Journal of Cosmetics, Dermatological Sciences and Applications
Vol.3 No.3(2013), Article ID:35858,3 pages DOI:10.4236/jcdsa.2013.33027
Subacute Toxicological Analysis of Excessive Staphylococcus epidermidis Administration in Mice
![]()
1Laboratory of Biochemistry, Department of Pharmacy, Faculty of Pharmaceutical Sciences, Nagasaki International University, Sasebo, Japan; 2Research and Development Department, BIOGENOMICS Co., Ltd., Omura, Japan; 3Department of Nutrition, Kyushu Women’s University, Kitakyushu, Japan.
Email: *nodake@niu.ac.jp
Copyright © 2013 Yuichi Nodake et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received June 10th, 2013; revised July 12th, 2013; accepted July 20th, 2013
Keywords: Staphylococcus epidermidis; Skin Microbiota; Skin Care; Cosmetics; Subacute Toxicity Analysis
ABSTRACT
We performed subacute toxicological analysis of mice that received repeated oral administration of excess Staphylococcus epidermidis every day for 4 weeks. No mouse died and no abnormalities of specific internal organs were induced by excessive S. epidermidis administration. Furthermore, acute toxicity analysis following oral and intraperitoneal administration of excess S. epidermidis showed no toxicity in the test mice.
1. Introduction
Elucidation of the relationships between skin microbiota and skin diseases has drastically advanced through the development of new detection methods [1,2]. Among the variety of bacteria that comprise the normal skin microbiota, Staphylococcus epidermidis is a Gram-positive and coagulase-negative cocci and a well-known beneficial bacterium that participates in the maintenance of skin health. The metabolic products of S. epidermidis, such as glycerin, organic acids, and antimicrobial agents, contribute to improve skin moisture retention, maintain low acidity of the horny layer, improve texture of the skin surface, and suppress S. aureus colonization [3-5]. The anti-aging properties of S. epidermidis for the skin has gained much attention, as the superoxide dismutase produced by S. epidermidis is a known quencher of reactive oxygen species [6]. Consequently, many cosmetics for facilitating the growth of S. epidermidis on the skin surface have recently been developed to exploit the skin care benefits induced by S. epidermidis.
S. epidermidis produces a glycocalyx that forms a hydrophobic biofilm, which acts as a glue allowing the microorganism to adhere to plastic and cells, and also induces resistance to phagocytosis and some antibiotics; however, S. epidermidis infections can lead to life-threatening conditions, such as sepsis and endocarditis [7]. In general, S. epidermidis on the skin surface frequently invades the inside of the body via the mouth or wounds, but it does not usually cause infections in healthy subjects. In fact, S. epidermidis infections are mainly restricted to those with compromised immune function or patients with indwelling catheters receiving various medical treatments [8,9]. However, no report has demonstrated the influence of excessive S. epidermidis invasion into a living body until now. Therefore, we performed the present study to evaluate the subacute toxicity of orally administered S. epidermidis in mice by monitoring feed intake, body weight, organ weight (liver and kidney), and histopathological findings. In addition, the acute toxicity of S. epidermidis, following oral and intraperitoneal administration to mice, was also evaluated.
2. Materials and Methods
2.1. Bacteria, Animals, and Chemical Reagents
The standard S. epidermidis strain JCM 2414, which was established from skin of the human face, was obtained from RIKEN BioResource Center (Tsukuba, Japan). Male, 5-week-old, Slc:ddY mice were purchased from Kyudo Company Limited (Kumamoto, Japan). Skim milk and trypticase soy (TS) broth was purchased from Snow Brand Milk Products Co., Ltd. (Sapporo, Japan) and Becton, Dickinson & Company (New Jersy, USA), respectively. All other reagents used in this study were of high-quality analytical grades.
2.2. Preparation of Sample Solution
The S. epidermidis standard strain was aerobically cultured at 37˚C for 48 h in TS medium and then centrifuged at 10,000 × g for 5 min and the supernatant was then discarded. After the resulting precipitate was washed three times with sterilized phosphate-buffered saline (PBS) at pH 7.4 containing 10% skim milk and resuspended in the same solution, the bacterial concentration was adjusted to 2.6 × 1011 cells/ml (“sample solution”).
2.3. Subacute Toxicity Analysis
Animal experiments were conducted in accordance with the “Standards Relating to the Care and Management of Laboratory Animals and Relief of Pain” (Notice no. 88, Ministry of the Environment, Government of Japan) and the experimental protocol in the animal experiments was approved by the Ethics Review Committee of Nagasaki International University (approval number 13). Male 5- week-old Slc:ddY mice were individually housed in an animal care facility under controlled temperature, relative humidity, and light (22˚C ± 2˚C, 55 ± 2%, and 08:00- 20:00 h, respectively). The mice had access to water and a CE-2 solid diet ad libitum (CLEA Japan, Inc., Tokyo, Japan).
After acclimatization for 1 week, all mice with a body weight of approximately 26 g were divided into two groups (groups I and II; n = 10 each). In groups I and II, the mice received repeated oral administration of the sample solution (0.5 ml) or PBS containing 10% skim milk (0.5 ml) every day for 4 weeks, respectively. General signs were observed daily and the body weight of each mouse was measured once a week. At the end of the experiment, the mice were anaesthetized with sodium barbital and signs at necropsy were visually observed. The excised liver and kidney tissues of each mouse were collected and weighed. All data were presented as the mean ± standard error and statistical significance was evaluated using the Student’s t-test for paired comparesons with p < 0.05 considered statistically significant.
3. Results and Discussion
No mouse died in group II owing to the intake of S. epidermidis during the test period. Tables 1 and 2 summarized changes in body weight and feed intake in groups I and II, respectively. The values obtained from the mice in both groups changed similarly and all mice grew well without marked differences in appearance. Table 3 lists some characteristics of the mice injected S. epidermidis for 4 weeks in the present test. An important aspect of our data is that there were no significant differences in
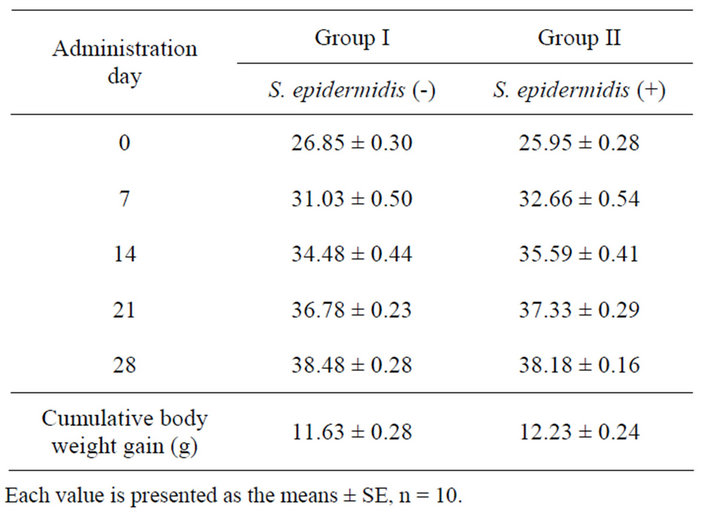
Table 1. Body weight change in mice.
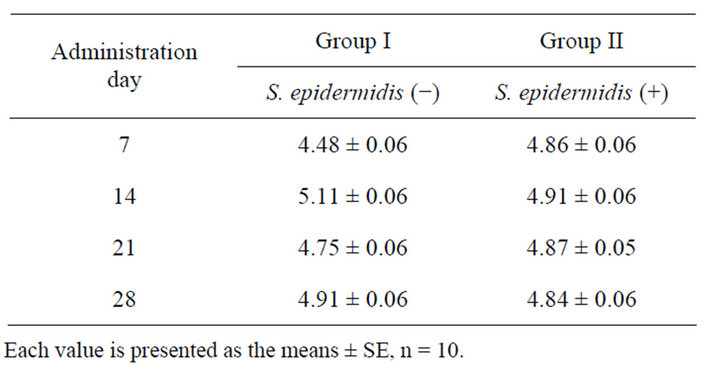
Table 2. Feed intake by mice.
Each value is presented as the means ± SE, n = 10.
the internal organ weights among the mice. Necropsies at the end of the experiment indicated neither pathological symptoms nor histopathological abnormalities in the internal organs.
The above mentioned results indicate that excessive S. epidermidis ingestion did not influence the growth of mice or induce organ-specific abnormalities. Considering that 1.0 × 103–4 cells/cm2 of S. epidermidis live on the skin surface and some will ultimately invade the body via the mouth or wounds, the number of S. epidermidis orally administered to the mice in group II was designed to be highly excessive. The results of the present study demonstrated a low toxicity of S. epidermidis under the experimental conditions, as mice were continuously administered 1.3 × 1011 cells/day of S. epidermidis. Since the bacterial number adopted in the present study was more than that in acute toxicity testing in newly discovered functional lactic acid bacteria strains, it was thought that the results obtained from the present analysis were sufficiently reliable. In addition, we also obtained clearly evidence supporting the low toxicity of S. epidermidis from acute toxicity testing, in which 0.3 − 1.3 × 1012 S. epidermidis cells were orally or intraperitoneally administered to the test mice in single doses (data not shown). In the acute toxicity tests of S. epidermidis, no mice died and exhibited significant disorders during
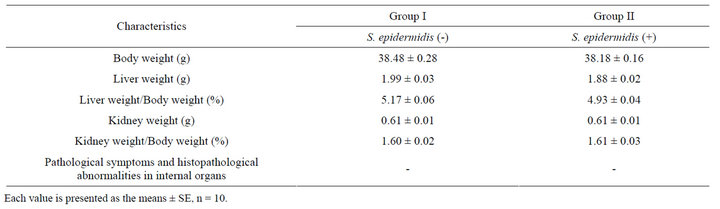
Table 3. Some characteristics of mice at the end of the experiment.
the test period (48 h). Moreover, whole genome analysis revealed that S. epidermidis strains ATCC 12228 and RP62A do not express several pathogenic factors, such as enterotoxins, pathogenicity islands, staphylocoagulase, exfoliative toxins, α-haemolysin, γ-haemolysin, clumping factors, staphylokinase, hyaluronidase, leukocidins, or leukotoxins, as compared with S. aureus [10,11]. Consequently, the biofilm-forming capacity of S. epidermidis is a major virulence factor, but it does not produce aggressive virulence determinants in healthy subjects. These findings suggested that the use of cosmetics to facilitate S. epidermidis growth on skin surface may allow the frequent invasion of S. epidermidis, but this is relatively safe in healthy subjects. The results of our present report regarding the safety of S. epidermidis should serve as a driving force to accelerate the development of novel cosmetics.
REFERENCES
- I. Dekio, H. Hayashi, M. Sakamoto, M. Kitahara, T. Nishikawa, M. Suematsu and Y. Benno, “Detection of Potentially Novel Bacterial Components of the Human Skin Microbiota Using Culture-Independent Molecular Profiling,” Journal of Medical Microbiology, Vol. 54, No. 12, 2005, pp. 1231-1238. doi:10.1099/jmm.0.46075-0
- I. Dekio, M. Sakamoto, H. Hayashi, M. Amagai, M. Suematsu and Y. Benno, “Characterization of Skin Microbiota in Patients with Atopic Dermatitis and in Normal Subjects Using 16S rRNA Gene-Based Comprehensive Analysis,” Journal of Medical Microbiology., Vol. 56, No. 12, 2007, pp. 1675-1683. doi:10.1099/jmm.0.47268-0
- T. Iwase, Y. Uehara, H. Shinji, A. Tajima, H. Seo, K. Takada, T. Agata and Y. Mizunoe, “Staphylococcus epidermidis Esp Inhibits Staphylococcus Aureus Biofilm Formation and Nasal Colonization,” Nature, Vol. 465, No. 7296, 2010, pp. 346-349. doi:10.1038/nature09074
- M. Al-Mahrous, S. K. Sandiford, J. R. Tagg and M. Upton, “Purification and Characterization of a Novel DeltaLysin Variant that Inhibits Staphylococcus Aureus and Has Limited Hemolytic Activity,” Peptides, Vol. 31, No. 9, 2010, pp. 1661-1668. doi:10.1016/j.peptides.2010.06.006
- A. L. Cogen, K. Yamasaki, K. M. Sanchez, R. A. Dorschner, Y. Lai, D. T. MacLeod, J. W. Torpey, M. Otto, V. Nizet, J. E. Kim and R. L. Gallo, “Selective Antimicrobial Action Is Provided by Phenol-Soluble Modulins Derived from Staphylococcus epidermidis, a Normal Resident of the Skin,” Journal of Investigative Dermatology, Vol. 130, No. 1, 2010, pp. 192-200. doi:10.1038/jid.2009.243
- A. Kaneko, S. Kondo, J. Akiyama and K. Okabe, “A New Cosmetic SOD Delivery System Using Skin Surface Resident Staphylococcus epidermidis by Lotions Containing Mn/Zn Ions,” Drug Delivery System, Vol. 12, No. 5, 1997, pp. 339-345. doi:10.2745/dds.12.339
- L. Nilsson, P. Flock and G. Lindberg, “A FibrinogenBinding Protein of Staphylococcus epidermidis,” Infection and Immunity, Vol. 66, No. 6, 1998, pp. 2666-2673.
- M. Otto, “Staphylococcus epidermidis the ‘Accidental’ Pathogen,” Nature Reviews Microbiology, Vol. 7, No. 8, 2009, pp. 555-567. doi:10.1038/nrmicro2182
- G. Hedin, “Staphylococcus epidermidis Hospital Epidemiology and the Detection of Methicillin Resistance,” Scandinavian Journal of Infectious Diseases. Supplementum, Vol. 90, No. 1, 1993, pp. 1-59.
- Y. Q. Zhang, S. X. Ren, H. L. Li, Y. X. Wang, G. Fu, J. Yang, Z. Q. Qin, Y. G. Miao, W. Y. Wang, R. S. Chen, Y. Shen, Z. Chen, Z. H. Yuan, G. P. Zhao, D. Qu, A. Danchin and Y. M. Wen, “Genome-Based Analysis of Virulence Genes in a Non-Biofilm-Forming Staphylococcus epidermidisstrain (ATCC 12228),” Molecular Microbiology, Vol. 49, No. 6, 2003, pp. 1577-1583. doi:10.1046/j.1365-2958.2003.03671.x
- S. R. Gill, D. E. Fouts, G. L. Archer, E. F. Mongodin, R. T. Deboy, J. Ravel, I. T. Paulsen, J. F. Kolonay, L. Brinkac, M. Beanan, R. J. Dodson, S. C. Daugherty, R. Madupu, S. V. Angiuoli, A. S. Durkin, D. H. Haft, J. Vamathevan, H. Khouri, T. Utterback, C. Lee, G. Dimitrov, L. Jiang, H. Qin, J. Weidman, K. Tran, K. Kang, I. R. Hance, K. E. Nelson and C. M. Fraser, “Insights on Evolution of Virulence and Resistance from the Complete Genome Analysis of an Early Methicillin-Resistant Staphylococcus Aureus Strain and a Biofilm-Producing Methicillin-Resistant Staphylococcus epidermidis Strain,” Journal of Bacteriology, Vol. 187, No. 7, 2005, pp. 2426- 2438. doi:10.1128/JB.187.7.2426-2438.2005
NOTES
*Corresponding author.

