Journal of Analytical Sciences, Methods and Instrumentation
Vol.05 No.03(2015), Article ID:59367,8 pages
10.4236/jasmi.2015.53004
Shale Oil Solvent Extraction of Central Jordan El-Lajjun Oil Shale
Hani M. Alnawafleh1,2*, Feras Y. Fraige1,3
1Faculty of Engineering, Al-Hussein Bin Talal University, Ma’an, Jordan
2Faculty of Engineering, Tafila Technical University, Tafila, Jordan
3Faculty of Engineering, King Saud University, Al-Muzahmiayah Branch, Riyadh, Saudi Arabia
Email: *hanialnawafleh@ahu.edu.jo, hani1995@yahoo.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 31 July 2015; accepted 29 August 2015; published 2 September 2015
ABSTRACT
The extraction of the organic matter (OM) from oil shale (OS) can be achieved by several pro- cessing techniques. Normally, these techniques can remove high proportion of the organic material contained in oil shale. In this work, organic solvents extraction experiments were implemented to investigate the effect of various parameters on Jordanian El-Lajjun oil shale extractability. Results indicate that the approximate organic matter content in studied El-Lajjun oil shale is 17.48%, and 75% of OS sample particles diameters are less than 270 μm. The grain size has minor effect on shale oil extraction via organic solvents. Among eleven solvents used, the highest yield is obtained via the tetrahedrofuran (THF), whereas, with the use of solvent mixtures, the highest bitumen yield is obtained through the mixture of THF and toluene. The solvation variability is related to mode of extraction and various physicochemical factors such as extraction temperature, pressure, solvent type and mixing time, which result in different OM yield. The results indicate that the solvent extraction could be potential for shale oil extraction from Jordanian El-Lajjun OS under certain conditions of temperature, pressure and solvent type used.
Keywords:
Oil Shale, Organic Matter, Bitumen, Solvent Extraction, El-Lajjun, Jordan

1. Introduction
Oil shale (OS) deposits are widely distributed in the world. Oil shales are sedimentary rocks contain hydrogen rich organic matter (OM) that is known as kerogen and bitumen [1] . Kerogen is OM that has not gone through oil window of elevated temperature and pressure necessary to generate conventional light oil [2] -[4] .
Jordanian oil shales belong to the upper cretaceous and lower tertiary formations [5] , which are widespread throughout the country [6] . The oil shale is generally not exposed, and all the geological investigations so far conducted are based mainly upon shallow boreholes [6] . The OS in Jordan is basically kerogen rich, bituminous limestone that is deposited in shallow marine environment [7] [8] . The OM content of the Jordanian OS varies from locality to the other, but the general trend is that the central Jordan deposits are richer than those of the Yarmouk Basin [9] . Kerogen, the insoluble part of the OM, makes more than 80% of the total OM content. Bitumen content varies from few percent up to 20% of the total OM content [7] .
The extraction of shale oil from its sources is usually undertaken above ground (ex-situ processing) and some recent technologies perform this in-situ. In both cases, the immature kerogen is converted to synthetic shale oil and OS gas. Most conversion technologies involve heating OS at temperature between 450˚C and 500˚C in the absence of oxygen [10] . Solvent extraction involves dissolving compounds from a solid into a solvent or from a solution into another solvent [11] [12] . Solvent extraction of an OM from OS consists of several steps, including solvation of the OM by extraction solvent, desorption from the matrix surface, and, finally, transportation of the OM into the bulk extraction liquid [13] . The kinetics of solvent extraction is a function of both the various chemical reactions occurring in the system and the rates of diffusion of the various species that control the chemistry of the extraction process [12] . One of the standard methods for shale oil extraction using solvent is the soxhlet extraction [13] [14] . Solvent extraction of shale oil can be carried out under super critical conditions in order to affect the extraction of kerogen from the raw OS material [15] -[17] . Stirrer tank reactors and accelerated solvent extractors can also be used. With the solvent extraction process, the effects of particle size, extraction time, extraction temperature, mixing rate, solvent-to-oil-shale ratio, and type of solvent are mostly important and should be considered [13] [18] .
There is limited number of evaluation studies that are carried out in the field of processing of Jordanian OS. Those evaluation studies aimed to investigate the suitability of the well known OS processing methodologies to the use, processing, and extraction of shale oil from the Jordanian oil shales, e.g. [19] -[23] . The work on shale oil extraction from Jordanian oil shale is still open and limited research has been undertaken (e.g. [19] [24] -[26] ). Still many aspects need to be investigated. This work presented here aims to investigate the extraction properties of El-Lajjun OS via organic solvents. Different methodologies have been implemented. Important factors controlled their extractability have been investigated.
2. Sampling and Methodology
Oil shale sample brought from open pit excavated in El-Lajjun in Central Jordan (Figure 1). The studied oil shale
Figure 1. Location of El-Lajjun oil shale deposit.
Table 1. Solvent mixtures used and their percentage.
from El-Lajjun is very hard bituminous limestone. The outer OS surface is buff creamy due to weathering. Full sample preparation methodology is reported in previous papers [9] [27] . The methodology implemented covered OS petrography, mineralogy, organic carbon (TOC) determination and fisher assay. The OS sample has been analyzed for its TOC content via Carbon Determinator available at the Natural Resources Authority (NRA) in
Solvent extraction adopted in this study has been carried out in three different modes; namely soxhlet extraction, extraction via mixing and stirring, and lastly supercritical fluid extraction. Oil shale solvent extraction was carried out with eleven different solvents and nine solvent mixtures (Table 1).
In soxhlet extraction, about four grams of comminuted oil shale were transferred to a thimble which was then placed in the soxhlet chamber (100 ml). The soxhlet chamber was fitted to a distillation flask containing 200 ml of the solvent. Soxhlet extraction was carried out for 24 hrs. The extract then condensed by rotary evaporator and then dried under a stream of N2 gas. The extractable organic matter (yield) was then determined.
To compare the performance of soxhlet extractor on shale oil yield with stirring performance, 200 ml of 1:1 mixture of THF-Acetone were used to extract the shale oil. Parameters investigated are temperature, time, and grain size effect. Under these conditions, OS sample was stirred on magnet hot plate at 1000 rpm. After the completion of each run, the sample was filtered and then the solvent was separated from liquor via rotary evaporator. Then the remaining part of the procedure was similar to that explained above for soxhlet extraction.
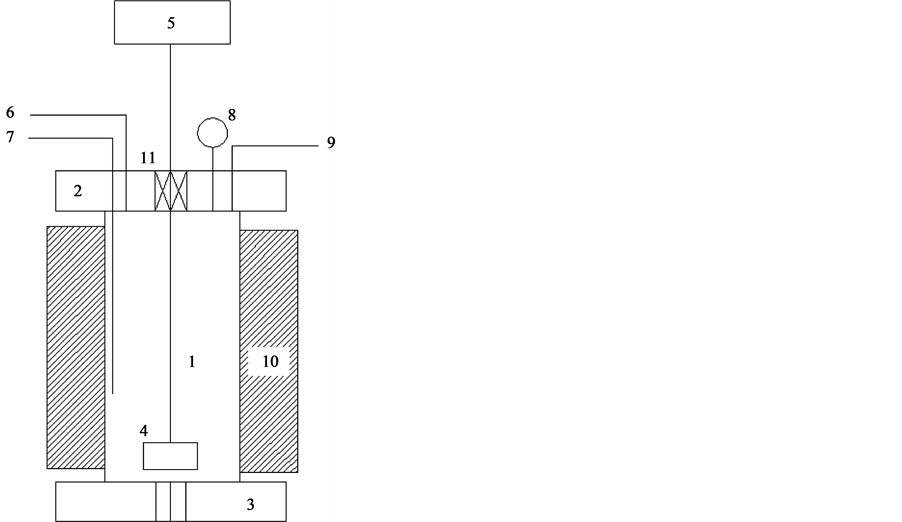
For elevated pressure and temperature conditions, a home-made reactor is designed by the authors and manufactured in the Royal Scientific Society, Jordan. Figure 2 shows schematic diagram of the reactor. A sample with specific weight is charged with solvent and temperature and pressure are raised to set points where it remains constant during the extraction time.
3. Results and Discussion
Material characterization; mineralogy, composition, texture and quality is reported in Alnawafleh and Fraige [27] . El-Lajjun oil shale is considered to be of high quality [27] . It is primarily carbonate rich in organic matter. The approximate OM content is 17.48%. Minor amounts of quartz are also present. The allochems or bioclasts are mainly foraminifera.
3.1. Grain Size Distribution
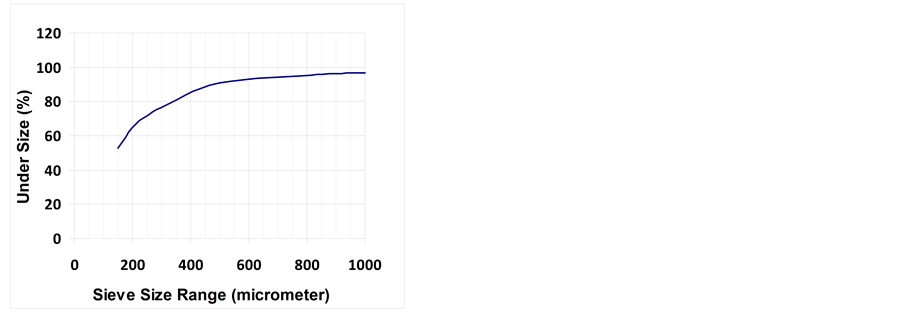
Grain size distribution of the studied El-Lajjun OS is shown in Figure 3. The % cumulative fraction passing (undersize) is plotted against size. 52.8% of the sample is less than 150 µm. The d50 is incorporated in this rage (i.e. < 150 µm). The d75 = 270 µm, which means that 75% of the sample particles diameters are less than 270 µm and 25% are greater. This could advantage for any future mining and utilization of OS since 50% - 70% of the total energy used to recover ore is consumed in grinding [28] .
3. 2. Effect of Solvent Type on Shale Oil Extractability
The effect of solvent type on the shale oil extractability is shown in Figure 4 and Figure 5, respectively. Results
Figure 2. Schematic diagram of solvent extraction reactor. 1: main reactor body; 2: top cap; 3: bottom cap; 4: stirrer; 5: stirrer display and control; 6: liquid reactant line; 7: temperature measuring probe; 8: pressure measuring tap; 9: nitrogen gas line; 10: nlectric heater; 11: stirrer rod bearing and sealing.
Figure 3. Grain size distribution curve for El-Lajjun OS.
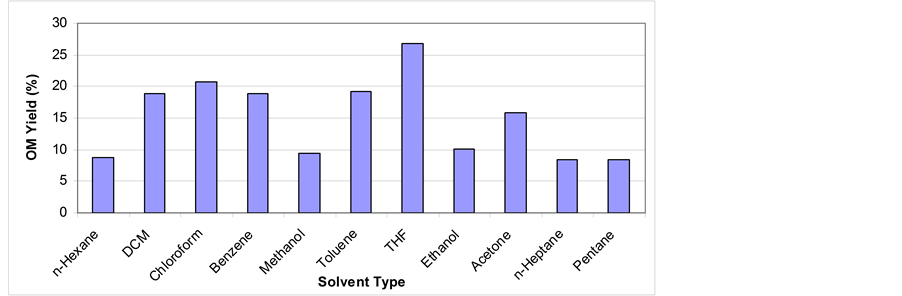
Figure 4. OM yield % of different solvent type via soxhlet extraction for 24 hrs; grain size < 150 µm.
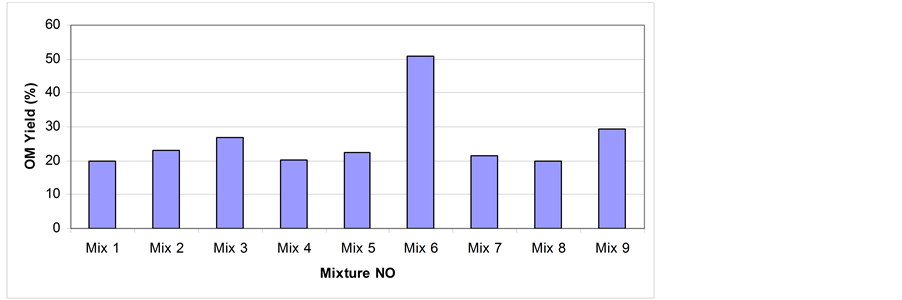
Figure 5. OM yield % of solvents mixtures via soxhlet extraction for 24 hrs; grain size < 150 µm.
show that different solvents have different OM yield and the polar organic solvents have better ability to dissolve the OM than non-polar solvents. This is due to several reasons include chemical composition of the solvent and the cohesion of atoms with each other which helps to attach OM and easily extract it. Another factor is that the polar solvents have low boiling points compared with non-polar ones which mean that the energy required to heat the non-polar is quite high compare with that of polar solvents [29] -[31] . As shown in Figure 4, the best polar solvent is THF (OM yield % = 27%) because of its complex chemical composition, cohesion between atoms, and strong structure that can dissolve the OM easily [31] . Due to the low boiling point of THF, more runs can be obtained via soxhlet extraction over 24 hrs. This might give more extraction yield. Quite similar results were obtained by Anabtawi and Uysal [32] . The THF also reported by Koel et al. [13] and Matouq et al. [33] gave the highest yield. A study by Shawaqfeh and Al-Harahsheh [26] and that by Bsieso [19] indicated that the yield was enhanced by the use of polar solvents. Among nine solvents mixtures used, mix 6 and mix 9 are considered to be the best yielding mixtures (Table 1, Figure 5).
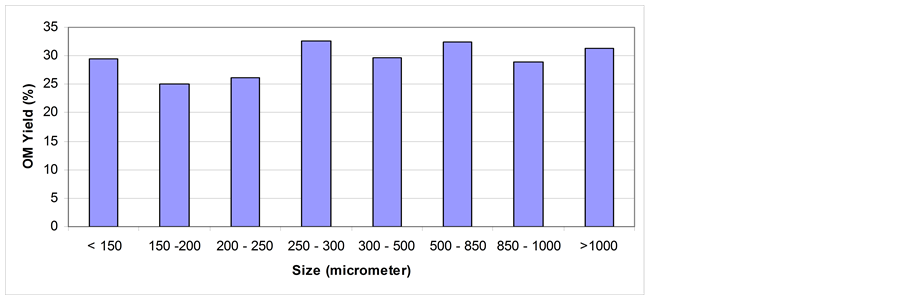
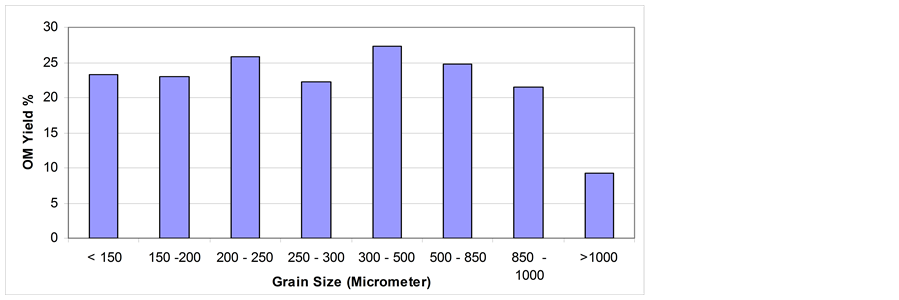
3.3. Effect of Grain Size on Shale Oil Extractability
The effect of grain size on shale oil extractability via soxhlet extraction and mixing and stirring extraction is shown in Figure 6 and Figure 7 respectively. Via soxhlet extraction the highest yield is obtained generally for gain size > 250 µm. However, clear fluctuation in OM yield on different grain sizes is obtained. This fluctuation indicates that the grain size has minor effect on OM yield. Quite similar result has been reported by Anabtawi and Uysal [32] .
In comparison with OM yield obtained with soxhlet extraction, the OM yield via mixing and stirring shows quite different trend especially for grain sizes > 300 µm. Beyond this size, the general trend in OM yield % is decreasing. This might be due to the mixing time of 10 mins that is not enough to dissolve much more quantities of OM. Compared with that of soxhlet extraction for 24 hrs, the yield via mixing and stirring is generally low. The extraction kinetics, dissolution thermodynamics, and the mode of mass transfer from the particles in different grain sizes can be differ resulted in different OM yield.
On fine grain sizes (i.e. < 250 µm) problems of agglomeration and coagulation could be raised which will impede agitation, hinder dissolution, and obstruct percolation processes. The solution drainage or settling velocity will be small due to high resistance to solvent flow inside the extractor under gravity settling condition [31] .
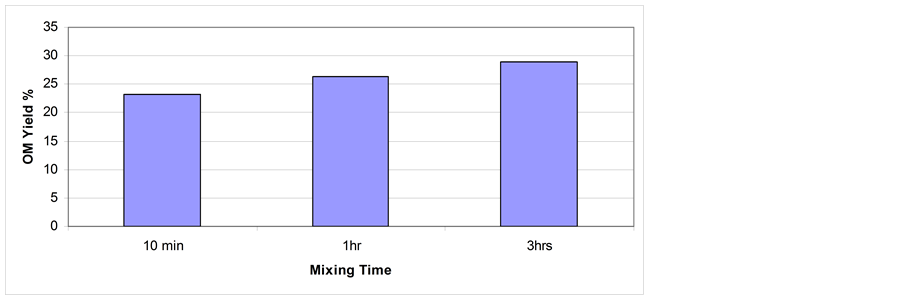
3.4. Effect of Mixing Time on Shale Oil Extractability
The effect of mixing time on shale oil extraction is shown in Figure 8. Experiments carried out using THF- Acetone mixture (1:1) via stirring at 50˚C and 1000 rpm of the grain size fraction < 150 µm. Results show that the OM yield is increased with increasing mixing time. With increasing mixing time, the interaction between the OS particles and solvent molecules will increase, leading to better OM dissolution [31] .
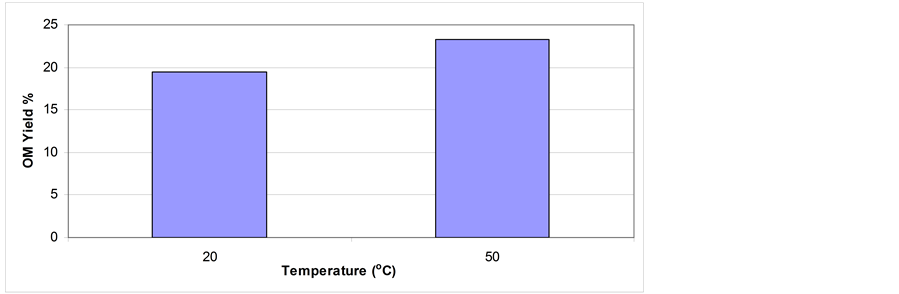
3.5. Effect of Extraction Temperature on Shale Oil Extractability
The effect of extraction temperature on shale oil extraction via mixing and stirring is shown in Figure 9. At nearly room temperature conditions, the OM yield is quite low compared with that at 50˚C in which the OM yield increased substantially. Preheating the OS to temperature below the boiling point of the solvent increases the OM solubility and extractability. Compared with these results from mixing and stirring experiments, significant OM release expressed as high OM yield is gained using supercritical fluid extraction (Figure 10). Higher extraction temperature especially in supercritical conditions resulted in higher oil yield [26] [34] . Increasing the temperature decreases the adhesiveness force, viscosity, and surface tension force of the oil contained in the shale and substantial extraction can be achieved in a relatively short time by heating the OS to a temperature of 50˚C [31] . The results presented previously show that the extraction behavior differs from one procedure to another under certain experimental conditions. Supercritical extraction is preferred to be followed.
Figure 6. Effect of grain size on OM yield using THF-Acetone mixture (1:1) via soxhlet extraction for 24 hrs.
Figure 7. Effect of grain size on OM yield using THF-Acetone mixture (1:1) via stirring for 10 mins at 50˚C and 1000 rpm.
Figure 8. Effect of stirring time on OM yield using THF-Acetone mixture (1:1) via stirring at 50˚C and 1000 rpm, grain size <150 µm.
Figure 9. Heating temperature effect on OM yield using THF-Acetone mixture (1:1) via stirring at 1000 rpm for 10 mins, grain size < 150 µm.
Figure 10. Yield increase via the designed reactor using THF-Acetone (1:1) at elevated temperature and increased mixing rate. Internal reactor pressure is 5 bars. Grain size < 150 µm. Extraction time 10 mins.
4. Conclusion
In this study the shale oil extraction from Jordanian OS via organic solvents has been investigated. 75% of El- Lajjun OS sample particles diameters are less than 270 µm. The grain size has minor effect of shale oil extraction via organic solvent and procedure implemented in this study. The THF is found to be the best solvent among eleven solvents (polar and non-polar) used in the extraction procedure via soxhlet extraction. The OM yield obtained by soxhlet extraction for 24 hrs is greater than that obtained via mixing and stirring. The OM yield is increased with increasing solvation/mixing time, pressure and temperature. Solvent extraction is better to be conducted under supercritical conditions. The solvent extraction could be potential for shale oil extraction under certain conditions of temperature and pressure and solvent used.
Acknowledgments
This research project is funded by the Al-Hussein Bin Talal University (Research fund # 6/2009) and The Scientific Research Support Fund (# E/2/23/2008) of the Ministry of Higher Education and Scientific Research in Jordan. The authors are also thankful for the NRA for their assistance during the lab work.
Cite this paper
Hani M.Alnawafleh,11,Feras Y.Fraige,11, (2015) Shale Oil Solvent Extraction of Central Jordan El-Lajjun Oil Shale. Journal of Analytical Sciences, Methods and Instrumentation,05,35-43. doi: 10.4236/jasmi.2015.53004
References
- 1. Dyni, J.R. (2006) Geology and Resources of Some World Oil-Shale Deposits. Scientific Investigations Report 2005- 5294, USGS, U.S. Geological Survey, Reston, VA, 42 p.
- 2. Hunt, J.M. (1996) Petroleum Geochemistry and Geology. W.H. Freeman and Company, New York, 332 p.
- 3. Peters, K.E., Walters, C.C. and Moldowan, J.M. (2005) The Biomarker Guide. Vols 1 & 2, Cambridge University Press, New York, NY, 1155 p.
- 4. Tissot, B.P. and Welte, D.H. (1984) Petroleum Formation and Occurrence. Springer Verlag, Berlin, 1155 p.
- 5. Abed, A.M. (2000) The Geology of JORDAN and Its Environment and Water (in Arabic). Publication of the Jordanian Geologists Association, Amman.
- 6. Alali, J. (2006) Jordan Oil Shale, Availability, Distribution, and Investment Opportunity. NO. rtos-A117, The NRA, Jordan.
- 7. Abed, A. and Arouri, K. (2006) Characterization and Genesis of Oil shale From Jordan. University of Jordan, Amman.
- 8. Alnawafleh, H.M. (2007) Geological Factors Controlling the Variability of Maastrichtian Bituminous Rocks in Jordan. PhD Thesis, The University of Nottingham, Nottingham.
- 9. Alnawafleh, H.M. and Fraige, F.Y. (2013) Characterization of South and Central Jordan Oil Shales. European Journal of Scientific Research, 102, 589-595.
- 10. Groppo, J.G. (1995) Oil Shale Beneficiation for Processing. In: Snape, C., Ed., Composition, Geochemistry and Conversion of Oil Shales, Kluwer Academic Publishers, Dordrecht, Boston and London, 175-189. http://dx.doi.org/10.1007/978-94-011-0317-6_11
- 11. Choppin, G.R., Liljenzin, J.O. and Rydberg, J. (2002) Radiochemistry and Nuclear Chemistry. 3rd Edition, Butterworth-Heinemann, Woburn.
- 12. Rydberg, J., Cox, M., Musikas, C. and Choppin, G.R. (2004) Solvent Extraction Principles and Practice. 2nd Edition, Lavoisier, Paris. http://dx.doi.org/10.1201/9780203021460
- 13. Koel, M., Ljovin, S., Hollis, K. and Rubin, J. (2001) Using Neoteric Solvents in Oil Shale Studies. Pure and Applied Chemistry, 73, 153-159. http://dx.doi.org/10.1351/pac200173010153
- 14. Razvigorova, M., Budinova, T., Minkova, V., Ekinci, E., Atakul, H. and Petrova, B. (2006) Investigations on the Structure and Composition of Oil Shale for Utilization as Energy and Chemical Sources. No. A111, Natural Resources Authority, Amman.
- 15. Hu, H.Q., Zhang, J., Guo, S.C. and Chen, G.H. (1999) Extraction of Huadian Oil Shale with Water in Sub- and Supercritical States. Fuel, 78, 645-651. http://dx.doi.org/10.1016/S0016-2361(98)00199-9
- 16. Olukcu, N., Yanik, J., Saglam, M., Yuksel, M. and Karaduman, M. (1999) Solvent Effect on the Extraction of Beypazari Oil Shale. Energy & Fuels, 13, 895-902. http://dx.doi.org/10.1021/ef9802678
- 17. Torrente, M.C. and Galan, M.A. (2011) Extraction of Kerogen from Oil Shale (Puertollano, Spain) with Supercritical Toluene and Methanol Mixtures. Industrial & Engineering Chemistry Research, 50, 1730-1738. http://dx.doi.org/10.1021/ie1004509
- 18. Amin, S.K., Hawash, S., El Diwani, G. and El Rafei, S. (2010) Kinetics and Thermodynamics of Oil Extraction from Jatropha curcas in Aqueous Acidic Hexane Solutions. Journal of American Science, 6, 293-300.
- 19. Bsieso, M.S. (2006) Jordan’s Experience in Oil Shale Studies Employing Different Technology. Proceedings of the 26th Oil Shale Symposium, Golden, CO, 16-20 October 2006, 20 p.
- 20. Besieso, M. (2007) Jordan’s Commercial Oil Shale Strategy. Proceedings of the 27th Oil Shale Symposium, Golden, CO, 15-19 October 2007, 15 p.
- 21. Jaber, J.O. and Probert, S.D. (1999) Pyrolysis and Gasification Kinetics of Jordanian Oil-Shales. Applied Energy, 63, 269-286. http://dx.doi.org/10.1016/S0306-2619(99)00033-1
- 22. Fainberg, V. (1998) Integrated Oil Shale Processing into Energy and Chemicals Using Combined Cycle Technology. Energy Sources, 20, 465-481.
- 23. Jaber, J.O., Probert, S.D. and Williams, P.T. (1999) Evaluation of Oil Yield from Jordanian Oil Shales. Energy, 24, 761-781.
- 24. Allawzi, M.A., Awni Al-Otoom, A., Allaboun, H., Ajlouni, A. and Nseirat, F. (2011) CO2 Supercritical Fluid Extraction of Jordanian Oil Shale Utilizing Different Co-Solvents. Fuel Processing Technology, 92, 2016-2023. http://dx.doi.org/10.1016/j.fuproc.2011.06.001
- 25. Guo, S.H. (2000) Solvent Extraction of Jordanian Oil Shale Kerogen. Oil Shale, 17, 266-270.
- 26. Shawaqfeh, A. and Al-Harahsheh, A. (2004) Solvation of Jordanian Oil Shale Using Different Organic Solvents by Continuous Contact Mixing. Energy Sources, 26, 1321-1330.
http://dx.doi.org/10.1080/00908310490442024 - 27. Alnawafleh, H.M. and Fraige, F.Y. (2015) Analysis of Selected Oil Shale Samples from El-Lajjun, Central Jordan. International Journal of Sciences and Technology (IJST), In Press.
- 28. Wills, B.A. and Napier-Munn, T. (2006) Wills’ Mineral Processing Technology. 7th Edition, Butterworth-Heinemann, Oxford.
- 29. Berkovich, A.J., Young, B.R., Levy, J.H., Schmidt, S.J. and Ray, A. (1997) Thermal Characterization of Australian Oil Shales. Journal of Thermal Analysis, 49, 737-743.
http://dx.doi.org/10.1007/BF01996757 - 30. Kök, M.V. (2001) Thermal Investigation of Seyitömer Oil Shale. Thermochimica Acta, 369, 149-155.
http://dx.doi.org/10.1016/S0040-6031(00)00764-4 - 31. Tamimi, A. and Uysal, B. (1990) Parametric Investigation of Oil-Shale Extraction with Organic Solvents. Separation Science and Technology, 25, 1151-1159.
http://dx.doi.org/10.1080/01496399008051844 - 32. Anabtawi, M. and Uysal, B. (2009) Extraction of El-Lajjun Oil Shale. Separation Science and Technology, 30, 3363- 3373.
- 33. Matouq, M., Koda, S., Maricela, M., Alayed, O. and Tagawa, T. (2009) Solvent Extraction of Bitumen from Jordan Oil Shale Assisted by Low Frequency Ultrasound. Journal of the Japan Petroleum Institute, 52, 265-269. http://dx.doi.org/10.1627/jpi.52.265
- 34. Liauw, M.Y., Natan, F.A., Widiyanti, P., Ikasari, D., Indraswati, N. and Soetaredj, F.E. (2008) Extraction of Neem Oil (Azadirachta indica A. Juss) Using N-Hexane and Ethanol: Studies of Oil Quality, Kinetic and Thermodynamic. ARPN Journal of Engineering and Applied Science, 3, 49-54.
NOTES

*Corresponding author.