio of bead: cells were 69.06 × 105 with 18% of CD9+ cells. If a lower number of beads, 2:1, were used, the recovery of the total cell isolated were 41.43 × 105 that contain 14% of CD9+ SSCs. When the bead:cell ratio was increased to 6:1, a total of 70.68 × 105 cells were isolated but the percentage of CD9+ spermatogonial stem cells recovery was not increased rather its percentage decreased to 8%, as more non target cells were trapped in cell-bead aggregates.
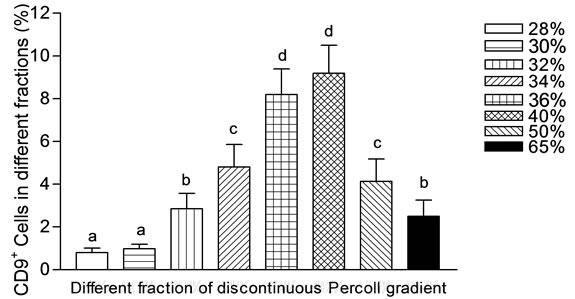
Figure 3. Number of CD9+ cells in different fractions of percoll discontinuous density gradient. The 36% and 40% fraction of the gradient contained the highest percentage of SSCs. Fraction 28% and 30% of the gradient contained the lowest percentage of SSCs. Values reported are the mean ± SEM. Mean showing different letters are significantly different at (P < 0.05).
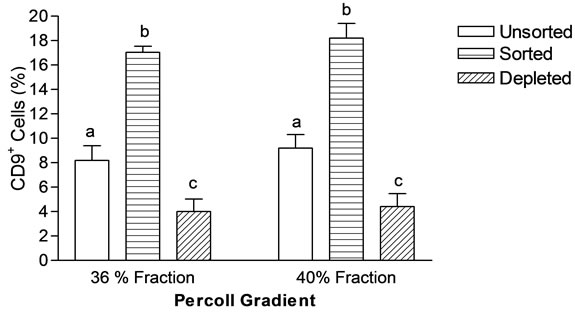
Figure 4. Magnetic sorting of specific fraction of percoll gradient (36% and 40%). Sorted fractions contain more number of CD9+ SSCs in comparison to unsorted fraction. Values are Mean ± SEM of three experiments. Mean showing different letters are significantly different at (P < 0.05).

Figure 5. Spermatogonial stem cells were isolated at various bead:cell ratios. The optimal ratio was 4:1 at which the yield of SSCs was highest.
3.6. Immunological Characterization of the Isolated Cell Fractions
The live cells in unsorted, sorted and depleted fractions were characterized using antibody directed against CD9 surface protein by immuno microscopy which revealed that the unsorted fraction contained 5.8% ± 0.66% CD9+ cells. The sorted fraction contained 18.2% ± 1.2% CD9+ cells, indicating three fold enrichment from unsorted fraction (Figure 6). The depleted fraction had no significant (P > 0.05) depletion of CD9+ cells as compared to the unsorted fraction.
4. DISCUSSION
The isolation of undifferentiated SSCs from mammalian testicular tissue will expand the knowledge of male fertility and aid in developing technologies to enhance reproductive efficiency along with further exploration of SSC characteristics and mechanisms involved in cell fate decisions between self-renewal and differentiation. The conventional techniques that were used in recent years for spermatogonial cell enrichment was either elutriation [20] or velocity sedimentation in a BSA gradient at unit gravity [21,22]. Out of the large population of differentiating germ cells within seminiferous tubules, only a small population of spermatogonia resides at the basement membrane of the adult and fully active seminiferous tubule. The isolation of this small spermatogonial population is technically challenging because of their small number, so magnetic beads are specifically useful for their isolation including stem cells from various tissues [23-25] and organ such as bone marrow, muscle and liver [26-28]. Many molecular markers have been used to identify and study undifferentiated spermatogonia and gonocytes such as promyelocytic leukemia zinc finger protein (PLZF) in rodents, non human primates and pigs [29-32], ubiquitin carboxyl esterase L1 (UCHL1) in the bull [33] and boar [32,34,35], VASA in many species
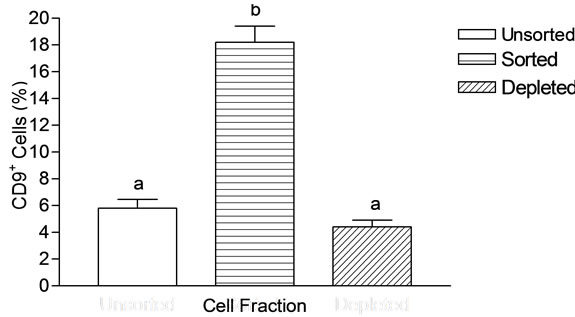
Figure 6. Bar chart of the number of CD9+ cells in the cell fractions obtained after magnetic separation using anti-CD9 antibody conjugated magnetic beads. Cells were isolated from goat testis. The results are shown as mean ± SEM. The unsorted fraction and depleted fraction consisted of low CD9+ cells. The sorted fraction showed high level of CD9+ cells.
including bulls [36], boars [37], primates [31] & mice [38], CD9 in mouse and rat [5]. The sorting efficiency of intact cells mainly rely on the availability of specific or particular surface markers on the membrane of stem cells. CD9 acts as a surface marker on the spermatogonial stem cells as several literatures reveals its presence [4,5]. Zou et al. reported enrichment of female germline stem cells using short-type pituitary gland and brain-cadherin (Stpbc), CD9 and interferon inducible transmembrane protein 3 (Iftm3, Fragilis) [39]. However, spermatogonial stem cells can be enriched by selection with an antibody against CD9 of these cell surface molecules. Magnetic cell separation has been described as a successful tool for enrichment of SSCs [40,41].
In the Present study, we sought to isolate enriched spermatogonial stem cell from goat testis. Our results clearly demonstrated that three step purification viz differential plating, percoll discontinuous density gradient followed by magnetic activated cell sorting (MACS) not only decontaminated mature spermatids, spermatozoa and other somatic cell but also substantially enriched the pool of proliferative SSCs from the enzymatic digested heterogeneous testicular cell population. Spermatogonial stem cells were enriched by conventional method of differential plating leading to 7% purification of CD9+ cells by eliminating the somatic cells (myoid and sertoli cells). This is in correlation with PLZF-positive cells of ovine testis study [42]. Thus, suggesting that efficiency of isolation and/or enrichment of cells appears to depend on the maturity of the testis, as at maturity CD9+ cells seem to be abundant [42]. Further an enrichment of SSCs by using discontinuous percoll gradients were performed and showed maximum enrichment at 36% and 40% fraction which was 8% and 9% respectively. Our result is very much consistent with other enrichment studies such as Polychromatin erythroblasts (PCE) from rat bone marrow [43] and Cardiomyocytes derived from human embryonic stem cells [44] in which percentage of PCE and cardiomyocytes were highest at (1.040/1.058 = 36%) and (40.5%/58.5% and within 58.5%) percoll fraction respectively. The maximum enriched percoll fraction namely 36% and 40% fraction were further enriched by immunomagnetic beads, showing significantly (P < 0.05) high SSCs enrichment in sorted fraction as compared to unsorted and depleted fraction. Thus, giving an overall enrichment upto 15% - 18% which is in correlation with other study in which spermatogonial cells were enriched upto 25% - 54% when normal testes from Djungarian hamsters, mice and marmoset monkeys were used [45]. The magnetic separation of these two fractions (36% and 40%) of percoll gradient did not show much more enrichment of CD9+ SSCs in comparison to magnetic separation after enzymatic digestion, which indicates that the efficiency of recovery is independent of the number of SSCs in the starting material but was dependent on the ratio of magnetic beads to SSCs. Hence, SSCs were recovered with appreciable efficiency and purity using a target bead cell ratio of 4:1. If a lower number of beads, 2:1, was used, the recovery decreased significantly. When the bead:cell ratio was increased to 6:1, the CD9+ SSCs recovery was not increased, but more non target cells were trapped in cell-bead aggregates. This is consistent with rat mast cell isolation studies where the optimal bead:cell ratio was 3:1 which gave the highest yield and purity of mast cell [19]. We therefore calculated and characterized the unsorted, sorted and depleted fractions indicating that the CD9+ cells were enriched upto three fold as compared to the presorted fraction and the depleted fraction is widely identical to the unsorted fraction because it contains approximately 98% of all the isolated cells and approximately 90% of the CD9+ cells that were present in the unsorted total cell suspension. This is in correlation with enrichment studies of GFRα1 + spermatogonia from adult primate testes where monkey and human testicular cells enriched GFRα1 + cells were threefold and fivefold respectively [46]. Although the procedure to isolate and to enrich SSCs takes about 5 - 6 hr, the viability of the cells is still high ranging between 65% - 70%. This makes these cells suitable for analysis of mRNA expression and protein synthesis and can also be used for culture, preservation and transplantation.
In this study it has been shown that spermatogonial stem cells were enriched upto 15% - 18% when testes from goats were used. The efficiency of separation is probably determined by the binding affinity of the antibody. Magnetic cell sorting is specifically useful for separation of a few cells from a larger number of unwanted cells in the cell preparation [47-49]. Magnetic cell sorting allows the separation of rare target cells with frequencies down to 1 in 1 × 108. These methodological features render this approach appropriate for the isolation of spermatogonia from mature testes. The availability of isolation and enrichment technique would help in studying underlying molecular mechanisms that regulate germ cell development, mitotic proliferation and differentiation of stem cells, meiosis and their regulation in a vertebrate. Additionally, these tools could provide a novel avenue for genetic modification of the male germline and subsequent generation of transgenic livestock with favourable traits such as disease resistance and production of meat or milk containing components beneficial for human consumption. This method will enable the preparation of enriched spermatogonial suspensions for exploration of physiology, reproductive medicine and therapeutic approaches for fertility preservation.
5. ACKNOWLEDGEMENTS
The authors are grateful to Indian Council of Agricultural Research for providing funds for this piece of work.
REFERENCES
- Creemers, L.B., den Ouden, K., van Pelt, A.M.M. and de Rooij, D.G. (2002) Maintenance of adult mouse type A spermatogonia in vitro: Influence of serum and growth factors and comparison with prepubertal spermatogonial cell culture. Reproduction, 124, 791-799. doi:10.1530/rep.0.1240791
- Creemers, L.B., Meng, X., den Ouden, K., van Pelt, A.M.M., Izadyar, F., Santoro, M., Sariola, H. and de Rooij, D.G. (2002) Transplantation of germ cells from glial cell line-derived neurotrophic factor-overexpressing mice to host testes depleted of endogenous spermatogenesis by fractionated irradiation. Biology of Reproduction, 66, 1579-1584. doi:10.1095/biolreprod66.6.1579
- Nagano, M., Ryu, B., Brinster, C.J., Avarbock, M.R. and Brinster, R.L. (2003) Maintenance of mouse male germ line stem cell in vitro. Biology of Reproduction, 68, 2207-2214. doi:10.1095/biolreprod.102.014050
- Shinohara, T., Avarbock, M.R. and Brinster, R.L. (1999) beta1- and alpha-6-integrin are surface markers on mouse spermatogonial stem cells. Proceedings of the National Academy of Sciences of the United States of America, 96, 5504-5509. doi:10.1073/pnas.96.10.5504
- Kanatsu-Shinohara, M., Toyokuni, S. and Shinohara, T. (2004) CD9 is a surface marker on mouse and rat germ line stem cells. Biology of Reproduction, 70, 70-75. doi:10.1095/biolreprod.103.020867
- Oka, M., Tagoku, K., Russell, T.L., Nakano, Y., Hamazaki, T., Meyer, E.M., Yokota, T. and Terada, N. (2002) CD9 is associated with leukemia inhibitory factor-mediated maintenance of embryonic stem cells. Molecular Biology of the Cell, 13, 1274-1281. doi:10.1091/mbc.02-01-0600
- Ikeyama, S., Koyama, M., Yamaoko, M., Sasada, R. and Miyake, M. (1993) Suppression of cell motility and metastasis by transfection with human motility-related protein (MRP/CD9) DNA. The Journal of Experimental Medicine, 177, 1231-1237. doi:10.1084/jem.177.5.1231
- Masellis-Smith, A. and Shaw, A.R. (1994) CD9-regulated adhesion: Anti-CD9 monoclonal antibody induces pre-B cell adhesion to bone marrow fibroblasts through de novo recognition of fibronectin. Journal of Immunology, 152, 2768-2777.
- Hadjiargyrou, M. and Patterson, P.H. (1995) An anti-CD9 monoclonal antibody promotes adhesion and induces proliferation of Schwann cells in vitro. The Journal of Neuroscience, 15, 574-583.
- Maecker, H.T., Todd, S.C. and Levy, S. (1997) The tetraspanin superfamily: Molecular facilitators. Federation of American Societies for Experimental Biology, 11, 428-442.
- Tachibana, I. and Hemler, M.E. (1999) Role of transmembrane 4 superfamily (TM4SF) proteins CD9 and CD81 in muscle cell fusion and myotube maintenance. The Journal of Cell Biology, 146, 893-904. doi:10.1083/jcb.146.4.893
- Avarbock, M., Brinster, C. and Brinster, R. (1996) Reconstitution of spermatogenesis from frozen spermatogonial stem cells. Nature Medicine, 2, 693-696. doi:10.1038/nm0696-693
- Nagano, M. and Brinster, R.L. (1998) Spermatogonial transplantation and reconstitution of donor cell spermatogenesis in recipient mice. Acta Pathologica Microbiologica et Immunologica Scandinavica, 106, 47-57. doi:10.1111/j.1699-0463.1998.tb01318.x
- Honaramooz, A., Megee, S. and Dobrinski, I. (2002) Germ cell transplantation in pig. Biology of Reproduction, 66, 21-28. doi:10.1095/biolreprod66.1.21
- van Pelt, A., Morena, A., van Dissel-Emiliani, F., Boitani, C., Gaemers, I., de Rooij, D. and Stefanini, M. (1996) Isolation of the synchronized A spermatogonia from adult vitamin A: Deficient rat testes. Biology of Reproduction, 55, 439-444. doi:10.1095/biolreprod55.2.439
- Morena, A., Boitani, C., Pesce, M., Felici, M. and Stefanini, M. (1996) Isolation of highly purified type A spermatogonia from prepubertal rat testis. Journal of Andrology, 17, 708-717.
- Miltenyi, S., Muller, W., Weichel, W. and Radbruch, A. (1990) High gradient magnetic cell separation with MACS. Cytometry, 11, 231-238. doi:10.1002/cyto.990110203
- Cristea, I.M., Williams, R., Chait, B.T. and Rout, M.P. (2005) Fluorescent proteins as proteomic probes. Molecular and Cellular Proteomics, 4, 1933-1941. doi:10.1074/mcp.M500227-MCP200
- Jamur, M.C., Grodzki, A.C.G., Moreno, A.N., Swaim, W.D., Siraganian, R.P. and Oliver, C. (1997) Immunomagnetic isolation of rat bone marrow-derived and peritoneal mast cells. The Journal of Histochemistry and Cytochemistry, 45, 1715-1722. doi:10.1177/002215549704501215
- Bucci, L.R., Brock, W.A., Johnson, T.S. and Meistrich, M.L. (1986) Isolation and biochemical studies of enriched populations of spermatogonia and early primary spermatocytes from rat testes. Biology of Reproduction, 34, 195-206. doi:10.1095/biolreprod34.1.195
- Bellve, A.R., Cavicchia, J.C., Millette, C.F., O’Brien, D.A., Bhatnagar, Y.M. and Dym, M. (1977) Spermatogenic cells of the prepuberal mouse: Isolation and morphological characterization. The Journal of Cell Biology, 74, 68-85. doi:10.1083/jcb.74.1.68
- Dym, M., Jia, M., Dirami, G., Price, J.M., Rabin, S.J., Mocchetti, I. and Ravindranath, N. (1995) Expression of c-kit receptor and its autophosphorylation in immature rat type A: Spermatogonia. Biology of Reproduction, 52, 8-19. doi:10.1095/biolreprod52.1.8
- Cammareri, P., Lombardo, Y., Francipane, M.G., Bonventre, S., Todaro, M. and Stassi, G. (2008) Isolation and culture of colon cancer stem cells. Methods in Cell Biology, 86, 311-324. doi:10.1016/S0091-679X(08)00014-9
- Liu, X.L., Yuan, J.Y., Zhang, J.W., Zhang, X.H. and Wang, R.X. (2007) Differential gene expression in human hematopoietic stem cells specified toward erythroid, megakaryocytic, and granulocytic lineage. Journal of Leukocyte Biology, 82, 986-1002. doi:10.1189/jlb.0107014
- Zhang, J., Duan, X., Zhang, H., Deng, Z., Zhou, Z., Wen, N., Smith, A.J., Zhao, W. and Jin, Y. (2006) Isolation of neural crest-derived stem cells from rat embryonic mandibular processes. Biology of the Cell, 98, 567-575. doi:10.1042/BC20060012
- Gangopadhyay, N.N., Shen, H., Landreneau, R., Luketich, J.D. and Schuchert, M.J. (2004) Isolation and tracking of a rare lymphoid progenitor cell which facilitates bone marrow transplantation in mice. Journal of Immunological Methods, 292, 73-81. doi:10.1016/j.jim.2004.06.015
- Le Grand, F., Auda-Boucher, G., Levitsky, D., Rouaud, T., Fontaine-Perus, J. and Gardahaut, M.F. (2004) Endothelial cells within embryonic skeletal muscles: A potential source of myogenic progenitors. Experimental Cell Research, 301, 232-241. doi:10.1016/j.yexcr.2004.07.028
- Qin, A.L., Zhou, X.Q., Zhang, W., Yu, H. and Xie, Q. (2004) Characterization and enrichment of hepatic progenitor cells in adult rat liver. World Journal of Gastroenterology, 10, 1480-1486.
- Buaas, F.W., Kirsh, A.L., Sharma, M., McLean, D.J., Morris, J.L., Griswold, M.D., de Rooij, D.G. and Braun, R.E. (2004) PLZF is required in adult male germ cells for stem cell self-renewal. Nature Genetics, 36, 647-652. doi:10.1038/ng1366
- Costoya, J.A., Hobbs, R.M., Barna, M., Cattoretti, G., Manova, K., Sukhwani, M., Orwig, K.E., Wolgemuth, D.J. and Pandolfi, P.P. (2004) Essential role of PLZF inmaintenance of spermatogonial stem cells. Nature Genetics, 36, 551-553. doi:10.1038/ng1367
- Hermann, B.P., Sukhwani, M., Lin, C.C., Sheng, Y., Tomko, J., Rodriguez, M., Shuttleworth, J.J., McFarland, D., Hobbs, R.M., Pandolfi, P.P., Schatten, G.P. and Orwig, K.E. (2007) Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells, 25, 2330-2338. doi:10.1634/stemcells.2007-0143
- Luo, J., Megee, S. and Dobrinski, I. (2009) Asymmetric distribution of UCH-L1 in spermatogonia is associated with maintenance and differentiation of spermatogonial stem cells. Journal of Cellular Physiology, 220, 460-468. doi:10.1002/jcp.21789
- Herrid, M., Davey, R.J. and Hill, J.R. (2007) Characterization of germ cells from pre-pubertal bull calves in preparation for germ cell transplantation. Cell and Tissue Research, 330, 321-329. doi:10.1007/s00441-007-0445-z
- Frankenhuis, M.T., Kramer, M.F. and de Rooij, D.G. (1982) Spermatogenesis in the boar. The Veterinary Quarterly, 4, 57-61. doi:10.1080/01652176.1982.9693840
- Luo, J., Megee, S., Rathi, R. and Dobrinski, I. (2006) Protein gene product 9.5 is a spermatogonia-specific marker in the pig testis: Application to enrichment and culture of porcine spermatogonia. Molecular Reproduction and Development, 73, 1531-1540. doi:10.1002/mrd.20529
- Bartholomew, R.A. and Parks, J.E. (2007) Identification, localization, and sequencing of fetal bovine vasa homolog. Animal Reproduction Science, 101, 241-251. doi:10.1016/j.anireprosci.2006.09.017
- Lee, G.S., Kim, H.S., Lee, S.H., Kang, M.S., Kim, D.Y., Lee, C.K., Kang, S.K., Lee, B.C. and Hwang, W.S. (2005) Characterization of pig vasa homolog gene and specific expression in germ cell lineage. Molecular Reproduction and Development, 72, 320-328. doi:10.1002/mrd.20320
- Toyooka, Y., Tsuenekawa, N., Takahashi, Y., Matsui, Y., Satoh, M. and Noce, T. (2000) Expression and intercellular localization of mouse vasa-homologue protein during germ cell development. Mechanisms of Development, 93, 139-149. doi:10.1016/S0925-4773(00)00283-5
- Zou, K., Hou, L., Sun, K., Xie, W. and Wu, J. (2011) Improved efficiency of female germline stem cell purification using fragilis-based magnetic bead sorting. Stem Cells and Development, 20, 2197-2204.
- Hofmann, M.C., Braydich-Stolle, L. and Dym, M. (2005) Isolation of male germ-line stem cells; influence of GDNF. Developmental Biology, 279, 114-124. doi:10.1016/j.ydbio.2004.12.006
- Kubota, H., Avarbock, M.R. and Brinster, R.L. (2004) Culture conditions and single growth factors affect fate determination of mouse spermatogonial stem cells. Biology of Reproduction, 71, 722-731. doi:10.1095/biolreprod.104.029207
- Borjigin, U., Davey, R., Hutton, K. and Herrid, M. (2010) Expression of promyelocytic leukaemia zinc-finger in ovine testis and its application in evaluating the enrichment efficiency of differential plating. Reproduction Fertility and Development, 22, 733-742. doi:10.1071/RD09237
- Asano, H., Deguchi, Y., Kawamura, S. and Inaba, M. (2011) A simple method for enrichment of polychromatic erythroblasts from rat bone marrow, and their proliferation and maturation in vitro. The Journal of Toxicological Sciences, 435, 435-444. doi:10.2131/jts.36.435
- Xu, C., Police, S., Rao, N. and Carpenter M.K. (2002) Characterization & enrichment of cardiomyocytes derived from human embryonic stem cells. Circulation Research, 91, 501-508. doi:10.1161/01.RES.0000035254.80718.91
- von Schonfeldt, V., Krishnamurthy, H., Foppiani, L. and Schlatt, S. (1999) Magnetic cell sorting is a fast & effective method of enriching viable spermatogonia from djungarian hamster, mouse, and marmoset monkey testes. Biology of Reproduction, 61, 582-589. doi:10.1095/biolreprod61.3.582
- Gassei, K., Ehmcke, J., Dhir, R. and Schlatt, S. (2010) Magnetic activated cell sorting allows isolation of spermatogonia from adult primate testes and reveals distinct GFRα1-positive subpopulations in men. Journal of Medical Primatology, 39, 83-91. doi:10.1111/j.1600-0684.2009.00397.x
- Semple, J.W., Allen, D., Chang, W., Castaldi, P. and Freedman, J. (1993) Rapid separation of CD4+ and CD19+ lymphocyte populations from human peripheral blood by a magnetic activated cell sorter (MACS). Cytometry, 14, 955-960. doi:10.1002/cyto.990140816
- Büsch, J., Huber, P., Pflüger, E., Miltenyi, S., Holtz, J. and Radbruch, A. (1994) Enrichment of fetal cells from maternal blood by high gradient magnetic cell sorting (double MACS) for PCR-based genetic analysis. Prenatal Diagnosis, 14, 1129-1140. doi:10.1002/pd.1970141206
- Schmitz, B., Radbruch, A., Kümmel, T., Wickenhauser, C., Korb, H., Hansmann, M.L., Thiele, J. and Fischer, R. (1994) Magnetic activated cell sorting (MACS)—A new immunomagnetic method for megakaryocytic cell isolation: Comparison of different separation techniques. European Journal of Haematology, 52, 267-275. doi:10.1111/j.1600-0609.1994.tb00095.x

