Journal of Analytical Sciences, Methods and Instrumentation
Vol.05 No.04(2015), Article ID:61968,7 pages
10.4236/jasmi.2015.54007
Determination of N and O-Atoms, of N2(A) and N2(X, v > 13) Metastable Molecules and
 Ion Densities in the Afterglows of Ar-N2 Microwave Discharges
Ion Densities in the Afterglows of Ar-N2 Microwave Discharges
Andre Ricard, Hayat Zerrouki, Jean-Philippe Sarrette
Laplace, Toulouse, France

Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).



Received 5 November 2015; accepted 14 December 2015; published 17 December 2015
ABSTRACT
Early afterglows of Ar-N2 flowing microwave discharges are characterized by optical emission spectroscopy. The N and O atoms, the N2(A) and N2(X, v > 13) metastable molecules and
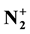 ion densities are determined by optical emission spectroscopy after calibration by NO titration for N and O-atoms and measurements of NO and N2 band intensities. For an Ar-xN2 gas mixture with × increasing from 2 to 100% at 4 Torr, 100 Watt and an afterglow time of 3 × 10−3 s at the 5 liter reactor inlet, it is found densities in the ranges of (2 - 6) × 1014 cm−3 for N-atoms, one order of magnitude lower for N2(X, v > 13) and for O-atoms (coming from air impurity), of 1010 - 1011 cm−3 for N2(A) and of 108 - 109 cm−3 for
ion densities are determined by optical emission spectroscopy after calibration by NO titration for N and O-atoms and measurements of NO and N2 band intensities. For an Ar-xN2 gas mixture with × increasing from 2 to 100% at 4 Torr, 100 Watt and an afterglow time of 3 × 10−3 s at the 5 liter reactor inlet, it is found densities in the ranges of (2 - 6) × 1014 cm−3 for N-atoms, one order of magnitude lower for N2(X, v > 13) and for O-atoms (coming from air impurity), of 1010 - 1011 cm−3 for N2(A) and of 108 - 109 cm−3 for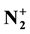 .
.
Keywords:
Ar-N2 Microwave Discharge, Flowing Afterglow, N-Atoms, N2 Metastables,
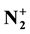 Ions
Ions

1. Introduction
Afterglows of N2 flowing microwave discharges have been studied at medium gas pressures (1 - 20 Torr) for sterilization of medical instruments by N-atoms [1] [2] . The mentioned project of sterilization in N2 afterglow is based on N-atom etching of bacteria without oxidation by O-atoms. A part of the present study is to detect the O-atoms from air impurity to appreciate their influence on the sterilization process.
The main part concerns a study of Ar-N2 gas mixtures to enhance the sterilization process in the early afterglow. The interest of N2 dilution into Ar is to increase the electron energy in the plasma at constant values of transmitted power and of gas pressure. Superelastic collisions of electrons on the Ar metastable atoms produced in the plasma could enhance the electron energy. It is mentioned here that in the present measurements of flowing afterglow, the Ar metastable atoms have disappeared after collisions on the tube wall (destruction probability of about 1). As a consequence, the excitation transfers of Ar metastable atoms on N2 can be discarded at a distance of about 1 cm after the discharge end. Another interest of Argon dilution is to maintain the plasma at high gas pressure, up to the atmospheric gas pressure while keeping a plasma power as low than 100 Watt [3] .
The early flowing afterglows produced from Ar-N2 microwave plasmas are presently studied by emission spectroscopy with the same experimental methods as in N2-H2 RF afterglow [4] [5] , in N2, N2-O2 [6] and in N2-H2, Ar-N2-H2, Ar-N2-O2 microwave early afterglows [6] .
The present paper is focused on Ar-N2 early afterglow by directly introducing the discharge tube of 5 mm dia. inside the 5 litre reactor. By this way, it is expected to add the metastable N2(A) and N2(X, v > 13) molecules and
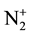 ions to the N-atoms in the surface treatments as previously experimented [1] [2] . The studied active species are as in [6] the N and O-atoms, the N2(A) and N2(X, v > 13) metastable molecules and
ions to the N-atoms in the surface treatments as previously experimented [1] [2] . The studied active species are as in [6] the N and O-atoms, the N2(A) and N2(X, v > 13) metastable molecules and
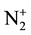 ions. The intensities emitted by the N2 first positive (1st pos.) and N2 second positive (2nd pos.) systems and by the NOβ bands are measured to obtain the mentioned active specie densities after NO titration to calibrate the N and O-atom densities [6] . The O-atoms are coming from air impurity in the discharge.
ions. The intensities emitted by the N2 first positive (1st pos.) and N2 second positive (2nd pos.) systems and by the NOβ bands are measured to obtain the mentioned active specie densities after NO titration to calibrate the N and O-atom densities [6] . The O-atoms are coming from air impurity in the discharge.
2. Experimental Setup and NO Titration
The experimental setup is changed in comparison to the one used in [6] . The dia. 5 mm discharge tube is now directly connected to the 5 litre reactor as shown in Figure 1. The Ar-N2 microwave plasmas is always produced by a surfatron cavity at 2450 MHz, 100 Watt, 1 slm, but lowering the gas pressure from 8 Torr in [6] to 4 Torr to allow a satisfactory diffusion of the afterglow inside the 5 litre reactor.
The plasma is located inside the dia.5 mm tube with a length after the surfatron gap varying from about 5 cm in pure N2 to 20 cm in the Ar-2%N2 gas mixture. With a discharge tube length of 30 cm after the surfatron gap, the residence time before the afterglow in the 5 litre reactor is 3 × 10−3 s.
The optical emission spectroscopy across the reactor is performed by means of an optical fiber connected to an Acton Spectra Pro 2500i spectrometer (grating 600 gr/mm) equipped with a Pixis 256E CCD detector (front illuminated 1024 × 256 pixels).
The N-atom density is obtained from the I580 measured intensity after calibration by NO titration as described in [6] .
Figure 1. Microwave discharge and post-discharge reactor of 5 liters.
3. The Ar-N2 Early Afterglow
3.1. N-Atom Density
As reported in [6] , the pure late afterglow emission is produced by reaction R1 in Table 1.
The N2 (580 nm) band head intensity (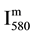 ) in arbitrary unit (a.u) was measured for constant parameters of the Acton spectrometer (grating 600 gr/mm, slit of 150 μm, integrating time 1 s).
) in arbitrary unit (a.u) was measured for constant parameters of the Acton spectrometer (grating 600 gr/mm, slit of 150 μm, integrating time 1 s).
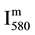 is then deduced from reaction R1 with v’ = 11 and hυ = hc/λ (580 nm), as follows:
is then deduced from reaction R1 with v’ = 11 and hυ = hc/λ (580 nm), as follows:
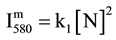 (1)
(1)
with k1 explicited in [4] - [6] .
The reaction R1 produced with an excess of Ar atoms results in a change of the N2(B, v') distribution as compared to pure N2 at a given aN+N value. The N + N recombination coefficient aN+N has been calculated in [6] in conditions of pink and late afterglows for Ar-xN2 gas mixture with x from 2% to 100%.
Equation (1) becomes:
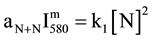 (2)
(2)
By NO titration,it has been verified the same k1 value inside the error bars as for pure N2 [6] :
k1 = 0.6 (+/− 0.3)10−26 cm6 counts/s with
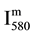 in counts/s and [N] in cm−3.
in counts/s and [N] in cm−3.
It is obtained aN+N = 0.9 for pure N2 and aN+N = 0.5 for the Ar-2% N2 mixture in the 5 litre reactor.
This result indicates that the early afterglow in N2 is dominated by the N+N recombination as expressed by R1.
The N-atom density is then obtained in the 5 litre reactor by taking into account the change of diameter from 2.1 cm in the tube to 15 cm in the reactor.
It is reported in Figure 2 the N-atom density variation with the %N2 into Ar
A slow increase of N-atom density is found in the range 2% - 10% N2 to reach a constant value of (5 - 6) × 1014 cm−3 between 10 and 100% N2. The uncertainty on N-atom density is estimated to be 30% [6] .
Table 1. Kinetic reactions in Ar-N2 afterglow.
Figure 2. Active species density versus the %N2 into the Ar-N2 early afterglow in the 5 litre reactor at 4 Torr, 1 Slm, afterglow time of 3 × 10−3 s, plasma 100 Watt.
3.2. Density of O-Atoms in Impurity in the Ar-N2 Early Afterglow
The NOβ bands are presently observed as a result of the recombination of N and O atoms by reaction R2. In a similar way than for Equation (1), the NO (320 nm) measured band intensity ( ) is deduced from reaction R2 as follows:
) is deduced from reaction R2 as follows:

The coefficients in k3 are explicited in ref. 6 as for k1.
The O atom density can be deduced from the N-atom density by considering the aN+N.


with k4 = k1/k3.
After several NO titration experiments, it was found in [6] : k4 = 1(+/−0.4). From k4 obtained by NO titration, the O-atom density in the Ar-N2 early afterglow inside the reactor was determined by Equation (4) after measurements of

As shown in Figure 2, there is a slow decrease of the O-atom density from 3 to 2 × 1013 cm−3 between 2% to 100%N2.
3.3. Density of N2(A) Metastable Molecules
It has been detected the N2(C, 1® B, 0) emission at 316 nm near the NOβ emission at 320 nm which is used as in [4] - [6] to determine the density of the N2(A) metastable molecule.
It is considered that the N2 2nd positive system in the early afterglow is produced by reaction R3.
The N2 (316 nm) measured intensity (

with k5 explicited in [6] .
From Equations (3) and (5), it comes the following


with k6 = k3/k5. The N2(A) density is then obtained from equation (6) with the N and O atom densities previously determined.
As shown in Figure 2, the N2(A) density kept a constant value in the Ar-N2 gas mixture. It is estimated that it is obtained the order of magnitude of N2(A) density in the range 1010 - 1011 cm−3.
3.4. Density of N2(X, v > 13) Molecules
The production of N2(B, 11) by R1 in the early afterglow is less than 1 ( aN+N < 1).
Other collisional processes in the pink afterglow [7] also excite the N2(B) states, in addition to reaction R1.
For this other part (1 − aN+N), it is considered the reactions R4 and R5 whose rate coefficients are reported in [4] - [6] . The contribution of reactions R4 and R5 on


where kR4, kR5 are the rate coefficients of reactions R4, R5. As

With the experimental values of aN+N and of N and N2(A) densities, it is found that


From the obtained values of N-atom and N2(A) density, it was deduced the values of [N2(X, v > 13)] as reproduced in Figure 2.
It is observed about one order of magnitude lower N2(X, v > 13) density as compared to N values.
Such values of [N2(X, v > 13)] can be considered as an estimated value depending on the R5 rate coefficient.
3.5. Density of

The emission of the


The


with




By comparing the intensities of Im316 from Equation (5) and Im391 from equation (10), it is calculated:

with k11 increasing from 6.6 10−2 in pure N2 to 0.17 in Ar-2%N2.
By assuming the equality [N2, X, v>12] = [N2, X, v > 13], it is found a


Compared to published data [11] [12] , the value of

4. Interest of Ar-N2 Gas Mixture for Surface Treatments
It is reported in Figure 3 the N/N2, N2(X, v > 13)/N2, N2(A)/N2 and

Figure 3. Density ratios of active species on N2 versus the %N2 in the Ar-N2 gas mixtures. In addition

Ar. Clearly, there is an interest of low %N2 to increase the active species density relative to N2 if it can be considered that the Ar atoms have no influence on the surface processes.
The

There is thus an interest of Ar-xN2 gas mixtures with x = 2% - 20% for surface treatments with high N, N2(A, Xv > 13) and

The

This increase of


5. Conclusions
Densities of N and O atoms (the O-are coming from air impurity), N2(A) and N2(X, v > 13) metastable molecules and

The density of these active species are obtained by comparing the N2 (580 nm), NOβ (320 nm), N2 (316 nm) and

It is found densities in the ranges of (2 - 6) × 1014 cm−3 for N-atoms, one order of magnitude lower for both N2(X, v > 13) and O-atoms (coming from air impurity), of 1010 - 1011 cm−3 for N2(A) and of 108 - 109 cm−3 for
The densities obtained by these line-ratio measurements are with an uncertainty of 30% for N-atoms and the order of magnitude for O-atoms and N2(A) metastable molecules. Estimated densities values are obtained for the N2(X, v > 13) metastable and

It is found that the main interest of N2 dilution into Ar is to increase the N/N2 dissociation from 0.5% in N2 to about 10% in the Ar-2%N2 which could be of interest for surface reactions of N-atoms with less N2 molecules. The other N2(A)/ N2, N2(X, v > 13)/N2 density ratios are also increasing at low %N2 into Ar. It is not the case for the


Cite this paper
AndreRicard,HayatZerrouki,Jean-PhilippeSarrette, (2015) Determination of N and O-Atoms, of N2(A) and N2(X, v> 13) Metastable Molecules and N2+ Ion Densities in the Afterglows of Ar-N2 Microwave Discharges. Journal of Analytical Sciences, Methods and Instrumentation,05,59-65. doi: 10.4236/jasmi.2015.54007
References
- 1. Villeger, S., Sarrette, J.P. and Ricard, A. (2005) Synergy between N and O Atom Action and Substrate Temperature in a Sterilization Process Using a Flowing N2-O2 Microwave Post-Discharge. Plasma Process and Polymers, 2, 709-711.
http://dx.doi.org/10.1002/ppap.200500040 - 2. Villeger, S., Sarrette, J.P., Rouffet, B., Cousty, S. and Ricard, A. (2008) Treatment of Flat and Hollow Substrates by a Pure Nitrogen Flowing Post Discharge. Application to Bacterial Decontamination in Low Diameter Tubes. European Physical Journal Applied Physics, 42, 25-32.
http://dx.doi.org/10.1051/epjap:2007177 - 3. Ricard, A., Gaboriau, F. and Canal, C. (2008) Optical Spectroscopy to Control a Plasma Reactor for Surface Treatments. Surface and Coatings Technology, 202, 5220-5224.
http://dx.doi.org/10.1016/j.surfcoat.2008.06.070 - 4. Ricard, A., Oh, S.G. and Guerra,V. (2013) Line-Ratio Determination of Atomic Oxygen and N2 Metastable Absolute Densities in an RF Nitrogen Late Afterglow. Plasma Sources Science and Technology, 22, Article ID: 035009. http://dx.doi.org/10.1088/0963-0252/22/3/035009
- 5. Ricard, A. and Oh, S.G. (2014) Densities of Active Species in N2 and N2-H2 RF Pink Afterglow. Plasma Sources Science and Technology, 23, Article ID: 045009.
http://dx.doi.org/10.1088/0963-0252/23/4/045009 - 6. Zerrouki, H., Ricard, A. and Sarrette, J.P. (2014) Determination of N and O-Atoms, of N2(A) and N2(X, v>13) Metastable Molecules and N2+ Ion Densities in the Afterglows of N2-H2, Ar-N2-H2 and Ar-N2-O2 Microwave Discharges. Journal of Physics: Conference Series, 550, Article ID: 012045.
http://dx.doi.org/10.1088/1742-6596/550/1/012045 - 7. Levaton, J. and Amorim, J. (2012) Metastable Atomic Species in the N2 Flowing Afterglow. Chemical Physics, 397, 9-17.
http://dx.doi.org/10.1016/j.chemphys.2011.11.010 - 8. Sa, P.A., Guerra, V., Loureiro, J. and Sadeghi, N. (2004) Self-Consistent Kinetic Model of the Short Lived Afterglow in Flowing Nitrogen. Journal of Physics D: Applied Physics, 37, 221-231.
http://dx.doi.org/10.1088/0022-3727/37/2/010 - 9. Piper, L.G. (1994) Further Observations on the Nitrogen Orange Afterglow. Journal of Chemical Physics, 101, 10229- 10236. http://dx.doi.org/10.1063/1.467903
- 10. Kang, N., Lee, M., Ricard, A. and Oh, S.G. (2012) Effect of Controlled O2 Impurities on N2 Afterglows of RF Discharges. Current Applied Physics, 12, 1448-1453.
http://dx.doi.org/10.1016/j.cap.2012.04.009 - 11. Sadeghi, N., Foissac, C. and Supiot, P. (2001) Kinetics of N2(A) Molecules and Ionization Mechanisms in the Afterglow of a Flowing N2 Microwave Discharge. Journal of Physics D: Applied Physics, 34, 1779-1788. http://dx.doi.org/10.1088/0022-3727/34/12/304
- 12. Ferreira, J.A., Stafford, L., Leonelli, R. and Ricard, A. (2014) Electrical Characterization of the Flowing Afterglow of N2 and N2/O2 Microwave Plasmas at Reduced Pressure. Journal of Applied Physics, 115, Article ID: 163303.
- 13. Fehsenfeld, F.C., Ferguson, E.E. and Schmeltekopf, A.J. (1966) Thermal Energy Ion-Neutral Reactions Rates VI. Some Ar+ Charge Transfer Reactions. Journal of Chemical Physics, 45, 404-405.
http://dx.doi.org/10.1063/1.1727351





