New Journal of Glass and Ceramics
Vol.07 No.02(2017), Article ID:75984,12 pages
10.4236/njgc.2017.72003
Advantage of NMR and FTIR Spectroscopy to Determine Structure Role of CeO2 in Complicated Borosilicate Glasses: New Approach
Gomaa El-Damrawi, Abdelmeguid K. Hassan, Salma Ehmead, Amal El Shahawy
Physics Department, Faculty of Science, Mansoura University, Mansoura, Egypt

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: February 3, 2017; Accepted: April 27, 2017; Published: April 30, 2017
ABSTRACT
Microstructure of complicated glasses in the system 30Na2O-2Al2O3-25 SiO2- xCeO2 (43-x) B2O3, x changes from 0.5 to 20 mol% have been extensively studied. Structural determination of glasses containing high cerium oxide content (≥8 mol% CeO2) was carried out by 11B NMR and FTIR spectroscopy. On the other hand, 29Si MAS NMR experiment is hardly to be applied to glasses of CeO2 > 8 mol%. This is due to the paramagnetic action which is raised by cerium cations causing dilution or delaying in the resonance phenomenon. It is evidenced from NMR data that sodium oxide is high enough to modify the glass forming units which constitute the skeleton of the glass. Ceria is as well as silica and B2O3 all are acting as glass forming species. Decreasing of both fraction of boron tetrahedral units (N4) and chemical shift of silicon nuclei (δ) confirm the role of CeO2 as a glass former. On the other hand, fast decrease in N4 and chemical shift of Si nuclei with further increasing CeO2 contents (≥8 mol%) gives a clear evidence that the ability of cerium oxide to participate as a network former increases with increasing its content. New approach is applied to determine the fraction of CeO4 as a glass forming units. In this approach, we use the common advantage of 11B NMR and FTIR spectroscopy to obtain Ce4 fraction. The latter species cannot be determined from NMR spectroscopy, since very high relaxation time and magnetization of ceria cause intensive spectral broadening which prevent resonance spectra to be appeared.
Keywords:
NMR Spectroscpy, Cerium Oxide, New Approach, Glasses

1. Introduction
It was reported previously [1] [2] [3] that some types of borosilicate glasses (BSG) have an interested academic and scientific priority as well as technical applications. Growing achievement in field microelectronics technology requires new types of glasses which may be used as sealants, particularly, for molten carbonate fuel cells (MCFC) [3] [4] . Specific types of borosilicate glasses have long been the subject of structural studies [2] [5] [6] [7] . This is because of their interest in both technical and academicals considerations.
The structure of borosilicate glasses is based on the base former units which are the main constituents of the glass network. These units are designed as, Qn in silicate [SiO4] and N4 or B4 in borate containing [BO3] and [BO4] structural species. The [BO3] species would be formed in both symmetric and asymmetric configurations. While [BO4] units are formed in the symmetric tetrahedral coordination. As a result of mixing between borate and silicate matrices, the oxygen atoms can be bonded to boron and silicon or in some cases to silicon atoms or boron only, and have a Na+ and/or Ce2+ as charge compensators. The distribution of the borate and silicate structural units depends on the ratios of Na2O/ B2O3 and SiO2/B2O3 [2] [5] [6] which are designed as R and K structural factor. For all K values, N4 increases up to a maximum in between 0.5 and 0.75 for R = 1, and the value of this maximum increases with increasing K values. At specific value of K, the proportion of N4 slowly decreases with increasing R values.
In order to test the possible quantitative use of NMR and FTIR spectroscopy, glasses of two individual composition regimes have been prepared and measured. One contains an extremely low concentration of CeO2 and the other enriched with it. The first region contains a limited concentration from CeO2 as a paramagnetic material. In such a case, 29Si NMR study can easily be applied to obtain Qn values of different borosilicate glasses. On the other hand, the second type of borosilicate glasses contains further high level from CeO2 (≥8 mol) which limit the advantage of 29Si NMR measurements to be used. This is because the high spins magnetic moment of magnetic cations such as cerium and iron produces sufficient broadening of Si NMR lines [2] [8] . As a result, different contributions cannot be resolved and broader non featured and unobservable spectra can be considered.
11B and FTIR spectroscopy are not affected by adding even more concentration from paramagnetic species from CeO2. Therefore, these tools would be simply applied to obtain complementary data. It can be used as a quantitative tool applied to determine structural fractions, such as N4 in borate network. In this study, 11B NMR & FTIR spectroscopy can be applied for all glass compositions while 29MAS NMR technique is limited to low CeO2 concentration (8 mol%).
2. Experimental
2.1. Sample Preparation
The glasses were prepared from reagent grade SiO2, H3BO3, Na2CO3, Al2O3 and CeO2 The melting process was carried out using alumina crucibles at a temperature ranging from 1250˚C to 1520˚C depending on composition. After swirling the melt several times to ensure good homogeneity and air bubble free, the melt was quenching over a stainless steel plate and pressed to obtain the desired shape.
2.2. Nuclear Magnetic Resonance
All NMR measurement have been carried out on glass sample in form of powder. The measurements were carried out via JEOL GSX-500 high-resolution solid-state MAS NMR spectrometer (Mansoura University-EGYPT) in a magnetic field of 11.75 T. A specific frequency of 99.3 MHz is applied to record 29Si MAS NMR spectra. A spinning frequency of 6 kHz is applied to a cylindrical zirconia sample holder to rotated at a speed depends on the type of the measured nuclei. The Signal of pulse length of 2.62 µs and a recycle delay of 30 s is applied to record 29Si NMR signal. Around 1000 - 2000 scans were accumulated to get good spectrum. 11B MAS NMR spectra were recorded at a frequency of 160.4 MHz and spinning rate of 15 KHz. The glass samples were measured with a single pulse length of 0.5 - 1.0 ms and a pulse delay of 2.5 s, and an accumulation of 100 - 200 scans. 27Al Mas NMR spectra were recorded at a frequency of 130.3 MHz and spinning rate of 6 KHz.
2.3. FTIR Measurements
Fourier transform infrared absorption signals of the studied glasses were measured at room temperature in the wavelength range 4000 - 400 cm−1 using a computerized recording FTIR spectrometer (Mattson 5000, USA). Fine powdered samples were mixed with KBr in the ratio 1:100 for quantitative analysis and the weighed mixtures were subjected to a load of 5 t/cm2 in an evocable i.e. to produce clear homogenous discs. Then, the IR absorption spectra were immediately measured after preparing the discs to avoid moisture attack.
3. Results and Discussion
The studied borosilicate glasses are investigated by 23Na, 29Si, 27Al and 11B NMR to offer deeper insight into the structure of a given glass. The fraction of bridging (BO) and nonbridging oxygen atoms (NBO) as a consequence can simply be determined. The NMR results also make a distinction for three-fold coordinated boron B3 between symmetric (B3 with 3BO or 3NBO) and asymmetric (B3 with one or two NBOs) boron, respectively B3s and B3a.
The structural features of modified borosilicate’ glasses have been shown to depend on the field strength (i.e. charge/radius) of the cation introduced. It is evidenced from NMR investigations that increasing CeO2/Na2O molar ratio will result in promotion the capacity of bonds between different forming species in the glass matrix. This is appeared from a continues decrease in both NBO and N4 fraction species. These features are correlated to the higher field strength of the Ce2+ cation as compared to Na+ one. NBOs are preferentially associated with the higher field strength cation Ce2+. which results in reducing its content in the investigated glasses.
3.1. Cerium Free Borosilicate Glass
23Na, 27Al, 11B and 29Si MAS NMR
NMR spectroscopy of sodium in glasses can be considered as a powerful measuring tool to follow the level of precipitation, crystallization and verification of structural species in glass matrix. For example, Figure 1(a) presents 23Na NMR spectra corresponding to Na2O distribution as a modifier in glass matrix, since Na2O is participated between the different species which forming the glass network. The feature of appeared spectrum is clearly differed from that of spectrum (b), leading to formation of additional structural groups in glass network. Presence of weak peak at 2 ppm is considered to be due to accumulation and precipitation of even little concentration from modifier cations in glass phase to form some types of clusters. This consideration is previously reported, since Na2SiO3 crystalline phase is structurally identified in ternary alkali silicate glasses by different techniques [2] [9] [10] [11] . The broadening of spectra is considered as good evidence for incorporation of sodium in the network as a glass modifier [2] [6] .
27Al NMR spectrum presented in Figure 2 showed that Al2O3 inters the glass in tetrahedral configuration with oxygen atoms as a first neighbor. The chemical shift of the 27Al spectrum is 59.6 ppm which is referred to AlO4 species as glass forming units. The well formed AlO4 units aren’t looking as isolated but it was linked with SiO4 groups and therefore, Al-O-Si mixed bridges are the product [2] [5] .
Figure 1. 23Na NMR spectra of borosilicate glass. (a) for glass containing clustered Na atoms, (b) represent Na as a modifier only.
Figure 3 is 29Si NMR spectrum of borosilicate glass free from CeO2. The chemical shift (σ) of base glass is listed at −86.6 ppm and is related to Q2 species [2] [5] [6] . This leads that Na2O as modifier oxide is consumed to break two bridging bond as an average per each tetrahedral SiO4 unit. Some of tetrahedral aluminum may shield silicon nuclei through forming Si-O-Al bonds. Then the chemical shift of (−86.6, ppm) would be related to Q2 species or 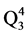 which means the 3 from oxygen atoms around the silicon are NB type, one connected to Al atom via BO.
which means the 3 from oxygen atoms around the silicon are NB type, one connected to Al atom via BO.
Figure 4 presents 11B NMR spectrum of cerium free borosilicate glass. The
Figure 2. 27Al NMR spectrum of cerium free borosilicate glasses.
Figure 3. 29Si MAS NMR spectrum of cerium free glass.
Figure 4. 11B NMR spectrum of borosilicate glass.
chemical shift (σ) of the main signal is appeared at 0 ppm and the broad signal is appeared between 5 - 22 ppm which is assigned to both symmetric and asymmetric BO3 species [11] . The determined value of fraction of transformed borons (N4) is equal to 0.64 which means that 64% from total boron is found in tetrahedral configuration with oxygen atoms.
3.2. Cerium Containing Borosilicate Glasses
11B and 29Si NMR Results
Figure 5 showed 29Si NMR spectra for two glasses containing different concentration from CeO2 (3 and 6 mol%). The chemical shift of glass containing 6 mol% CeO2 is lower (−92.4 ppm) than that of glass containing 3 mol%. (−87 ppm). The difference between the two values of chemical shift is extremely high (6 ppm) which confirm the effective role of cerium in changing the network structure even upon a small addition. In terms of chemical shift consideration, we conclude that CeO2 plays a role of strong glass former, since more shielded silicate units are formed upon CeO2 addition. In such situation Q3 species are the more formed unit. As a consequence, tetrahedral silicate units containing only one non bridging oxygen atom are the main product in sample containing 6 mol% CeO2.
The experimental 11B MAS NMR spectra for glasses involving different concentration from CeO2 are shown in Figure 6. The lowest spectrum is related to sodium borosilicate glass (free from CeO2). NMR spectra for glasses of higher CeO2 concentrations are also presented in the same figure. It can be seen from this figure that there is a remarkable changes in the spectral features upon increasing CeO2 contents. Increasing CeO2 at expense of B2O3 is noticed to have no remarked effect on the chemical shifts (σ) of tetrahedral boron (BO4), since (σ) is
Figure 5. (a) and (b) NMR spectrium of glass containing 3 and 6 mol% CeO2, respectively.
still fixed around 0 ppm for all investigated samples. But the fraction of boron tetrahedral units (N4) is only affected, since it changed from 0.63 to 0.32 upon addition of 20 mol% CeO2, See Figure 7. This means that substitution of B2O3 with CeO2 should result in decreasing the concentration of BO4 units in the borate network through lowering transformation of BO3 triangle units to BO4 groups. It can be seen from Figure 7 that the relative area characterizing BO3 units increases with increasing CeO2 contents and reverse behavior is shown indicating decreasing BO4 concentration. This means that portion of Na2O which is responsible for boron transformation is also decreased. This may because the major part of the cerium inters as a glass former. As a result, most of modifier would be firstly consumed to form CeO4 groups and the rest can be distributed between the borate and silicate network. Therefore, the fraction of tetrahedral boron N4 is hardly decreased with increasing CeO2 content which support that CeO2 has great ability to withdraw Na2O from borate network. CeO2 is therefore
Figure 6. 11B NMR spectra of selected composition of modified borosilicate glasses containing CeO2.
Figure 7. Fraction of boron tetrahedral as a function of CeO2 concentration.
played the role of glass former, since it consumes some of Na2O as a modifier to build CeO4 groups. Formation of the latter groups is expected to grow at expense of both BO4 and NBO units which may result in reducing the concentration of fraction of tetrahedral boron N4 with increasing CeO2 concentration. In comparison, glass of higher CeO2 content has lower fraction of BO4 than that of cerium free glass. Generally, N4 showed abrupt decreasing trend upon increasing CeO2 concentrations, Figure 7.
All NMR spectra are analyzed to obtain a quantitated values representing BO4 and BO3 groups. The relative area representing each type (BO4 and BO3) has been determined and .presented graphically by Figure 8 Reverse behavior is shown between BO4 and BO3 concentration with increasing CeO2 contents. This behavior is closely matches with that presented by Figure 7. Both figures showed a reduction in N4 concentration. In the same time the area representing BO3 groups is increased with increasing CeO2 concentration. These changes support that the concentration of Na2O which required to modify borate network is deeply reduced upon increasing CeO2 concentration. As a result, concentration of well transformed BO3 to BO4 is also decreased which in turns result in decreasing N4.
3.3. FTIR Spectroscopy
Figure 9 showed FTIR absorption spectra of boroesilicate glasses containing different concentrations from CeO2. It is hardly to extract specific information refered to each element shared in performing the spectra. This is because of great overlap between mixed vibration modes of Si-O, B-O, Ce-O which may be hardly to be separated. Therfore, for example, we take the advantage of 11B NMR spectrocopy in correlation to FTIR [11] [12] [13] [14] to get complete information about strucural role of Ce nuclei which cannot measured by NMR. In this respect, we analyzed FTIR spectra and dtermine the fraction of all foure coordinated species, which is termed B4, since
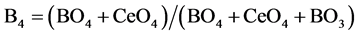 (1)
(1)
Figure 8. Change of relative areas of both BO3 and BO4 units with CeO2 concentration.
Figure 9. FTIR spectra of borosilicate glasses containing different CeO2 concentrations.
From the above equation, the fraction of both boron and ceium terahedral units can be detrmined, since (BO4 + CeO4) is represented by the spectral area which is resolved between 800 - 1200 cm−1. By using the advantage of 11B NMR spectroscopy, the fraction o f boron in terahedral coordination
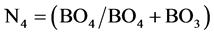 (2)
(2)
can be simply drmined. Subtracting the numercal data detrmined from Equation (2) from that of Equation (1), the concentation or fraction of CeO2 as a former CeO4 units can be simply obtained.
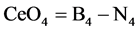 (3)
(3)
Figure 10 represented B4 and N4 fraction detrmined from equations1 and 2 respectively. The difference between the B4 and N4 is sined by Ce4 (CeO4) represented by the graph of Figure 11. It can be shown from this figure that concentration of CeO4 as a glass former is increased with increasing CeO2 content.
4. Conclusion
Structural role of cerium is determined in borosilicate glasses by different modern techniques. Cerium inters the network of the investigated glasses as a strong network former. Increasing CeO2 concentrations result in decreasing both N4 and NBO in the whole glass network. New approach has been applied to determine
Figure 10. Change of both N4 and B4 fraction with CeO2 concentration.
Figure 11. Change of fraction of ceria (Ce4) = (B4 − N4) with increasing ceria contents.
CeO4 fraction which can not be determined by NMR spectroscopy.
Cite this paper
El-Damrawi, G., Hassan, A.K., Ehmead, S. and El Shahawy, A. (2017) Advantage of NMR and FTIR Spectroscopy to Determine Structure Role of CeO2 in Complicated Borosilicate Glasses: New Approach. New Journal of Glass and Ceramics, 7, 22-33. https://doi.org/10.4236/njgc.2017.72003
References
- 1. Schaeffer, H.A. and Langfeld, R. (2014) Werkstoff Glas—Alter Werkstoff mit groβer Zukunft Reihe Technik im Fokus. Springer Verlag, Heidelberg.
- 2. El-Damraei, G., Hassan, A.K., Ramadan, R. and El-Jadal, S. (2016) Nuclear Magnetic Resonance and FTIR Structural Studies on Borosilicate Glasses Containing Iron Oxide. New Journal of Glass and Ceramics, 6, 47-56.
https://doi.org/10.4236/njgc.2016.64006 - 3. Pascual, M.J., Duran, A. and Pascual, L. (2002) Sintering Process of Glasses in the System Na2O-B2O3-SiO2. Journal of Non-Crystalline Solids, 306, 58.
- 4. Schaeffer, H.A. Langfeld, R. and Benz-Zauner, M., Eds. (2012) Werkstoff Glas. Deutsches Museum Verlag, München.
- 5. El-Damrawi, G., Müller-Warmuth, W., Doweida, H. and Gohar, I.A. (1993) 11B, 29Si and 27Al Nuclear Magnetic Resonance Studies of Na2O-Al2O3-B2O3-SiO2 Glasses. Physics and Chemistry of Glasses, 34, 52.
- 6. El-Damrawi, G., Müller-Warmuth, W. and Doweidar, H. (1992) Structure and Heat Treatment Effects of Sodium Borosilicate Glasses as Studied by 29Si and 11B NMR. Journal of Non-Crystalline Solids, 146, 137-144.
- 7. MacKenzie, K.J.D. and Smith, M.E. (2002) Multinuclear Solid-State Nuclear Magnetic Resonance of Inorganic Materials. Pergaman Materials Series 4, Pergamon, 218.
- 8. Muñoz, F., Montagne, L. and Delevoye, L. (2006) Phosphate Speciation in Sodium Borosilicate Glasses Studied by Nuclear Magnetic. Journal of Non-Crystalline Solids, 352, 2958-2968.
- 9. El Damrawi, G., Hassan, A.K., El-Gadal, S. (2017) Morphological and Structural Investigations on Iron Borosilicate Glasses. New Journal of Glass and Ceramics, in press.
- 10. El Damrawi, G., Doweidar, H. and Kamal, H. (2013) Structure and Crystallization Behavior of Silicate-Based Bioactive Glasses. Australian Journal of Basic and Applied Science, 7, 573-582.
- 11. El-Damrawi, G., Gharghar, F., Ramadan, R. and Aboelez, M (2016) 11B NMR Spectroscopy of Lead Borate Glasses: Additive Effect of Cerium Oxide. New Journal of Glass and Ceramics, 6, 57-63.
- 12. El Damrawi, G., Hassan, A.K., Kamal, H., Aboelez, M. and Labeeb, S. (2016) Structural Investigations on Na2O-CaO-V2O5-SiO2 Bioglass Ceramics. British Journal of Applied Science & Technology, 16, 1-9.
https://doi.org/10.9734/BJAST/2016/26683 - 13. Magdas, D.A., Cozar, O., Chis, V., Ardelean, I. and Vedeanuc, N. (2008) The Structural Dual Role of Fe2O3 in Some Lead-Phosphate Glasses. Vibrational Spectroscopy, 48, 251-254.
- 14. Affatigato, M., Feller, S.A., Howes, A.P. and Scales, C.R. (2008) Quantitative Measurement of Q3 Species in Silicate and Borosilicate Glasses Using Raman Spectroscopy. Journal of Non-Crystalline Solids, 354, 1936-1942.












