American Journal of Molecular Biology
Vol.2 No.2(2012), Article ID:18944,7 pages DOI:10.4236/ajmb.2012.22011
The effects of astragalus polysaccharide on zebrafish cell apoptosis and senescence
![]()
1Department of Biology, Tonghua Normal University, Tonghua City, China
2Department of Life Science, North-East Normal University, Changchun City, China
3Department of Marine and Science, Life Ocean Uinversity of China, Qingdao City, China
Email: *qingguangx@163.com
Received 24 October 2011; revised 8 November 2011; accepted 4 December 2011
Keywords: Astragalus; Polysaccharide; Zebrafish; Cell Proliferation; Senescence
ABSTRACT
Astragalus polysaccharide (AP) is the extraction of astragalus, which is a plant used in traditional Chinese herb medicine and may increase an orgainism’s resistance to stress. Several earlier studies in vitro have indicated that AP has anti-aging activities, however the mechanism underlyling these activities was unclear and remained to be elucidated. In this study, Using the zebrafish (Danio rerio), we evaluated molecular mechanism of the effect of AP on zebrafish growth, development and apoptosis. 30 zebrafish embryos (24 hours post fertilization (hpf)) were exposed to varying concentrations of AP (from 0.125 mg/ml to 0.5 mg/ml) continuously for 3 days. The results of β-galactosidase (SA-β-gal) and acridine orange fluorescence showed that AP can delay zebrafish embryos apoptosis under the concentration of 0.125 mg/ml. In addition, the differential gene expression of AP treated zebrafish embryos was examined by RT-PCR analysis. We found that the gene expression of mdm2 and tert were up-regulated while bax, p21 and p53 gene expression were down-regulated during early apoptosis of the zebrafish embryos mediated by AP. These results demonstrated that AP may play a role during the induction of senescence and this function might by p53-mediated pathway.
1. INTRODUCTION
Chronic oxidative stress has been shown to reduce lifespan in many sopecies and lead to accelerated aging [1- 3]. Thus, it is understandable that many suppose that understanding the aging process might play a majore role in the development of therapies to slow or alleviate agerelated diseases, and cognitive disorders. The elucidation of the genetic mechanism regulating aging and senescence will greatly enhance our understanding of some of the most fundamental properties of higher organisms.
With the advent of successful genetic interventions in aging among model organisms over the past 3 decades, pathways regulating aging and potential molecular targets for interventions have been identified [4]. On cell level there are two kinds of senescence, replicative senescence and premature senescence (SIPS), different types of intrinsic and extrinsic stress signals are likely to converge on the activation of the p53 protein, the Rb protein, or both. In this manner, these two key tumor suppressor proteins might act as integrators of stress signals, and their combined level of activation would determine the onset of senescence [5-8].
Medical herb has recently become attractive as health beneficial foods and as a source material for drug development [9]. Current studies suggest that development of anti-aging drugs from Chinese herbs may be one of the possible interventions [10,11]. Crude extractions or fractions from edible antioxidant sources may help prevent or alleviate many reactive oxygen species (ROS) related diseases [12,13]. Oriental herbal medicine has been widely investigated for drug development because it has fewer side effects [14].
Astragalus is an important invigorating medicine in traditional Chinese medicine, their use dates back more than 2000 years, and are recorded in Shen Nong’s Materia Medica. Astragalus polysaccharide (AP) is the extraction of astragalus. Modern pharmacology indicated that AP has anti-decrepit effect in brain and heart tissues in mice [15,16]. To access more fundamental biology of aging and longevity in higher complex organism such as vertebrate, it is desirable to expand the range of vertebrate model systems for laboratory studies.
Zebrafish are teleosts of the cyprinid family the class of ray-finned fish and are strong conservation with humans, which makes it an excellent model organism for studying complex biological processes, such as angiogenesis, senescence, and toxicity response [17,18]. And unlike costly and laborious mouse toxicity assays, or cell-based assays that do not exhibit metabolic responses, zebrafish bioassays permit easy evaluation of drug effects on growth and development [19]. However, few studies to date have investigated the gerontology of this organism, which has great potential to give insight into organismal aging and associated diseases common to vertebrates [20-22]. Future studies that uncover the fundamental timing senescence can take advantage of the genetic approaches and fundamental genomics that are possible in zebrafish. Moreover, rapid increases in zebrafish resources will greatly assist such studies, but will necessitate core aging research in this animal such as that undertaken in our current research. In our studies, we performed experiments to elucidating the characteristics of AP on zebrafish during embryos development to explore baseline information of normal senescence at their onset. We followed various senescence-associated processes in our experiments that pertained to cell proliferation, senescence and some gene that might be affected by age or other senescence-inducing stresses expression.
2. MATERIALS AND METHOD
2.1. Reagents
Purified astragalus polysaccharide (AP) bought from Shanxi Undersum Biomedtech Co., Ltd, China. Polysaccharide from a membranaeceus was prepared by the method of Wang et al. [23], the dried samples (100 g) were ground to fine powder and put in 1.5 liter of boiling water and decocted for 2 h by a traditional method for Chinese medicinal herbs. The decoction was left to cool at room temperature, filtered and then freeze-dried to obtain crude polysaccharides were refluxed three times to remove lipids with 150 ml of chloroform: methanol solvent (2:1) (v/v). After filtering the residues were airdried. The result product was extracted three times in 300 ml of hot water (100˚C) and then filtered. The combined filtrate was precipitated using 150 ml of 95% ethanol, 100% ethanol and acetone, respectively. After filtering and centrifuging, the precipitate was collected and vacuum-dried, giving desire polysaccharide (13 g).
The content of the polysaccharide was measured by phenol sulfuric method [24]. Result showed that the content of the polysaccharide in the extract may reach 98.61%.
2.2. Embryos Handling and Treatment
Zebrafish embryos were generated by natural pairwise mating as described by Westerfied. 8-hour embryos were distributed into 24-well microplate (MILLIPORE Co., Bedford, MA), 30 embryos were exposed to varying concentrations of AP (from 0.125 mg/ml to 0.5 mg/ml) continuously for 3 days, the compounds were renewed daily. Each series of dilutions was repeated three times and the standard deviation calculated for each treatment.
2.3. SA-β-Gal Assay and Quantitation
Zebrafish embryos were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) at 4˚C overnight. After fixation, the fish samples were washed four times in PBS, and incubated in 37˚C (without CO2) for 12 - 16 h with SA-β-gal staining solution (5 mM postassium ferricyanide, 5 mM postassium ferrocyanide, 2 mM MgCl2, and 1 mg/ml X-gal in PBS at pH 6.0). Quanlitative analysis was done by stereo zoom microscope. The SA-β-gal pixels were selected, filtered and counted, and the net total number of pixels from each image was determined by subtracting both the black/brown melanocyte pixels and the white pixels indicating light reflection from the total number. The filters used were tested against control unstained fish samples to ensure that the pixels filtered accurately represented SA-β-gal staining.
2.4. AO Staining Assay and Quantitation
Zebrafish embryos were immersed in 1 ug/ml AO (acridinium chloride hemi[zinc chloride]) in egg water for 15 - 30 min at 28˚C and rinsed thoroughly 8 times in egg water. Stained embryos were anesthetized with MESAB (0.5 mM 3-aminobenzoic acid ethyl ester, 2 mM Na2HPO4) and mounted in methylcellulose in a depression slide for observation. Embryos without AO staining were used to determine baseline fluorescence. The fluorescence value was expressed as a relative fluorescence units (RFU = fluorescence reading of experimental group minus baseline reading of control group).
2.5. Quantitative RT-PCR
Total RNA was extracted from 72 hpf embryos using TRIZOL reagent. Quantitative RT-PCR was carried out on 200 ng RNA using the LightCycler RNA Amplication kit SYBR Green in a LighrCycler 2.0 instrument, following manufacturer’s protocols. The primers used are shown in the reference [25]. The samples were quantified by comparative cycle threshold method for relative quantification of gene expression, normalized to β-actin. All experiments were performed at least three independent experiments for each RNA preparation.
2.6. Statistical Analyses
Statistical in figure are expressed as mean ± S.D. (n = 30) and differences between groups were assessed by analysis of variance (ANOVA) and Student t-test. Difference were considered to be statistically significant if P < 0.05. All statistical analyses were carried out using SPSS for Windows, Version 11.5 (SPSS, Chicago, IL).
3. RESULTS
3.1. Zebrafish Embryos Bioassay for Assessing AP Toxicity
HDFs exposed to repeated sublethal stress under t-BHP display the morphological phenotype of senescence. The first studies were based on the description of the successive HDF morphotypes observed during in vitro aging [7,26]. Using this type of classification, it was possible to show that, after treated with AP which has anti-oxidant effect. The treated embryos acquired the morphological features of anti-aging. After following a series concentration of AP treatment, we found that if the concentration higher than 0.5 mg/ml, almost all the embryos were dead, and there is almost no difference among the lower concentration (0.025 mg/ml, 0.05 mg/ml, 0.75 mg/ml and 1.0 mg/ml, data not show), so in our experiment we choose three different concentration of AP, and the results indicate that the growth and development of zebrafish were inhibited at the concentration of 0.5 mg/ml (Figure 1).
3.2. Zebrafish Embryos for Assessing Senescence
3.2.1. SA-β-Gal Staining
Cytochemically and histochemically detectable SA-β-gal at pH 6.0 has been shown to increase during the replactive senescence of cells in culture and in tissue samples [27], and has subsequently been used most widely as a marker of cellular senescence in several vertebrate animal system, both in vivo and in vitro [28-30]. We stained AP treatment embryos and compared to the level of SA- β-gal staining to that non-treatment embryos. A majority of AP treatment embryos showed lighter staining than contrast. (Figures 2(A) and (B)), and this is equivalent to that observed in senescent mass in HDFs with an extended life-span [31].
3.2.2. Acriding Orange (AO) Staining
In fact, cells that are exposed to stress in culture will respond either by entry into senescence, by apoptosis which serves to constrain cell proliferation, or by a transient growth arrest; the choice among these three responses depends on the cell type, the type of stress, and the level of stress. Hence, senescence seems to represent one of several programs that can be activated by the cell when physiologic stress is encountered [32].
Following the results of SA-β-gal staining for cellular senescence, we adopted AO staining to test apoptosis for living cells. Because inappropriate cell death can easily be determined using acriding orange, which selectively labels apoptotic cells in live zebrafish embryos without complicated processing. Moreover, acridine orange can be extracted from whole embryos and quantitated using a fluorescence microplate reader [33]. The results indicate that like SA-β-gal staining, AP reduced apoptosis during embryos development (Figure 2(C)). But there is no significant difference among the treatment (data is not shown).
3.3. Zebrafish Embryos for Assessing Some Relevant Gene Expression during Senescence
The apoptoic processes in zebrafish and mammals are similar. The likelihood that the apoptosis machinery is conserved in zebrafish is supported by the identification of many apoptosis genes. Different types of intrinsic and extrinsic stress signals are likely to converge on the activation of the p53 protein, the Rb protein, or both [8]. To further investigate p53-dependent transcriptional responses in the treated zebrafish embryos, we examined the expression of p53 targeted genes using RT-PCR after 3-days AP treated embryos. These p53-response genes included p21, based on its central involvement in cellcycle arrest; bax, for its role in the apoptosis pathway; and p53 and mdm2 to explore the feedback loops down-
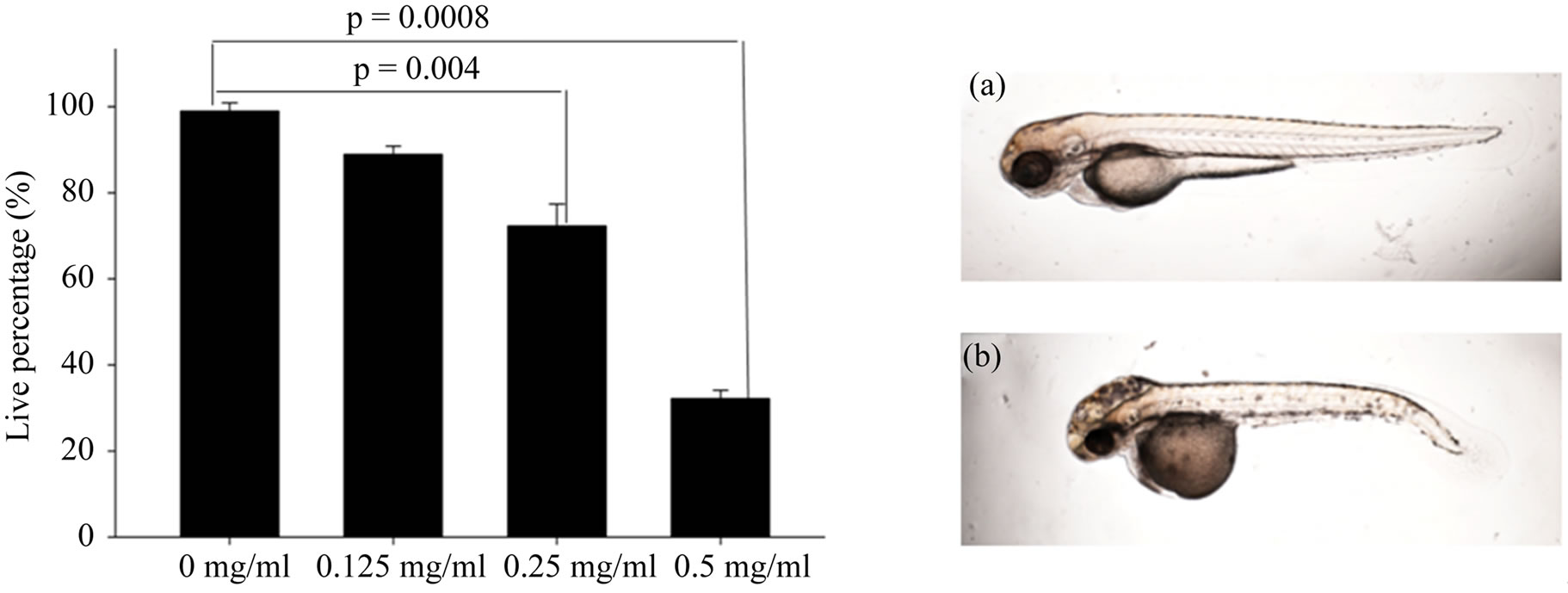
Figure 1. The effect of AP on zebrafish embryos growth and development at 24 hpf. Data represent the mean ± SD (n = 30). Zebrafish embryos development is normal at lower concentration of AP (from 0.125 mg/ml to 0.25 mg/ml) (a); while at high concentration of AP, the development of zebrafish embryos becomes abnormal (b).
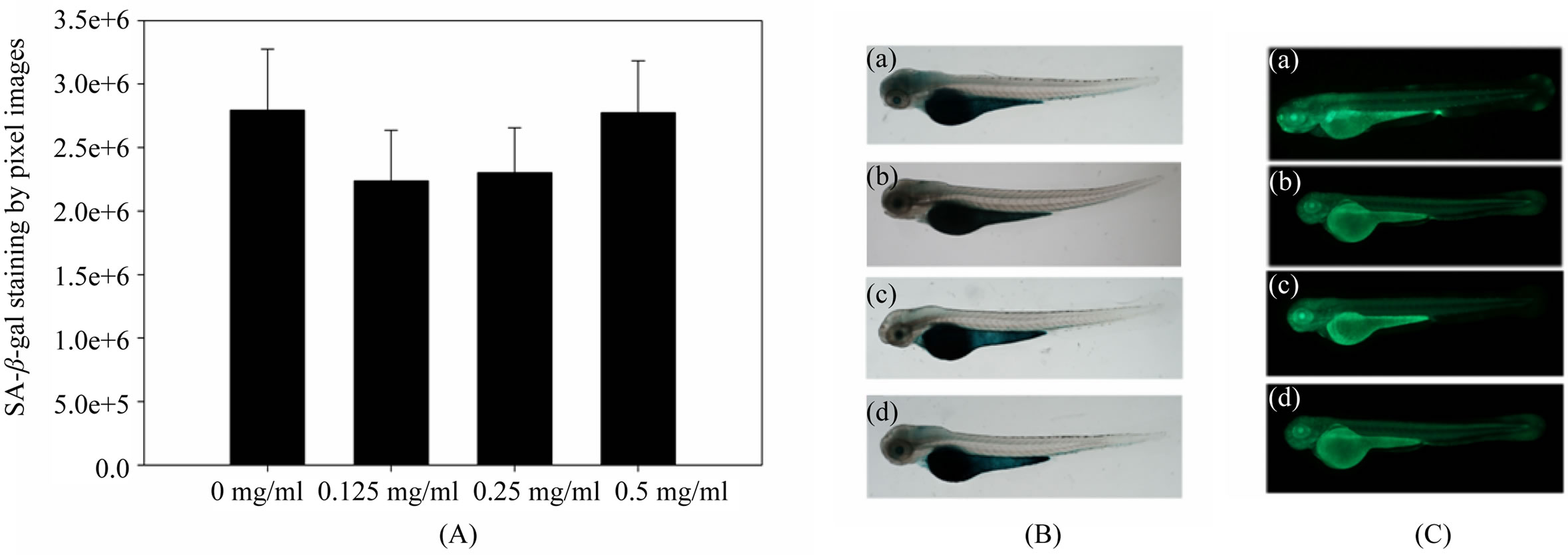
Figure 2. (A) Quantative apoptosis. SA-β-gal staining activity were performed in zebrafish embryos (72 hpf data represent the mean ± SD, n = 10) by whole body staining in control and AP treatment. Quantative analysis revealed that apoptosis decrease in a dose dependent; (B) Decreased apoptosis were observed by microscope in the head and yolk extension, at lower concentration of AP treatment decrease SA-β-gal staining; (C) AO staining were observed by microscope indicating that high concentration of AP (0.5 mg/ml) induced senescence ((a), (b), (c) and (d) represent the concentration of AP at 0, 0.125 mg/ml, 0.25 mg/ml and 0.5 mg/ml respectively).
stream of p53. Bax p21 and p53 gene showed downregulated in treated zebrafish embryos, while mdm2 showed up-regulated (Figure 3).
Telomerase are responsible for repairing damaged tissues and must continually self-renew themselves and regenerate the progenitors for which they are programmed [34,35]. Findings build on seminal cell culture studies showing that enforced tert expression can endow primary human cells with unlimited replicative potential [10]. Importantly, tert overexpression in epithelial tissues of cancer-resistant mice leads to extended median lifespan [11]. In our studies, after AP treatment, tert gene expression was also up-regulated (Figure 3).
4. DISCUSSION
Current studies suggest that development of anti-aging drugs from Chinese medical herbs may be one of the possible interventions [10,35]. AP has a variety of biological activities and pharmacological functions and play an important role in preventing and treating various chronic diseases, such as diabetes, hyperlipidemia, cancer, heaptitis, hypo-immunity function and thromobosis [36]. Modern pharmacology indicates that AP has anti-decrepit effect in brain and heart tissues in mice by increasing the activity of superoxide dismutase (SOD) [37,38]. It can also still delay cell senescence of aged mice. Although numerous studies have been published on human cells and mice examining the health aspects of AP, to our knowledge, there have been scarce studies to investigate its beneficial effects on health from the aspects of its antioxidant activity in zebrafish.
Zebrafish,.which have a low background incidence of tumors, have been shown to be an effective model for testing apoptosis and toxicity response [17,18]. And some studies demonstrate that exposures of zebrafish embryos to different stress induction can trigger the appearance of several independent biomarkers of senescence, which suggest a novel concept for low-dose drug testing [39,40]. Moreover, homologues of most of the apoptosis genes have been identified in zebrafish, and many drug targets are components of complex signaling pathway, and activation of signaling pathways lead to changes in multiple mRNA expression, thus the result of RT-PCR analysis can be used to identify the target of drug action efficiently.
In multicellular organisms, the tumor suppressor gene p53 plays a major role in maintaining the integrity of the genome by responding to various types of cellular stresses and inducing cell-cycle arrest or apoptosis [41-43]. Mechanistically, p53 causes an arrest of the cell cycle by trans-activating key downstream effector’s genes, such as the p21 cyclin-dependent kinase inhibitor, and allowing time to repair damaged DNA [44]. Furthermore, augmented levels of p53 protein can activate apoptotic pathways through both transcription-dependent and independent mechanisms [45]. Thus down-regulated of p53 gene could lead to reduce ability to activate apoptosis in cells that are compromised in DNA damage repair, which can lead to further accumulation of mutations during oncogenesis [46].
In this study, we are using a new animal model-zebrafish as a vertebrate model to develop whole-animal bioassays for exploring the mechanism of senescence, and our data leads us to the conclusion that AP delay zebrafish senescence by inhibition cell senescence and apoptosis in the early development, and also inhibition the
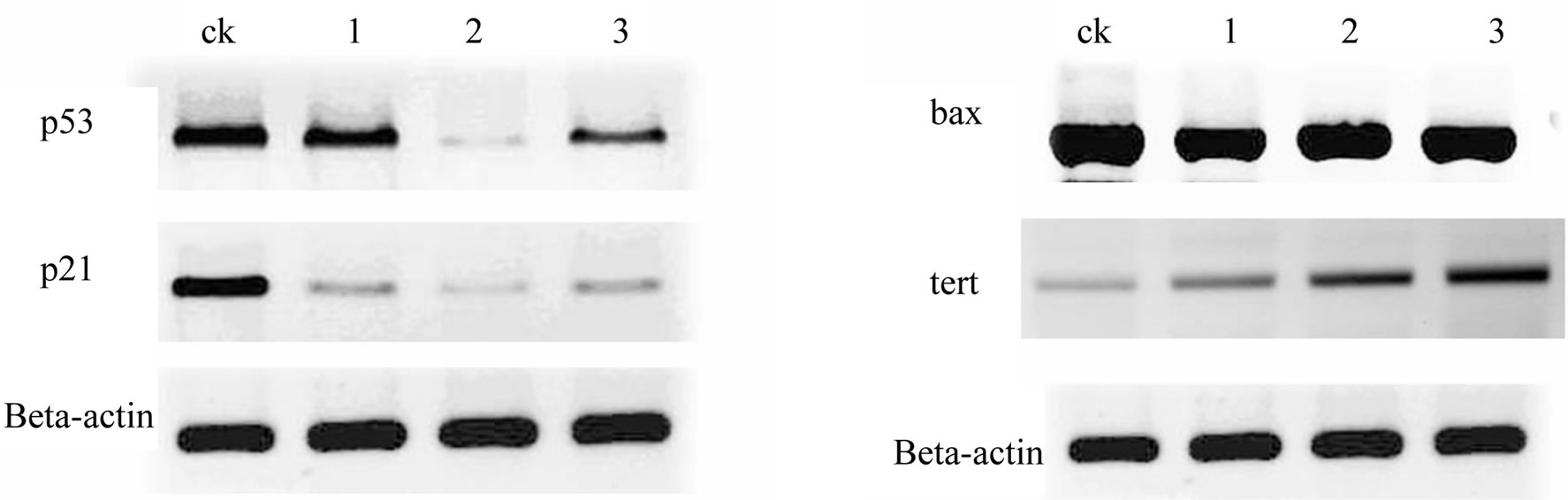
Figure 3. Zebrafish embryos lack regulated of key downstream target genes involved in the p53 regulatory (p53, mdm2), cell cycle checkpoint (p21), and apoptotic (bax, tert) pathways were analyzed in different concentration of AP treatment. Gene expression was assayed at 72 hpf. Beta-actin was used to control gene expression.
expression of p53 and p21 genes, while activation mdm2 and tert gene expression. And in our studies we use zebrafish embryos without any stress-induction to study the mechanism of AP function, but if these finding are the same after stress-induction and how it leads to longterm stress-specific changes in the expression level of specific proteins still need to be explored. Drug targets and apoptosis pathways for anti-aging therapy also need to be elucidated in the future studies.
REFERENCES
- Ku, H.H. and Sohal, R.S. (1993) Comparison of mitochondrial pro-oxidant generation and anti-oxidant defenses between rat and pigeon: Possible basis of variation in longevity and metabolic potential. Mechanisms of Ageing and Development, 72, 67-76. doi:10.1016/0047-6374(93)90132-B
- Sohal, R.S. and Weindruch, R. (1996) Oxidative stress, caloric restriction, and aging. Science, 273, 59-63. doi:10.1126/science.273.5271.59
- Finkel, T. and Holbrook, N.J. (2000) Oxidants, oxidative stress and biology of aging. Nature, 408, 239-247. doi:10.1038/35041687
- Jafari, M., Felgner, J.S., Bussel, I.I., Hutchili, T., Khodayari, B., Rose, M.R., Vince-Cruz, C. and Mueller, L.D. (2007) Rhodiola: A promising anti-aging Chinese herb. Rejuvenation Research, 10, 587-602. doi:10.1089/rej.2007.0560
- Ian, M.S. (2006) Oxidative damage and age-related functional declines. Mechanisms of Ageing and Development, 127,411-423. doi:10.1016/j.mad.2006.01.008
- Hayflick, L. and Moorhead, P.S. (1961) The serial cultivation of human diploid cell strains. Experimental Cell Research, 25, 585-621. doi:10.1016/0014-4827(61)90192-6
- Toussaint, O., Medrano, E.E. and Von Zglinicki, T. (2000) Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Journal of Experimental Gerontology, 35, 927-945. doi:10.1016/S0531-5565(00)00180-7
- Ikegami, R., Zhang J., Rivera-Bennetts A.K. and Yager T.D. (1997) Activation of the metaphase checkpoint and an apoptosis programme in the early zebrafish embryo by treatment with the spindle-destabilising agent nocodazole. Zygote, 5, 329-350. doi:10.1017/S0967199400003919
- Zheng, R., Jie, S., Hanchuan, D. and Mouncheng, W., (2005) Characterization and immunomodulating activities of polysaccharide from Lentinus edodes. International Immunopharmacology, 5, 811-820. doi:10.1016/j.intimp.2004.11.011
- Chang, I.M. (2001) Anti-aging and health-promoting constituents derived from traditional oriental herbal remedies: Information retrieval using the TradiMed 2000 DB. Annals of the New York Academy of Sciences, 928, 281-286. doi:10.1111/j.1749-6632.2001.tb05657.x
- Bastianetto, S. and Quirion, R. (2002) Nutural extracts as possible protective agents of brain aging. Neurobiology of Aging, 23, 891-897. doi:10.1016/S0197-4580(02)00024-6
- Berg, D., Youdim, M.B. and Riederer, P. (2004) Redox imbalance. Cell and Tissue Research, 318, 201-213. doi:10.1007/s00441-004-0976-5
- Kang, K.A., Lee, K.H., Zhang, R., Piao, M.J., Kang, M.Y., Kwak, Y.S., Yoo, B.S., You, H.J. and Hyun, J.W. (2007) Protective effects of castanopsis cuspidate through activation of ERK and NF-kappaB on oxidative cell death induced by hydrogen peroxide. Journal of Toxicology and Environmental Heath, Part A, 70, 1319-1328. doi:10.1080/15287390701429315
- Wong, C.K., Leung, K.N., Fung, K.P. and Choy, Y.M. (1994) Immunomodulatory and anti-tumor polysaccharide. Acta Nutrimenta Sinica, 24, 189-191.
- Xu, L. (2009) Research on pharmacological functions and clinical application of astragalus. Modern Medical (in Chinese), 25, 3312-3313.
- Huang, N.-L. and Zhang B.-Y. (2009) Research on pharmacological functions and clinical application of astragallus. Strait Pharmaceutical, 21, 137-139.
- Chen, J.N. and Fishman M.C. (1996) Zebrafish tinman homolog demarcates the heart field and initiates myocardial differentiation. Development, 123, 293-302.
- Granato, M. and Nusslein-Volhard, C. (1996) Fishing for genes controlling development. Current Opinion in Genetics and Development, 6, 461-468. doi:10.1016/S0959-437X(96)80068-2
- Parng, C., Seng, W. L., Semino, C. and McGrath, P. (2002) Zebrafish: A preclinical model for drug screening. ASSAY and Drug Development Technologies, 1, 41-50. doi:10.1089/154065802761001293
- Gerhard, G.S., Kauffman, E.J., Wang, X., Stewart, R., Moore, J.L., Kasales, C.J., Demidenko, E. and Cheng, K.C. (2002) Life spans and senescent phenotypes in two strains of zebrafish (Danio rerio), Experimental Gerontolog Experimental Gerontology Experimental Gerontology, 37, 1055-1068. doi:10.1016/S0531-5565(02)00088-8
- Keller, E.T. and Muurtha, J.M. (2004) The use of mature zebrafish (Danio rerio) as a model for human aging and disease. Comparative Biochemistry Physiology C: Toxicology and Pharmacology, 138, 335-341.
- Tsai, S.B., Tucci, V., Uchiyama, J., Fabian, N.J., Lin, M.C., Bayliss, P.E., Neuberg, D.S., Zhdanova, I.V. and Kishi, S. (2007) Differential effects of genotoxic stress on both concurrent body growth and gradual senescence in the adult zebrafish. Aging Cell, 6, 209-224. doi:10.1111/j.1474-9726.2007.00278.x
- Wang, S.-P., Li, X.-J. and Zhang, G.-Z. (2008) Study on optimization of the technology for extraction and purifycation from astragalus membranaceus. Journal of Molecular Science, 24, 60-65.
- Masuko, T., Minami, A., Iwasaki, N., Majima, T., Nishimura, S.I. and Lee, Y.C. (2005) Carbohydrate analysis by a phenol-sulfuric acid and method in microplate format. Analytical Biochemistry, 339, 69-72. doi:10.1016/j.ab.2004.12.001
- Robu, M.E., Arson, J.D., Nasevicius, A., Beiraghi, S., Brenner, C., Farber, S.A. and Ekker, S.C. (2007) p53 activation by knockdown technologies. PLoS Genetics, 3, 787-801.
- Rodemann, H.P., Bayreuther, K., Dittmann, F., Albiez, M. and Francz, P.I. (1989) Selective enrichment and biochemical characterization of seven human skin fibroblasts cell types in vitro. Experimental Cell Research, 180, 84- 93. doi:10.1016/0014-4827(89)90214-0
- Dimri, G.P., Lee, X., Basile, G., Acosta, M., Scott, G., Roskelley, C., Medrano, E.E., Linskens, M., Rubelj, I., Pereira-Smith, O., Peacocke, M. and Campisi, J. (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proceedings of National Academy of Sciences of the USA, 92, 9363-9367. doi:10.1073/pnas.92.20.9363
- Cao, L., Li, W., Kim, S., Brodie, S.G. and Deng, C.X. (2003) Senescence, aging, and malignant transformation mediated by p53 in mice lacking the Brca1 full-length isoform. Genes and Development, 17, 201-203. doi:10.1101/gad.1050003
- Keyes, W.M., Wu, Y., Vogel, H., Guo, X., Lowe, S.W. and Mills, A.A. (2005) p63 deficiency activates a program of cellular senescence and leads to accelerated aging. Gene and Development, 19, 1986-1999. doi:10.1101/gad.342305
- Ikegami, R., Zhang, J., Rivera-Bennetts, A.K. and Yager, T.D. (1997) Activation of the metaphase checkpoint and an apoptosis programme in the early zebrafish embryo by treatment with the spindle-destabilising agent nocodazole. Zygote, 5, 329-350. doi:10.1017/S0967199400003919
- Bodnar, A.G., Ouellette, M. and Frolkis, M. (1998) Extension of life-span by introduction of telomerase into normal human cells. Science, 279, 349-352. doi:10.1126/science.279.5349.349
- Ben-Porath, I. and Weinberg, R.A. (2004) When cells get stressed: An integrative view of cellular senescence. Journal of Clinical Invest, 113, 8-13.
- Meyerson, M. (2000) Role of telomeras in normal and cancer cells. Journal of Clinical Oncology, 18, 2626- 2634.
- Jaskelioff, M., Muller, F.L., Paik, J.H., Thomas, E., Jiang, S., Adams, A.C., Sahin, E., Kost-Alimova, M., Protopopov, A., Cadinanos, J., Horner, J.W., Maratos-Flier, E. and Depinho, R.A. (2011) Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature, 469, 102-106. doi:10.1038/nature09603
- Wyllie, F.S., Jones, C.J., Skinner, J.W., Haughton, M.F., Wallis, C., Wynford-Thomas, D., Faragher, R.G. and Kipling, D. (2000) Telomerase prevents the accelerated cell ageing of Werner syndrome fibroblasts. Nature Genet, 24, 16-17. doi:10.1038/71630
- Wang, Y. and Li, Y.-D. (2011) Research astragalus and cell on aging. Medical Information (in Chinese), 24, 2168- 2169.
- Xu, L. (2009) Research on pharmacological functions and clinical application of astragalus. Modern Medical (in Chinese), 25, 3312-3313.
- Huang, N.-L. and Zhang, B.-Y. (2009) Research on pharmacological functions and clinical application of astragallus. Strait Pharmaceutical (in Chinese), 21, 137-139.
- Stoletov, K., Montel, V., Lester, R.D., Gonias, S.L. and Klemke, R. (2007) High-resolution imaging of the dynamic tumor cell vascular interface in transparent zebrafish. Proceedings of National Academy of Sciences of the USA, 104, 17406-17411. doi:10.1073/pnas.0703446104
- Scholz, S., Fischer, S., Gundel, U., Kuster, E., Luckenbach, T. and Voelker, D. (2008) The zebrafish embryo model in environmental risk assessment—Applications beyond acute toxicity testing. Environmental Science and Pollution Research, 15, 394-404. doi:10.1007/s11356-008-0018-z
- Levine, A.J. (1997) p53, the cellular gatekeeper for growth and division. Cell, 88, 323-331. doi:10.1016/S0092-8674(00)81871-1
- Vousden, K.H. (2000) p53: Death star. Cell, 103, 691-694. doi:10.1016/S0092-8674(00)00171-9
- Vogelstein, B., Lane, D. and Levine, A.J. (2000) Surfing the p53 network. Nature, 408, 307-310. doi:10.1038/35042675
- Deng, C., Zhang, P., Harper, J.W., Elledge, S.J. and Leder, P. (1995) Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell, 82, 675-684. doi:10.1016/0092-8674(95)90039-X
- Fridman, J.S. and Lowe, S.W. (2003) Control of apoptosis by p53. Oncogene, 22, 9030-9040. doi:10.1038/sj.onc.1207116
- Berghmans, S., Murphey, R.D., Wienholds, E., Neuberg, D., Kutok, J.L., Fletcher, C.D., Morris, J.P., Liu, T.X., Schulte-Merker, S., Kanki, J.P., Plasterk, R., Zon, L.I. and Look, A.T. (2005) tp53 mutant zebrafish develop malignnant peripheral nerve sheath tumors. Proceedings of National Academy of Sciences of the USA, 102, 407-412. doi:10.1073/pnas.0406252102
NOTES
*Corresponding author.

