Journal of Analytical Sciences, Methods and Instrumentation
Vol.3 No.1(2013), Article ID:28975,5 pages DOI:10.4236/jasmi.2013.31002
Ultra Sensitive Determination and Preconcentration of Cd2+, Cu2+ and Pb2+ after Multi-Walled Carbon Nanotubes Adsorption
![]()
1Central Laboratory for Elemental and Isotopic Analysis, Nuclear Research Center, Atomic Energy Authority, Cairo, Egypt; 2Physics Department, Faculty of Science and Human Studies, Shaqra University, Shaqra, KSA; 3Chemistry Department, Faculty of Science, Jazan University, Jazan, KSA.
Email: rabuelkher@yahoo.com
Received September 10th, 2012; revised December 1st, 2012; accepted December 25th, 2012
Keywords: Multi-Walled Carbon Nanotubes; Graphite Furnace Atomic Absorption Spectrometry; Inductively Coupled Plasma Optical Emission Spectrometry
ABSTRACT
Multi-Walled carbon nanotubes are used as preconcentrating probes for the quantitative determination of trace cadmium, copper and lead in environmental and biological sample using graphite Furnace Atomic Absorption Spectrometry and inductively coupled Plasma Optical Emission spectrometry. The method is based on the electrostatic interactions of positively charged Cd+, Cu+ and Pb+ with the negatively charged multi-walled carbon nanotubes (MWCNTs) for the preconcentration and isolation of analytes from sample solutions. Effective preconcentration of trace cadmium, copper and lead was achieved in a pH range of 5 - 7, 5 - 7 and 4 - 7, respectively. The retained cadmium, copper and lead were efficiently eluted with 0.3 mol∙L−1 HCl for graphite Furnace Atomic Absorption Spectrometry determination. The multi-walled carbon nanotubes packed micro-column exhibited fairly fast kinetics for the adsorption of cadmium, copper and lead, permitting the use of high sample flow rates up to at least 3 mL∙min−1 for the flow injection on micro-column preconcentration without the loss of the retention efficiency. The detection limits (3σ) were 0.03, 0.01 and 0.5 ng∙mL−1 for Cd, Cu and Pb, respectively. The relative standard deviation under optimum condition is less than 2.9% (n = 10). The developed method was successfully applied to the determination of trace Cd, Cu and Pb in a variety of environmental and biological samples.
1. Introduction
Graphite furnace atomic absorption spectrometry (GFAAS) still continues to be one of most attractive approaches for trace element analysis in most laboratories due to its relatively low operational and instrumental costs, easy operation, and low sample throughput. However, direct GF-AAS determination of trace amounts of elements is usually difficult owing to its insufficient detection power. For this reason, the preliminary separation and preconcentration of trace elements from matrix is often required [1]. The majority of works published on flow injection (FI) on-line preconcentration for graphite furnace atomic absorption spectrometry (GF-AAS) has been executed with packed columns either by ion exchange or by adsorption [2]. Although CNTs have been proved to possess great potential applications in environmental protection by some investigators; the studies on the adsorption of trace pollutants with CNTs are still limited in the literature. The purpose of the present work is to assess the feasibility of the use of multi-walled carbon nanotubes as an adsorbent for determination of the trace elements Cd, Cu and Pb in environmental and biological samples with GF-AAS.
Multi-Walled carbon nanotubes have great potential as effective solid phase adsorbent for chelates, metal ions, organic compounds and organometallic compounds. This can be divided into single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs) according to the carbon atom layers in the wall of the nanotubes. Recently, functionalized carbon nanotubes have attracted more and more attention because of their interesting capabilities in different applications. When these materials are treated with the mixture of acids H2SO4 and HNO3, some of their physical properties can be changed, owing to the carboxyl group is partially induced at the sidewall of CNT, where the presence of oxygenated functional groups is the starting point for binding with a positive species [3-8].
Atomic Emission Spectroscopy (ICP-AES) [9] is one of several techniques available in analytical atomic spectroscopy [10]. ICP-AES utilizes plasma as the atomization and excitation source [10]. A plasma is an electrically neutral, highly ionized gas that consists of ions, electrons, and atoms [10]. ICP-OES has additional [11] advantages over the other techniques in terms of detection limits as well as speed of analysis. In ICP-OES sample experiences temperatures estimated to be in the vicinity of 10,000 K. This results in atomization and excitation of even most refractory elements with high efficiency so that detection limits for these elements with ICP-OES can be well over and order of magnitude better than the corresponding values of other techniques. The limit of quantitation values of most of the elements in ICP-OES in parts per million and even parts per billion. In number of analytical applications speed can be an important factor. Those advocating simultaneous ICP-OES regard it as the only method worth considering for this task because so much analyses sample in minutes is only fast enough if the sample preparation time takes only a few minutes [12,13].
Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP-AES) is one of the most common techniques for elemental analysis. Its high specificity, multielement capability and good detection limits result in the use of the technique in a large variety of applications [14]. All kinds of dissolved samples can be analyzed, varying from solutions containing high salt concentrations to diluted acids [14].
2. Experimental
2.1. Instrumentation
The measurements are performed using graphite furnace atomic absorption spectrometry (VARIO6, Analytik Jena). Hollow cathode lamps were used as the radiation sources and the operating parameters for GF-AAS are summarized in Table 1. A microwave digestion system was employed to digest the samples used for the validation of the developed method.
2.2. Reagents
All reagents were analytical grade. All metal solutions (1000 mg∙L−1) were purchased from MERCK for Certified Reference Materials. The working solutions were prepared by series dilution of stock solutions immediately prior to their use. Doubly de-ionized water (DDW, 18 MΩ∙cm−1) obtained from a Millipore system was used throughout the experiments. Multi-wall carbon nanotubes were used as sorbent: main range of diameter, 20 - 40 nm;
Table 1. The instrumental operating conditions of GF-AAS.
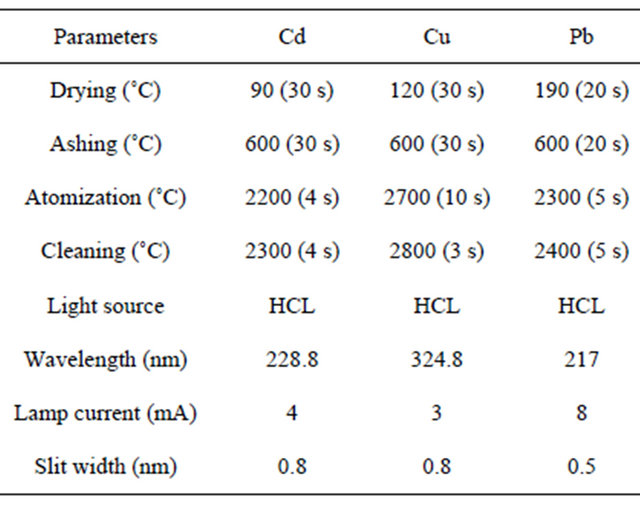
length, 1 - 2 μm; special surface area, 40 - 300 m2∙g−1.
2.3. Column Procedure
The preconcentration micro-column was made from a PTFE tube with effective length of 2 cm and inner diameter 3 mm. Next, 50 mg of multi-walled carbon nanotubes were packed into the micro-column. The micro-column plugged with a small portion of glass wool at both ends. Before use, 0.5 mol−1 HCL and doubly distilled deionized water successively passed through the micro-column in order to equilibrate, clean and neutralize it. A suitable aliquot of the sample solution containing 1.0 μg∙mL−1 of Cd (II), Cu (II) and Pb (II) in a volume of 50 ml was passed through the column after adjusting its pH to 6.0, at a flow rate of 3.0 ml∙min−1 controlled with a peristaltic pump. The bound metal ions were stripped off from the column with 0.1 mol HCl. The concentration of the metal ions in the elute was determined by GF-AAS.
2.4. Sample Preparation
River Nile water samples were collected and filtered through a 0.45 μm PTFE Millipore filter and acidified to a pH of about 1 with concentrated HCL prior to storage for use. The sediment samples from the River Nile were dissolved by adding HF, HNO3 and HClO4 to 1 g sample in closed vessel microwave system. The following certified reference materials were analyzed to check the accuracy of the developed method: Bovine liver 15776, Rice flour 1568a, GBW 07601 (human hair) and Fresh water SRM 1643d. The standard reference material was dissolved by adding HNO3 to 1 g sample in closed vessel microwave system (Myleston Mega 1200).
3. Results and Discussion
The adsorption of trace metals on MWCNTs depend on some parameters as, which must be adjusted before the analysis. These parameters are:
3.1. Effect of pH
The pH value plays an important role with respect to the adsorption of different ions on MWCNTs. The effect of the pH of the sample solution was evaluated using 10 μg·L−1 Cd (II), 10 μg·L−1 Cu (II) and 10 μg·L−1 Pb (II) in a pH range of 1 - 10. The results are shown in Figure 1. The optimum pH of the sample solution was in the range from 5 to 7. Therefore an average of a pH of 6 was selected in further experiments.
3.2. Effect of Shaking Time
Different shaking time (rang from 10 - 60 min) was studied for the percentage extraction of Cd (II), Cu (II) and Pb (II) by MWCNTs. The results showed that it took only 15, 18 and 20 min for Cd (II), Cu (II) and Pb (II) to reach maximum recovery (>90%), respectively, which indicated that, the equilibrium process is very fast.
3.3. Effect of Flow Rate
The flow rate of the sample solution affects the retention of cations on the adsorbent and the duration of complete analysis. Therefore, the effect of the flow rate of sample solution was examined under the optimum conditions (pH, eluent, etc.) by passing 50 ml of sample solution through the micro-column with a peristaltic pump. The flow rates were adjusted in rang of 1 - 5 ml∙min−1. It was found that the retention of the studied ions was practically not changed up to 3 ml∙min−1 flow rate. The recoveries of analytes decrease slightly when the flow rate is over 3 ml∙min−1. Thus, a flow rate of 3 ml∙min−1 is employed in this work.
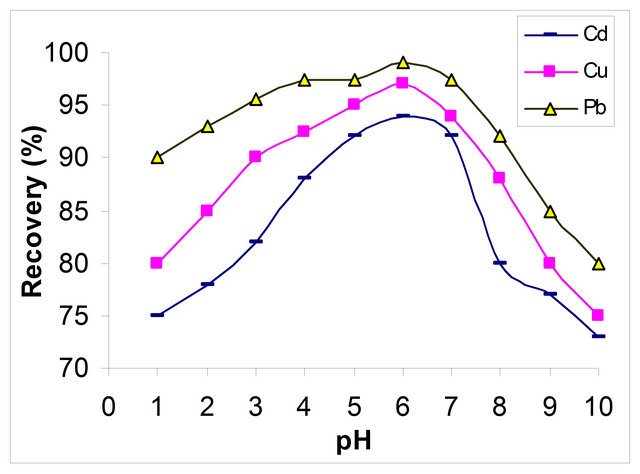
Figure 1. Effect of pH on adsorption of 10 μg∙L−1 Cd (II), Cu (II) and Pb (II) on MWCNTs (shaking time 20 min, temperature 27˚C).
3.4. Effect of Eluent Concentration, Maximum Sample Volume and Enrichment Factor
The eluent concentration was studied by using various concentrations and volumes HCl for adsorptions of retained Cd (II), Cu (II) and Pb (II). The obtained results are shown in Table 2. It was found that 2 ml of 0.3 mol∙L−1 HCl was sufficient for complete elution for these ions. Therefore, 2 ml of 0.3 mol∙L−1 HCl was used as eluent in further experiments.
In order to explore the possibility of enriching low concentrations of analytes from large volume of solution, the effect of sample volume on the retention of metal ions was also investigated. For this purpose, 50, 100, 150, 200, 250 and 300 ml of sample solutions containing 1 μg∙ml−1 of Cd (II), Cu (II) and Pb (II) were passed through the micro-column at the optimum flow rate. Quantitative recovery (>98%) of Cd (II), Cu (II) and Pb (II) was obtained for sample volume of 150 ml and at higher volume percent of recovery decreased. Therefore, 150 ml of sample solution was adopted for the preconcentration of analytes from sample solutions. The adsorbed metal ions could be eluted with 2 ml 0.3 mol∙L−1 HCl, so the enrichment factor achieved by this method was 150.
3.5. Effects of Coexisting Ions
The effects of common coexisting ions on the absorption of Cd (II), Cu (II) and Pb (II) on MWCNTs were investigated. In these experiments, solutions of 1 μg·L−1 of Cd (II), Cu (II) and Pb (II) containing the added interfering ions were treated according to the recommended procedure. The tolerance limit was set as the amount of ions causing recoveries of the examined elements to be less than 90%. The results showed that in excess of 1000- fold K (I), Na (I), Ca (II), 50-fold Fe (III), Co (II), Cr (III) and 30-fold Mn (II), Ni (II), Zn (II), Hg (II) ions had no significant interferences in the determination of the analytes. This is due to the low adsorbing capacity or rates for interfering ions. It can be seen that the presence of major cations has no obvious influences on the determination of traces of Cd (II), Cu (II) and Pb (II) in the
Table 2. Eluent data (%) for Cd (II), Cu (II) and Pb (II) adsorbed on MWCNTs (Eluent volume 2 ml).

environmental and biological studied samples.
3.6. Analytical Precision and Detection Limits
Under the selected conditions, the standard solutions were enriched and analyzed following the general procedure. The detection limits (3σ) were found to be 0.03, 0.01, 0.5 ng∙ml−1 for Cd (II), Cu (II) and Pb (II), respectively. The relative standard deviation (RSD) of the eight replicate determinations were lower by 1.9%, which indicated that the method had good precision for the analysis of trace Cd (II), Cu (II) and Pb (II) in solution samples.
3.7. Application of the Method
The proposed method has been applied to the determination of trace Cd (II), Cu (II) and Pb (II) in standard reference material (Bovine liver 1577b, Organics in FreezeDried Mussel Tissue (Mytilus edulis) 2974, Human hair GBW07601, Rice flour 1568a, Fresh water 1643d and Lake sediment IAEA-SL-1) and River Nile water, sediment samples. The results were listed in Tables 3-5. The analytical results for the standard reference material were in good agreement with the certified values. For the analysis of natural River Nile water and sediment the GF-AAS and ICP-OES were used for the determination of traces Cd (II), Cu (II) and Pb (II). According to the results which are shown in Tables 5 and 6, the results showed an agreement between GF-AAS and ICP-OES.
Table 3. Analysis results for determination of Cd (II), Cu (II) and Pb (II) in biological standard reference material (SRM).
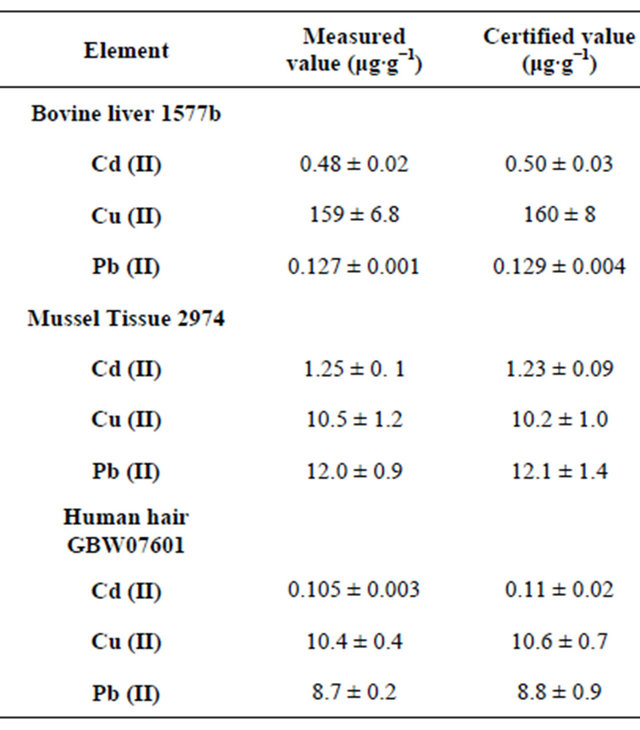
Table 4. Analysis results for determination of Cd (II), Cu (II) and Pb (II) in environmental standard reference material (SRM).

Table 5. Analysis results for determination of Cd (II), Cu (II) and Pb (II) in River Nile water.

Table 6. Analysis results for determination of Cd (II), Cu (II) and Pb (II) in River Nile sediment.

These results indicated the suitability of MWCNTs for selective solid-phase extraction and determination of traces Cd (II), Cu (II) and Pb (II) in biological and environmental samples.
4. Conclusion
In this study, a simple, rapid, selective, accurate and reliable method for the determination of trace levels of Cd (II), Cu (II) and Pb (II) was developed using MultiWalled carbon nanotubes (MWCNTs) as a solid-phase extractant. The most important characteristic of MWCNTs is its faster sorption and desorption for studied ions over other sorbents. This new developed method has been successfully applied to analyze traces Cd (II), Cu (II) and Pb (II) in biological and environmental samples. The precision and accuracy of the method are satisfied.
REFERENCES
- E. L. da Silva, E. M. Ganzarolli and E. Carasek, “Use of Nb2O5-SiO2 in an Automated On-Line Preconcentration System for Determination of Copper and Cadmium by FAAS,” Talanta, Vol. 62, No. 4, 2004, pp. 727-733. doi:10.1016/j.talanta.2003.09.014
- M. Sperling, X.-P. Yan and B. Welz, “Electrothermal Atomic Absorption Spectrometric Determination of Lead in High-Purity Reagents with Flow-Injection On-Line Microcolumn Preconcentration and Separation Using a Macrocycle Immobilized Silica Gel Sorbent,” Spectrochimica Acta Part B: Atomic Spectroscopy, Vol. 51, No. 14, 1996, pp. 1875-1889.
- E. Ballesteros, M. Gallego and M. Valcárcel, “Analytical Potential of Fullerene as Adsorbent for Organic and Organometallic Compounds from Aqueous Solutions,” Journal of Chromatography A, Vol. 869, No. 1-2, 2000, pp. 101-110. doi:10.1016/S0021-9673(99)01050-X
- J. R. Baena, M. Gallego and M. Valcárcel, “Group Speciation of Metal Dithiocarbamates by Sorption on C60 Fullerene,” Analyst, Vol. 125, No. 8, 2000, pp. 1495-1499. doi:10.1039/b004217j
- J. R. Baena, M. Gallego and M. Valcarcel, “Speciation of Lead in Environmental Waters by Preconcentration on a New Fullerene Derivative,” Analytical Chemistry, Vol. 74, No. 7, 2002, pp. 1519-1524. doi:10.1021/ac0111457
- H. X. Zhao, L. P. Wang, Y. M. Qiu, Z. Q. Zhou, W. K. Zhong and X. Li, “Multiwalled Carbon Nanotubes as a Solid-Phase Extraction Adsorbent for the Determination of Three Barbiturates in Pork by Ion Trap Gas Chromatography—Tandem Mass Spectrometry (GC/MS/MS) Following Microwave Assisted Derivatization,” Analytica Chimica Acta, Vol. 586, No. 1-2, 2007, pp. 399-406. doi:10.1016/j.aca.2006.12.003
- X. Y. Liu, Y. S. Ji, Y. H. Zhang, H. X. Zhang and M. C. Liu, “Oxidized Multiwalled Carbon Nanotubes as a Novel Solid-Phase Microextraction Fiber for Determination of Phenols in Aqueous Samples,” Journal of Chromatography A, Vol. 1165, No. 1-2, 2007, pp. 10-17. doi:10.1016/j.chroma.2007.07.057
- J.-X. Wang, D.-Q. Jiang, Z.-Y. Gu and X.-P. Yan, “Multiwalled Carbon Nanotubes Coated Fibers for Solid-Phase Microextraction of Polybrominated Diphenyl Ethers in Water and Milk Samples before Gas Chromatography with Electron-Capture Detection,” Journal of Chromatography A, Vol. 1137, No. 1, 2006, pp. 8-14. doi:10.1016/j.chroma.2006.10.003
- A. A. Warra and W. L. O. Jimoh, “Overview of an Inductively Coupled Plasma (ICP) System,” International Journal of Chemical Research, Vol. 3, No. 2, 2011, pp. 41-48.
- T. J. Manning and W. R. Grow, “Inductively Coupled Plasma—Atomic Emission Spectrometry,” The Chemical Educator, Springer Verlag, New York, Vol. 2, No. 1, 1997, pp. 1-19.
- V. V. Vallapragada, G. Inti and J. S. Ramulu, “A Validated Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES) Method to Estimate Free Calcium and Phosphorus in In Vitro Phosphate Binding Study of Eliphos Tablets,” American Journal of Analytical Chemistry, Vol. 2, No. 6, 2011, pp. 718-725.
- C. B. Boss and K. J. Fredeen, “Concepts, Instrumentation and Techniques in Inductively Coupled Plasma Optical Emission Spectrometry,” 3rd Edition, Perkin Elmer, Waltham, 1997.
- P. W. J. M. Boumans, “Inductively Coupled Plasma Emission Spectroscopy: Part 2,” In: P. J. Elving and J. D. Winefordner, Eds., Chemical Analysis, Vol. 90, John Wiley & Sons, New York, 1987.
- MiPlaza Materials Analysis, “Inductively Coupled PlasmaAtomic Emission Spectrometry (ICP-AES),” Tech. Note 112, Koninklijke Philips Electronics N.V., 2008.

