American Journal of Molecular Biology
Vol.4 No.3(2014), Article ID:47867,9 pages
DOI:10.4236/ajmb.2014.43012
Leptin Causes the Early Inhibition of Glycolysisand TCA Cycle-Related Genes in the Brain of Ob/Ob Mice to Restore Fertility
Carlos Fernandes Baptista1,2, Samuel Marcos Ribeiro de Noronha3, Maria de Nazareth Gamboa Ritto1,2, Eduardo Henrique da Silva Freitas1,4, Melquíades Pereira Júnior1, Mauro Abi Haidar1, Ismael Dale Cotrim Guerreiro da Silva1, Silvana Aparecida Alves Corrêa de Noronha3, Marisa Teresinha Patriarca1
1Gynecology Department, Escola Paulista de Medicina/Universidade Federal de São Paulo (EPM-UNIFESP), São Paulo, Brazil
2Departmento de Cirurgia Geral e Especializada, Universidade Federal do Rio de Janeiro (UNIRIO), Rio de Janeiro, Brazil
3Surgery Department, Escola Paulista de Medicina/Universidade Federal de São Paulo (EPM-UNIFESP), São Paulo, Brazil
4Departmento de Medicina Geral, Universidade Federal do Rio de Janeiro (UNIRIO), Rio de Janeiro, Brazil
Email: labgineco@globo.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 28 April 2014; revised 27 May 2014; accepted 26 June 2014
ABSTRACT
Introduction: Polycystic ovarian syndrome (PCOS) is undoubtedly the commonest androgen disorder in woman’s fertile period and certainly one of the most prevalent causes of anovulation. The syndrome has an estimated prevalence of 4% - 10% among women of childbearing age. Previously, our group demonstrated the effect of gonadal white adipose tissue transplantation from wild-type lean and fertile female mice to isogenic obese anovulatory ob/ob mice. These complex metabolic interrelationships between obesity and PCOS have yet to be fully understood. The aim of this study was to evaluate the effect of gonadal white adipose tissue (WAT) transplantation from the wildtype lean and fertile female mice to isogenic obese, anovulatory mice (Lep ob/Lep ob) on the expression of glycolysisand TCA cycle-related genes and obtain a general view of the glucose metabolism in the brain of these animals. Methods: Fifteen ob/ob mice ranging from 2 to 3 months of age were divided into 3 experimental groups: control normal weight (n = 5), obese control (n = 5) and obese 7 days leptin treated (n = 5). The whole brains of the mice were processed for RNA extraction. The samples from each group were used to perform PCR assays using an array plate containing 84 primers to study the glucose metabolism-related genes. Results: The glycolysisand TCA cycle-related genes were significantly downregulated. The most significantly affected genes were as follows: for glycolysis (fold regulation with p < 0.05): Pgm1, Bpgm, Aldob, and Eno3 (119, 45, 18, and 28 times less, respectively); and for the TCA cycle (fold regulation with p < 0.05): Cs, Idh3b, and Mdh2 (84, 27, and 37 times less, respectively). Conclusion: The seven-day leptin treated mice show a decrease in the glucose metabolism. These results confirm the ability of the adipose tissue-derived hormone leptin to regulate early crucial genes that are related to glycolysis mechanisms and to the TCA cycle. This hormone seems to revert early the central physiological conditions that are associated with PCOS; however, the morphological alterations can only be observed within a 45-day treatment.
Keywords:PCOS, Obesity, Leptin, Glycolysis, TCA Cycle, Gene Expression

1. Introduction
Polycystic ovarian syndrome (PCOS) is undoubtedly the commonest androgen disorder during a woman’s fertile period and certainly one of the most prevalent causes of anovulation [1] -[4] . PCOS is the classic example of the loss of functional cyclicity rhythm associated with anomalous feed-back or, as it is also known as inappropriate feed-back [5] . In this case, the excessive production of androgens and their subsequent extra glandular conversion into estrogens form the pathophysiological basis for chronic anovulation [6] -[9] . Additionally, the excessive androgen production can be explained by extraand intra-ovarian factors [10] . This syndrome has an estimated prevalence of 4% - 10% among women of childbearing age [11] -[14] and is remarkable for the heterogeneity of its symptoms, reflected by the presence or by the absence of insulin resistance (IR) among these women, as well as, by individual differences regarding the magnitude of the symptoms that are observed in women that are affected by the syndrome [14] -[16] .
The clinical manifestations of PCOS include classic menstrual disorders, obesity, adrenal enzyme deficiencies, hirsutism, metabolic syndrome, diabetes, infertility, and hyperandrogenism, which may also be associated with insulin resistance [13] [17] -[19] . A significant number of women with PCOS have an abnormally high IR when compared to those of matched controls. Moreover, PCOS can be considered the initial manifestation of metabolic syndrome [20] .
In fact, PCOS is often associated with obesity in women. Obesity is a worldwide problem that threatens the lives of adults, adolescents and children. Obesity has many associated comorbidities, such as the well-known components of metabolic syndrome (MetS), which harms the health of men and women [21] .
Obesity is associated with several metabolic disorders and has reproductive consequences that are complex and not well understood [22] . Adipose tissue produces leptin, which has dominated the literature on female fertility complications, but other adipokines, such as adiponectin and resistin, seem to be important as well, as our understanding of their biological functions improves. Leptin influences the developing embryo and the functioning of the ovary and of the endometrium and modulates the release and the activity of gonadotropins and of the hormones that control their synthesis (inhibin, GnRH and kisspeptins). The biological actions and the potential roles of the adipokines leptin, adiponectin and resistin are frequently studied in the context of female fertility and its interplay with the complexity of the obese metabolic state [23] .
Until recently, PCOS has been recognized as a hyperandrogenic disorder originating from hypothalamic pituitary gonadotropin secretion or ovarian dysfunction [24] . Lately, however, at least some cases of PCOS have been regarded as disorders of metabolic origin that impair reproduction [24] . Because obesity, particularly in the abdominal region, is found in approximately 50% of women with PCOS and appears in mid-childhood and increases during puberty [25] , excess adiposity has generated a great deal of attention. Furthermore, the clinical phenotype and the development of PCOS are thought to be reinforced by obesity. Additionally, PCOS also has a genetic component, and different genetic polymorphisms have also been linked to the syndrome or its associated disorders [26] . Altogether, these associations highlight the importance of disentangling the relationship between obesity and PCOS and in particular their common metabolic disorders.
Leptin, which is a product of the ob gene, is a major hormone that is secreted by white adipose tissue (WAT) and is involved in the regulation of body weight, glucose metabolism, and fertility. Leptin null mice (Lep ob/ Lep ob) are obese, hyperphagic, insulin resistant, and sterile. In addition, ob/ob females are invariably sterile, and few ob/ob males have been reported to occasionally reproduce. These ob/ob mice develop many pathological features that are common to human obesity, which is also marked by disturbances of reproductive function [27] .
Therefore, the complex metabolic interrelationships between obesity and PCOS have yet to be fully understood. The action of leptin in the brains of ob/ob mice seems to regulate early two key metabolic biological processes, glycolysis and the TCA cycle, especially in those nervous cells that express leptin receptors. Curiously, these receptors are not expressed by GnRH-releasing neurons. Leptin controls GnRH release by acting in other hypothalamic nuclei. Leptin regulates kiss1 neurons, making of these neurons probable targets for the hormonal control of the interaction between nutrition and reproduction [28] . Aiming to better understand these complex neuronal networks, the aim of this study was to evaluate the effect of gonadal WAT transplantation from wildtype lean and fertile female mice to isogenic obese, anovulatory mice (ob/ob) on the expression of glycolysisand TCA cycle-related genes and to obtain a general view of glucose metabolism in the brains of these animals.
2. Methodologies
2.1. Experimental Animals and Surgical Procedure
In this study, ten transgenic obese and anovulatory leptin-deficient mice (B6.V-Lep ob/J, designated as ob/ob mice) and five isogenic lean ovulatory littermates (wild-type) were obtained from the Center for the Development of Experimental Models, Federal University of São Paulo, Brazil (CEDEME). These animals were maintained in a temperature-controlled environment at approximately 24˚C under a 12/12-h light-dark cycle and were handled at least once a week. Five ob/ob mice received a white adipose tissue (WAT) transplant as described by Gavrilova and Marcus-Samuels et al. (2000) [29] and Pereira et al. (2011) [9] : the adipose gonad tissue samples that were obtained from the wild-type mice were placed in phosphate buffer solution (PBS) and fragmented into small pieces. The WAT grafts were implanted in the subcutaneous tissue through small, shaved skin incisions on the dorsal region of the animal, which was anesthetized with isoflurane. The brains of all of the animals were removed by the reported procedure and kept in liquid nitrogen until use.
2.2. Experimental Groups
The animals that were described in the previous section were divided into three experimental groups as follows:
2.2.1. Control Group (CG)
Five (5) normal-weight B6.V-Lep ob/J mice at two to three months of age and ovulatory cycles.
2.2.2. Obese Group (OG)
Five (5) ob/ob mice at two to three months of age and anovulatory cycles.
2.2.3. 7-Day Transplanted Mice Group (7dTM)
Five (5) ob/ob mice at two to three months of age and anovulatory cycles were implanted with adipose gonadal tissue from mice with ovulatory cycles. These animals were sacrificed seven (7) days after the surgical procedure.The template is used to format your paper and style the text. All margins, column widths, line spaces, and text fonts are prescribed; please do not alter them. You may note peculiarities. For example, the head margin in this template measures proportionately more than is customary. This measurement and others are deliberate, using specifications that anticipate your paper as one part of the entire journals, and not as an independent document. Please do not revise any of the current designations.
2.3. RNA Extraction
After using liquid nitrogen for cryogenic soaking, the tissues were homogenized in TrizolTM reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. After the complete dissociation of the nucleoprotein complexes, phase separation was achieved with chloroform and centrifugation. The precipitated RNA from the aqueous phase was washed with 75% ethanol. The RNA was dried and dissolved in RNase-free water. The total RNA was then purified with the Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA) and submitted to DNase treatment. The amount and quality of the extracted RNA were assessed by spectrophotometry using NanoDrop v3.3.0 (NanoDrop Technologies Inc., Rockland, DE).
2.4. QPCR
The total RNA (1.0 μg) per plate/array from each experimental group pool was used to synthesize the cDNA. The samples were treated with buffers from the kit, and reverse transcription reactions were performed using the RT2 First Strand Kit from SA Biosciences (Qiagen Company) according to the manufacturer’s protocol. The qPCR array was performed using the RT2 ProfilerTM PCR array of SA Biosciences
(http://www.sabiosciences.com/ArrayList.php). For each experimental group, 51 genes (29 for the TCA cycleand 22 for the glycolysis-related genes) were examined in triplicate (PAHS-006). The amplification, data acquisition and analysis curves were performed on an ABI Prism 7500 Fast Sequence Detection System (Applied Biosystems, Foster City, CA). In turn, each gene was checked for the efficiency and the minimum and maximum threshold curve pattern. To ensure accurate comparisons between the curves, the same threshold was established for every gene. Three genes were used for normalization (Hsp90ab1, Gapdh and Actb), and the average qC values were used to standardize the gene expression (2-CT change table) and to determine the difference between the groups. To consider a gene differentially expressed, we used a differential cut-off of two-fold (up or downregulated).
2.5. Histology
For the histological evaluation of all of the study groups, the uteri were obtained and processed immediately after euthanasia. The tissues were fixed in 4% formalin in saline, embedded in paraffin, and cut to a thickness of 5 mm. The sections were deparaffinized, rehydrated, stained with hematoxylin/eosin (H.E.), and evaluated under light microscopy.
2.6. Statistical Analysis
The p values were calculated based on Student’s t-test of the three replicate 2(−Δct) values for each gene in the control and treatment groups, and p values less than 0.05 were considered significant. The qPCR reactions were processed through the online software RT2 ProfilerTM PCR Array Data Analysis (SA Biosciences).
2.7. Ethics
The procedures were performed in accordance with the ethical standards of the institution and national guidelines for the care and use of laboratory animals. The study protocol for the use of laboratory animals in research was approved by the local ethics committee (CEP/UNIFESP, number 0017/12).
3. Results
In general, we observed a marked downregulation of the glycolysisand TCA cycle-related genes in the brain of 7dTM mice compared to those of the CG mice and a marked upregulation of these same groups of genes in the brains of the non-treated ob/ob mice (OG) compared to those of the CG mice. In the brains of the 7dTM mice, the most significantly downregulated genes were Pgm1, Bpgm, Aldob and Eno3, which were downregulated 119, 45, 18, and 28 times, respectively, for the glycolysis-related genes (fold regulation with p < 0.05), and Cs, Idh3b, and Mdh2, which were 84, 27, and 37 times, respectively, for the TCA cycle-related genes (fold regulation with p < 0.05). Curiously, Pcx was the only upregulated gene and was upregulated 27 times (fold regulation with p < 0.05). In contrast, the endometrial and ovarian morphologies could only be observed after a 45-day period.
To better describe the gene expression profiles that were obtained for each functional group of genes (glycolysis and TCA cycle) and in each experimental group comparison (7dTM with CG and OG with CG), the glycolysisand TCA cycle-related genes are discussed separately in the following two distinct subsections.
3.1. 7dTM versus CG
Glycolysis—Seventeen out of the twenty-two (77%) glycolysis-related genes were differentially expressed in the 7dTM group, using the CG group as a calibrator. All of these seventeen genes were downregulated, and none were upregulated. The downregulated genes included aldolases a, b and c; Bpgm; Enolases 1, 2 and 3; Gpi1; Pfkl; Pgam2; Pgk1; Pgms1, 2 and 3; Pklr; and Tpi1. Among the non-differentially expressed genes, Gapdhs and Hk3 were detected in both of the groups (7dTM and CG), while Gck and Hk2 were only detected in the CG group, and Pgk2 could not be detected in any of the groups. The fold-regulation values of each assessed glycolysis-related gene can be observed in Table1
Table 1. Fold-regulation values that were obtained for the glycolysisand TCA cycle-related genes in two different comparisons, 7dTM and OG, using the CG group as a calibrator. The data were processed with the online program Data AssistTM SA Biosciences (Qiagen Company). nde = non-differentially expressed.
TCA Cycle—Twenty out of the twenty-nine (67%) TCA cycle-related genes were differentially expressed in the 7dTM group, using the CG group as a calibrator. Nineteen of these genes were downregulated, and only one was upregulated. The downregulated genes included Aco1; Cs; Dlat; Dld; Dlst; Idh3a; Idhg; Mdhs 1, 1b and 2; Suclg1; Ogdh; Pcks 1 and 2; Pdhb; Sdhs a, b and c; Sucla2; and Suclg1. As mentioned earlier, Pcx was the only upregulated gene. Among the nine non-differentially expressed genes, Acly; Aco2; Idhs 1, 2 and 3b; Pdha1; Sdhd; and Suclg2 were detected in both of the groups (7dTM and CG), while Fh1 could only be detected in the CG. The fold-regulation values of each assessed TCA cycle-related gene can be observed in Table1
3.2. OG versus CG
Glycolysis—Sixteen out of the twenty-two (72%) glycolysis-related genes were differentially expressed in the OG group, using the CG group as a calibrator. All of these sixteen genes were upregulated, and none were downregulated. The upregulated genes included aldolases b and c; Bpgm; Enolases 1, 2 and 3; Gck; Gpi1; hexokinases 2 and 3; Pfkl; Pgam2; Pgk1; Pgms2 and 3; and Tpi1. Among the non-differentially expressed genes, Aldoa, Galm, Gapdhs, Pgm1 and Pklr were detected in both of the groups (OG and CG), while Pgk2 could not be detected in any of these groups. The fold-regulation values of each assessed glycolysis-related gene can be observed in Table1
TCA Cycle—Eighteen out of the twenty-nine (62%) TCA cycle-related genes were differentially expressed in the OG group, using the CG group as a calibrator. All of these eighteen genes were upregulated, and none were downregulated. The upregulated genes included Acly; Aco1; Aco2; Cs; Dlst; Fh1; Idh1; Idh3a; Idh3b; Mdhs 1b and 2; Pck2; Pdha1; Pdhb; Sdhs a and b; Sucla2; and Suclg2. All of the eleven non-differentially expressed genes (Dlat; Dld; Idhs 2 and 3g; Mdh1; Ogdh; Pck1; Pcx; Sdhc; Sdhd; and Suclg1) were detected in both of the groups (OG and CG). The fold-regulation values of each assessed TCA cycle-related gene can be observed in Table1
3.3. Histomorphology of the Uterus
With respect to the histology of the uteri, after a 7-day treatment period, the endometrium was similar to that of the OG group, with an absence of glandular tissue (Figure 1). However, Pereira et al., 2011 demonstrated that after a 45-day treatment period, these mice presented an endometrium similar to that of the CG group, with the presence of numerous leukocytes in the endometrium, signaling the restoration of hormonal control and the surface epithelial renewal that are typical of the estrous phase (Figure 1).
4. Discussion
We believe that investigating the molecular changes underlying the fertility restoration of leptin-treated ob/ob mice is crucial to better understanding the intracellular pathways controlling the interplay between metabolism and the female sexual cycle. With this in mind, we observed a lack of studies on glucose-related gene alterations in the brain of leptin-induced fertile ob/ob mice and decided to assess the expression of glycolysisand TCA cycle-related genes in these animals.
It is largely known that leptin plays a primary role in regulating homeostasis, obesity and fertility [23] [28] . Moreover, in a previous work, Pereira et al. (2009) clearly demonstrated that WAT transplantation decreases insulin resistance and restores fertility in female ob/ob mice [9] . Behind these physiological and morphological changes lay complex molecular interactions that deserve to be better understood.
As such, the aim of this work was to investigate the central effects of leptin on glucose metabolism accompanying neuropeptide Y inhibition and the restoration of fertility in the same animal model that was used by Pereira et al. [9] . Additionally, this work seems to be a pioneer in presenting an overall profile of glycollysisand TCA cycle-related gene expression in the brains of leptin-treated ob/ob mice.
Indicating the weak points, the results shown herein are restricted to mRNA quantifications and whole brain extracts, instead of specific brain nuclei, and were used to assess early central actions of leptin. However, as mentioned earlier, there is a lack of studies of these genes in the brains of leptin-treated ob/ob mice. In addition, we believe that differential gene expression will reflect specific adipokine hormone actions in leptin receptor-expressing cells, such as kiss1 neurons.
Leptin deficiency, which was observed in the ob/ob mice, caused obesity and led these animals to develop metabolic disorders such as insulin resistance and type II diabetes, as well as hypertension and infertility [27] - [29] . We do not know exactly in which cells in the brains of these 7-day leptin-treated mice have altered metabolic processes, but our results demonstrate an important early downregulation of glycolysisand TCA cy-
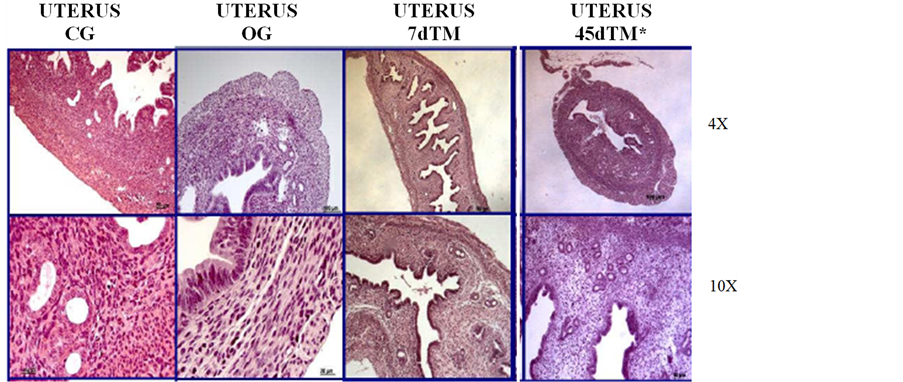
Figure 1. Histomorphological features of the uterus of animals from each experimental group at two different magnifications (4× and 10×). The 45dTM mice were obtained by Pereira et al. (2011) [9] .
cle-related genesin the brains of ob/ob mice caused by this adipokine.
Khan et al., 1998 have demonstrated through biochemical assays (glucose-6-phosphatase activity) that glucose cycling is increased in the brains of ob/ob mice [30] . Our gene expression results show the same, that is, except for Pcx, all of the other glycolysis-related genes assessed herein are upregulated in these mice, in contrast to the 7dTM and OG mice. Therefore, after 1 week of leptin treatment, there seems to be a marked reduction in the activity of these genes. We believe that among the early central neuronal circuitary changes caused by leptin and that will eventually lead to fertility restoration after a 45-day treatment, glucose cycling reduction, most likely in the hypothalamus, seems to be critical.
In general, similar to the profile that was observed for the glycolysis-related genes, the TCA cycle-related genes displayed a general downregulation in the 7dTM group compared to those of the CG group. However, these genes are upregulated in the brains of the OG mice compared to those in the CG mice. This profile matches previous reports, in which untreated ob/ob mice exhibited increased hepatic and central B-oxidation [31] [32] , especially in the hypothalamic Arcuate and Ventromedial nuclei [33] .
5. Conclusion
Overall, the data presented here indicate an early decrease in the central glucose metabolism after leptin treat ment. These results confirm the ability of the adipose tissue-derived hormone leptin to regulate crucial genes that are related to glycolysis mechanisms and to the TCA cycle. In summary, this hormone seems to revert early the central physiological conditions that are associated with PCOS in the central nervous system; however, the morphological alterations that are associated with fertility in the peripheral tissues can only be observed within a 45-day treatment. The extrapolation of these results to patients with metabolic syndrome must await further investigation.
References
- Edman, C.D., Aiman, E.J., Porter, J.C. and MacDonald, P.C. (1978) Identification of the Estrogen Product of Extraglandular Aromatization of Plasma Androstenedione. American Journal of Obstetrics and Gynecology, 130, 439-447.
- Edman, C.D. and MacDonald, P.C. (1978) Effect of Obesity on Conversion of Plasma Androstenedione to Estrone in Ovulatory and Anovulator Young Women. American Journal of Obstetrics and Gynecology, 130, 456-461.
- Ehrmann, D.A. (2005) Polycystic Ovary Syndrome. The New England Journal of Medicine, 352, 1223-1236. http://dx.doi.org/10.1056/NEJMra041536
- Pasquali, R. and Gambineri, A. (2006) Polycystic Ovary Syndrome: A Multifaceted Disease from Adolescence to Adult Age. The New York Academy of Sciences, 1092, 158-174. http://dx.doi.org/10.1196/annals.1365.014
- Goldzieher, J.W. and Green, J.A. (1962) The Polycystic Ovary. I. Clinical and Histologic Features. The Journal of Clinical Endocrinology and Metabolism, 22, 325-338. http://dx.doi.org/10.1210/jcem-22-3-325
- Vigil, P., Contreras, P., Alvarado, J.L., Godoy, A., Salgado, A.M. and Cortes, M.E. (2007) Evidence of Subpopulations with Different Levels of Insulin Resistance in Women with Polycystic Ovary Syndrome. Human Reproduction, 22, 2974-2980. http://dx.doi.org/10.1093/humrep/dem302
- Ingalls, A.M., Dickie, M.M. and Snell, G.D. (1950) Obese, a New Mutation in the House Mouse. Journal of Heredity, 41, 317-318.
- Jones, N. and Harrison, G.A. (1957) Genetically Determined Obesity and Sterility in the Mouse. In: Harrison, R.G., Ed., Studies on Fertility, Blackwell Scientific, Oxford, 51-64.
- Pereira Jr., M., Vidotti, D.B., Borra, R.C., Simões Mde, J., Da Silva, I.D. and Haidar, M.A. (2011) Involvement of GDF-9, Leptin, and IGF1 Receptors Associated with Adipose Tissue Transplantation on Fertility Restoration in Obese Anovulatory Mice. Gynecological Endocrinology, 27, 759-766. http://dx.doi.org/10.3109/09513590.2010.534330
- Vilmann, L.S., Thisted, E., Baker, J.L. and Holm, J.C. (2012) Development of Obesity and Polycystic Ovary Syndrome in Adolescents. Hormone Research in Paediatrics, 78, 269-278. http://dx.doi.org/10.1159/000345310
- Buggs, C. and Rosenfield, R. (2005) Polycystic Ovary Syndrome in Adolescence. Endocrinology and Metabolism Clinics of North America, 34, 677-705. http://dx.doi.org/10.1016/j.ecl.2005.04.005
- Yildiz, B.O. and Azziz, R. (2010) Ovarian and Adipose Tissue Dysfunction in Polycystic Ovary Syndrome: Report of the 4th Special Scientific Meeting of the Androgen Excess and PCOS Society. Fertility and Sterility, 94, 690-693. http://dx.doi.org/10.1016/j.fertnstert.2009.03.058
- Azziz, R., Woods, K.S., Reyna, R., Key, T.J., Knochenhauer, E.S. and Yildiz, B.O. (2004) The Prevalence and Features of the Polycystic Ovary Syndrome in an Unselected Population. The Journal of Clinical Endocrinology and Metabolism, 89, 2745-2749. http://dx.doi.org/10.1210/jc.2003-032046
- Lecke, S.B., Mattei, F., Morsch, D.M. and Spritzer, P.M. (2011) Abdominal Subcutaneous Fat Gene Expression and Circulating Levels of Leptin and Adiponectin in Polycystic Ovary Syndrome. Fertility and Sterility, 95, 2044-2049. http://dx.doi.org/10.1016/j.fertnstert.2011.02.041
- Glueck, C.J., Morrison, J.A., Daniels, S., Wang, P. and Stroop, D. (2011) Sex Hormone-Binding Globulin, Oligomenorrhea, Polycystic Ovary Syndrome, and Childhood Insulin at Age 14 Years Predict Metabolic Syndrome and Class III Obesity at Age 24 Years. The Journal of Pediatrics, 159, 308-313.
- Nair, M.K., Pappachan, P., Balakrishnan, S., Leena, M.L., George, B. and Russell, P.S. (2012) Menstrual Irregularity and Poly Cystic Ovarian Syndrome among Adolescent Girls—A 2 Year Follow-Up Study. The Indian Journal of Pediatrics, 79, S69-S73.
- de Zegher, F., Lopez-Bermejo, A. and Ibáñez, L. (2009) Adipose Tissue Expandability and the Early Origins of PCOS. Trends in Endocrinology Metaboilism, 20, 418-423. http://dx.doi.org/10.1016/j.tem.2009.06.003
- Hickey, M., Doherty, D.A., Atkinson, H., Sloboda, D.M., Franks, S., Norman, R.J., et al. (2011) Clinical, Ultrasound and Biochemical Features of Polycystic Ovary Syndrome in Adolescents: Implications for Diagnosis. Human Reproduction, 26, 1469-1477. http://dx.doi.org/10.1016/j.tem.2009.06.003
- Reinehr, T., de Sousa, G., Roth, C.L. and Andler, W. (2005) Androgens before and after Weight Loss in Obese Children. The Journal of Clinical Endocrinology & Metabolism, 90, 5588-5595. http://dx.doi.org/10.1210/jc.2005-0438
- Zhang, Y., Proenca, R., Maffei, M., Barone, M., Leopold, L. and Friedman, J.M. (1994) Positional Cloning of the Mouse Obese Gene and Its Human Homologue. Nature, 372, 425-432. http://dx.doi.org/10.1038/372425a0
- Mantzoros, C.S. (2001) The Role of Leptin and Hypothalamic Neuropeptides in Energy Homeostasis: Update on Leptin in Obesity. Growth Hormone and IGF Research, 11, S85-S89. http://dx.doi.org/10.1016/S1096-6374(01)80014-9
- Ceddia, R.B., Koistinen, H.A., Zierath, J.R. and Sweeney, G. (2002) Analysis of Paradoxical Observations on the Association between Leptin and Insulin Resistance. The FASEB Journal, 16, 1163-1176. http://dx.doi.org/10.1096/fj.02-0158rev
- Moschos, S., Chan, J.L. and Mantzoros, C.S. (2002) Leptin and Reproduction: A Review. Fertility and Sterility, 77, 433-444. http://dx.doi.org/10.1016/S0015-0282(01)03010-2
- Coleman, D.L. (1978) Obese and Diabetes: Two Mutant Genes Causing Diabetes-Obesity Syndromes in Mice. Diabetologia, 14, 141-148. http://dx.doi.org/10.1007/BF00429772
- Hummel, K.P., Dickie, M.M. and Coleman, D.L. (1966) Diabetes, a New Mutation in the Mouse. Science, 153, 1127-1128. http://dx.doi.org/10.1126/science.153.3740.1127
- Kashima, K., Yahata, T., Fujita, K. and Tanaka, K. (2013) Polycystic Ovary Syndrome: Association of a C/T Single Nucleotide Polymorphism at Tyrosine Kinase Domain of Insulin Receptor Gene with Pathogenesis among Lean Japanese Women. Journal of Reproductive Medicine, 58, 491-496.
- Burcelin, R., Thorens, B., Glauser, M., Gaillard, R.C. and Pralong, F.P. (2003) FP Gonadotropin-Releasing Hormone Secretion from Hypothalamic Neurons: Stimulation by Insulin and Potentiation by Leptin. Endocrinology, 144, 4484-4491. http://dx.doi.org/10.1210/en.2003-0457
- Lake, J.K., Power, C. and Cole, T.J. (1997) Women’s Reproductive Health: The Role of Body Mass Index in Early and Adult Life. International Journal of Obesity and Related Metabolic Disorders, 21, 432-438. http://dx.doi.org/10.1038/sj.ijo.0800424
- Gavrilova, O., Marcus-Samuels, B., Graham, D., Kim, J.K., Shulman, G.I., Castle, A.L., Vinson, C., Eckhaus, M., Reitman, M.L. (2000) Surgical Implantation of Adipose Tissue Reverses Diabetes in Lipoatrophic Mice. Journal of Clinical Investigation, 105, 271-278.
- Khan, A., Zong-Chao, L., Efendic, S. and Landau, B.R. (1998) Glucose-6-Phosphatase Activity in the Hypothalamus of ob/ob Mice. Metabolism, Clinical and Experimental, 47, 627-629.
- Hartz, A.J., Barboriak, P.N, Wong, A., Katayama, K.P. and Rimm, A.A. (1979) The Association of Obesity with Infertility and Related Menstural Abnormalities in Women. International Journal of Obesity, 3, 57-73.
- Brady, L.J., Silverstein, L.J., Hoppel, C.L. and Brady, P.S. (1985) Hepatic Mitochondrial Inner Membrane Properties and Carnitine Palmitoyltransferase A and B. Effect of Diabetes and Starvation. Biochemical Journal, 232, 445-450.
- Delgado, T.C., Violante, I.R., Nieto-Charques, L. and Cerdan, S. (2011) Neuroglial Metabolic Compartmentation Underlying Leptin Deficiency in the Obese ob/ob Mice as Detected by Magnetic Resonance Imaging and Spectroscopy Methods. Journal of Cerebral Blood Flow & Metabolism, 31, 2257-2266. http://dx.doi.org/10.1038/jcbfm.2011.134


