Paper Menu >>
Journal Menu >>
 Vol.2, No.6, 528-531 (2010) Health doi:10.4236/health.2010.26079 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ Local cerebral blood perfusion correlates with nerve fibre integrity in transient ischemic attack patients with middle cerebral artery stenosis: a pilot study Jiang Wu1, Ping Liu2, Jie Lei3, Jia Liu1,4, Hong-Liang Zhang1,4* 1Department of Neurology, First Hospital of Jilin University, Changchun, China 2Department of Orthopaedics, Fourth Hospital of Jilin University, Changchun, China 3Department of Paediatrics, First Hospital of Jilin University, Changchun, China 4Department of Neurobiology, Care Sciences and Society, Karolinska Institute, Stockholm, Sweden; *Corresponding Author: Hongliang.Zhang@ki.se Received 21 January 2010; revised 23 February 2010; accepted 26 February 2010. ABSTRACT Recent advances in neuroimaging contribute a lot to the accurate diagnosis and evaluation of cerebrovascular diseases. To explore the rela- tionship among blood perfusion, metabolism and brain structure integrity, 6 Chinese transient ischemic attack (TIA) patients with middle cere- bral artery (MCA) stenosis were examined by xenon-enhanced computed tomography (Xe-CT), magnetic resonance spectroscopy (MRS) and diffusion tensor imaging (DTI) to compare cere- bral blood flow (CBF) values, (choline + creatine)/ N-acetyl aspartate [(Cho + Cr)/NAA] values and fractional anisotropy (FA) values in the MCA territory. Our results showed that CBF values significantly decreased in the ipsilateral basal ganglion regions in all 5 cases with unilateral MCA stenosis, with a corresponding decrease of FA values in the same region. In conclusion, decreased blood perfusion may indicate nerve fibre damage in the dominating regions of st- enosed arteries. Keywords: Transient Ischemic Attack; Magnetic Resonance Imaging; Xenon-Enhanced Computed Tomography 1. INTRODUCTION Knowledge on recurrence, optimal evaluation and effect- tive prevention strategy of transient ischemic attack (TIA) is still lacking. An increasing body of evidence has revealed that TIA in Chinese population is more re- lated to intracranial artery stenosis [1]. Recent advances in neuroimaging contribute a lot to the accurate diagno- sis and evaluation of TIA and cerebrovascular stenosis. To address the relation between blood perfusion, struc- ture integrity and metabolism in the brain, we enrolled Chinese TIA patients with middle cerebral artery (MCA) stenosis to compare cerebral blood flow (CBF), (choline + creatine)/N-acetyl aspartate [(Cho + Cr)/NAA] and fractional anisotropy (FA) values of MCA territory measure by xenon-enhanced computed tomography (Xe-CT) and magnetic resonance imaging (MRI). 2. SUBJECTS AND METHODS A total of 6 consecutive TIA patients with MCA stenosis detected by TCD and confirmed by MRA, were enrolled to undertake Xe-CT, MRS and DTI examinations 3 days after the most recent attack in the department of radiol- ogy from January to June in 2009. TIA was as previ- ously defined [2]. Study protocol and informed consent were obtained from all participants in the study, which was conducted in accordance with institutional guide- lines. Patients with lacunar infarction in the basal gan- glion of CT or MRI were excluded from this research. All patients had a full clinical assessment, including electrocardiogram (ECG), carotid duplex Doppler Ul- trasonography, and mini mental status examination (MMSE) and National Institutes of Health Stroke Scale (NIHSS). American Nicolet TC8080 TCD machine, German Siemens MAGNETOM Avanto 1.5 T MR ma- chine was used to perform MRI, MRA, DTI and MRS examination, and American Diversified Diagnostic Products Xe-CT CBF system with German Simens PLUS4 CT were used to perform Xe-CT examination. MRI Protocol: MRI scanning, including conventional MR images, including T2-weighted, T1-weighted, and  J. Wu et al. / HEALTH 2 (2010) 528-531 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 529 529 fluid-attenuated inversion recovery (FLAIR) images, MRA, MRS and DTI were performed with the use of a clinical 1.5 T whole-body MR system Siemens MAG- NETOM Avanto with a conventional gradient system (Magnetom Vision, Siemens Medical System). Parame- ters are as follows: TSE/T1WI: TR 400 ms, TE 7.8 ms; TSE/T2WI: TR 3250 ms, TE 99 ms; Time-of-flight (TOF)-MRA, scanning time 6′01″, TR 25 ms, TE 4.6 ms, thickness 0.9 mm, band width 85 Hz; MRS(1H): two- dimensional MRS of basal ganglion, scanning time 7′12″, TR 1500 ms, TE 135 ms, band width 35 Hz; DTI: TR 3900 ms, TE 76 ms; DTI: TR 3900 ms, TE 76 ms, ma- trix 64 × 64, in-plane resolution was 1.875 × 1.875 mm. Xe-CT protocol: Xe-CT studies used a 28 to 33% concentration of medical-grade xenon gas mixed with O2. At the start of each study, a face mask was used to de- liver the mixed gas and the patient was closely moni- tored. The procedure used within the DDPI system in- volved obtaining two baseline scans before Xe inhala- tion and six scans during Xe inhalation for each of four axial planes of the brain, each 20 mm thick. CT scanning was performed at intervals of 1 min at the basal ganglial level. The resulting image was shown as a CBF map, on which CBF values could be extracted by placing regions of interest (ROIs) on corresponding brain tissue. The results of TCD and MRA were independent of the patients’ clinical manifestations, given by professional TCD and MRI technicians. And the (Cho + Cr)/NAA values, FA values and CBF values of the basal ganglion regions were measured and calculated by three respect- tive professional radiological physicians blinded to the clinical data of the subjects. Results are presented as mean ± SD. The data of FA and (Cho + Cr)/NAA values were analysed with nonparametric analysis, Wilcoxon Signed Ranks Test by software of SPSS 11.5 (for Win- dows OS). And CBF values were analysed with PEMS 3.0 to compare means of two samples. A probability value of < 0.05 was considered significant. 3. RESULTS All 6 subjects were male, aged 55.7 ± 10.3; ECG, carotid duplex Doppler ultrasonography, MMSE and NIHSS showed negative results. Average CBF values in bilateral ROIs were different, with the maximum difference of 12.2 ml/(100 g·min), and the minimum of 1.5 ml/(100 g·min); CBF values significantly decreased in the ipsi- lateral basal ganglion regions of stenosed MCA in all 5 cases with unilateral MCA stenosis (P < 0.05). (Table 1) FA values in stenosed MCA territory were significantly lower than those in the contra-lateral counterparts in 5 subjects with unilateral MCA stenosis. There was no Table 1. CBF values measured by Xe-CT [ml/(100 g·min)]. Subject No. Ipsilateral Contralateral p value 1 45.6 ± 17.0 51.9 ± 19.9 0.0000 2 45.2 ± 17.1 56.7 ± 20.9 0.0000 3 59.0 ± 20.3 63.4 ± 24.8 0.0000 4 32.9 ± 17.7 40.0 ± 14.7 0.0003 5 52.7 ± 17.5 64.9 ± 23.0 0.0000 *6 56.1 ± 18.4 57.6 ± 23.3 0.1270 * Bilateral MCA stenosis. difference when comparing (Cho + Cr) /NAA values of bilateral MCA territory. 4. REPRESENTATIVE CASE A 53-years old male with a history of untreated hyper- tension and diabetes mellitus, presented ictal left hemi- paresis for the first time. Dizziness, vertigo, nausea and dysarthria were absent. TCD suggested a reduced blood flow velocity of right MCA. Stenosed right MCA was confirmed by MRA. Xe-CT showed decreased CBF in the right basal ganglion, with CBF of 45.6 ± 17.0 ml/ (100 g·min) compared with 51.9 ± 19.9 ml/(100 g·min) in the right counterpart (p < 0.05) FA values in stenosed MCA territory were significantly lower than the counterpart. (p < 0.05) There was no significant difference between bilat- eral (Cho + Cr) /NAA values (p > 0.05) (Figure 1). 5. DISCUSSION Xe-CT is a quantitative method of CBF analysis, and multiple studies have validated the accuracy of CBF values obtained with Xe-CT [3]. MRS can evaluate the metabolism of brain tissues in vivo [4]. Abnormalities of neuronal structures lead to reductions in NAA quantity [5]. An increase in the Cho peak is associated with con- ditions such as demyelinating disease and brain tumors [6]. Also alterations in the NAA/Cho ratio have been reported with findings suggestive of neuronal damage caused by neuronal disorders [7]. In theory, the chronic or acute ischemia of brain tissue may result in the changes of (Cho + Cr)/NAA due to axonal degeneration or demyelination secondary to artery stenosis, while in the present study, no significant changes in (Cho + Cr)/ NAA value could be seen. The similar results can be seen in a previous MRS study in TIA without stenosed cerebral artery [8]. One reason may be argued that al- though there is a reduction of CBF in the territory of 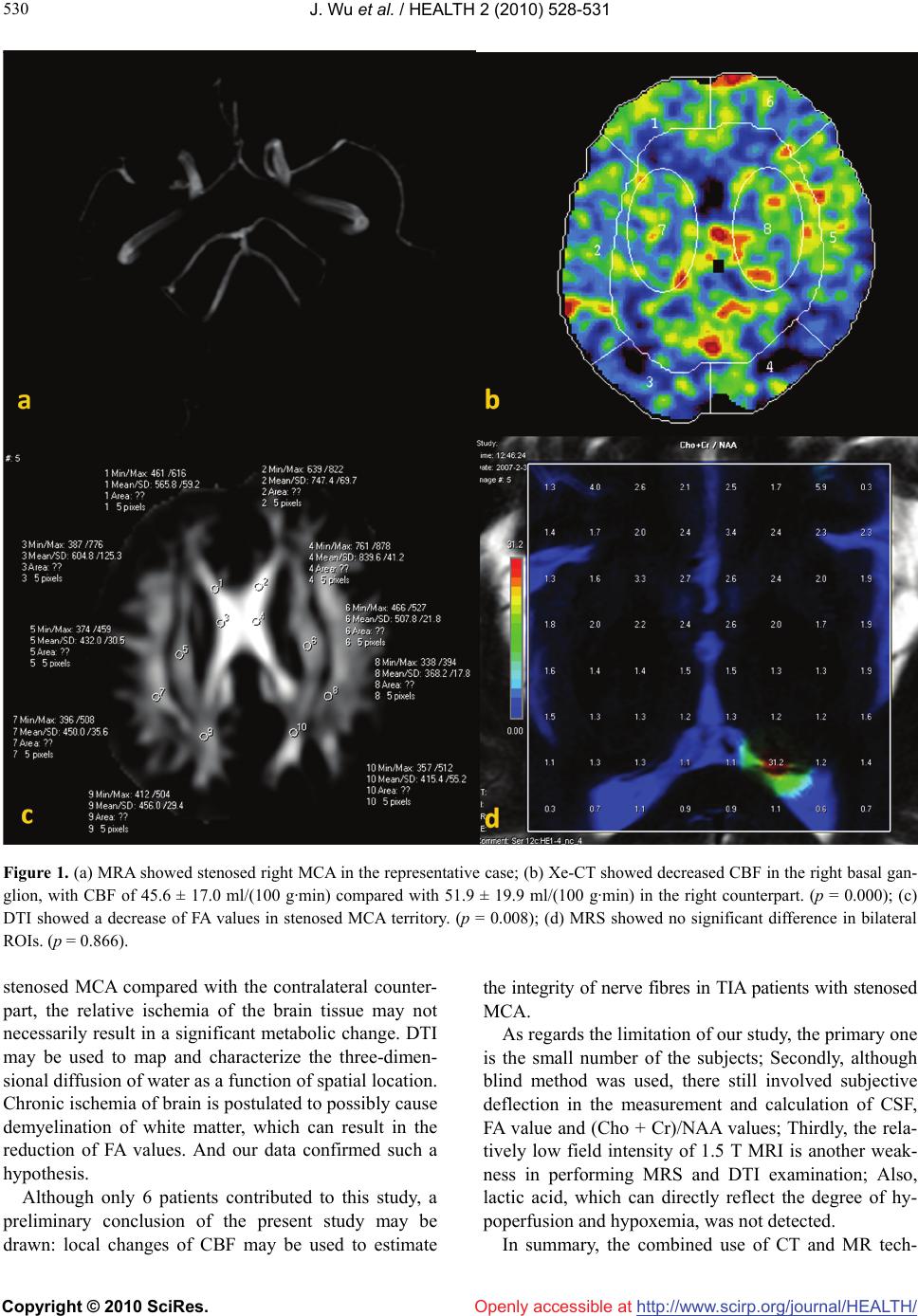 J. Wu et al. / HEALTH 2 (2010) 528-531 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 530 Figure 1. (a) MRA showed stenosed right MCA in the representative case; (b) Xe-CT showed decreased CBF in the right basal gan- glion, with CBF of 45.6 ± 17.0 ml/(100 g·min) compared with 51.9 ± 19.9 ml/(100 g·min) in the right counterpart. (p = 0.000); (c) DTI showed a decrease of FA values in stenosed MCA territory. (p = 0.008); (d) MRS showed no significant difference in bilateral ROIs. (p = 0.866). stenosed MCA compared with the contralateral counter- part, the relative ischemia of the brain tissue may not necessarily result in a significant metabolic change. DTI may be used to map and characterize the three-dimen- sional diffusion of water as a function of spatial location. Chronic ischemia of brain is postulated to possibly cause demyelination of white matter, which can result in the reduction of FA values. And our data confirmed such a hypothesis. Although only 6 patients contributed to this study, a preliminary conclusion of the present study may be drawn: local changes of CBF may be used to estimate the integrity of nerve fibres in TIA patients with stenosed MCA. As regards the limitation of our study, the primary one is the small number of the subjects; Secondly, although blind method was used, there still involved subjective deflection in the measurement and calculation of CSF, FA value and (Cho + Cr)/NAA values; Thirdly, the rela- tively low field intensity of 1.5 T MRI is another weak- ness in performing MRS and DTI examination; Also, lactic acid, which can directly reflect the degree of hy- poperfusion and hypoxemia, was not detected. In summary, the combined use of CT and MR tech-  J. Wu et al. / HEALTH 2 (2010) 528-531 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 531 531 niques to quantitatively assess blood perfusion, metabo- lism and water molecule diffusion may be a first step towards accurate diagnosis and evaluation of TIA and intracranial artery stenoses and may in the future allow effective prevention of stroke and monitoring of treat- ment strategy. 6. ACKNOWLEDGEMENTS This study was supported by the China Scholarship Council. We thank Hongwei Zhou, Jing Wang and Jing Xu, for kindly help in subject screening. REFERENCES [1] Li, D., Wang, M.L., Li, S.M. and Ling, F. (2008) Distri- bution and risk factors of steno-occlusive lesions in pa- tients with ischemic cerebrovascular disease. National Medical Journal of China, 88(17), 1158-1162. [2] Albers, G.W., Caplan, L.R., Easton, J.D., Fayad, P.B., Mohr, J.P., Saver, J.L., Sherman, D.G. and TIA Working Group (2002) Transient ischemic attack-proposal for a new definition. New England Journal of Medicine, 347 (21), 1713-1716. [3] Latchaw, R.E., Yonas, H., Hunter, G.J., Yuh, W.T., Ueda, T., Sorensen, A.G., Sunshine, J.L., Biller, J., Wechsler, L., Higashida, R., Hademenos, G. and Council on Cardio- vascular Radiology of the American Heart Association (2003) Guidelines and recommendations for perfusion imaging in cerebral ischemia: a scientific statement for healthcare professionals by the writing group on perfu- sion imaging, from the Council on Cardiovascular Radi- ology of the American Heart Association. Stroke, 34(4), 1804-1104. [4] Alkan, A., Sarac, K., Kutlu, R., Yakinci, C., Sigirci, A., Aslan, M. and Baysal, T. (2003) Early and late-state subacute sclerosing panencephalitis: chemical shift im- aging and single-voxel MR spectroscopy. American Journal of Neuroradiology, 24(3), 501-506. [5] Cecil, K.M. and Jones, B.V. (2001) Magnetic resonance spectroscopy of the pediatric brain. Top Magn Reson Imaging, 12(6), 435-452. [6] Irwan, R., Sijens, P.E., Potze, J.H. and Oudkerk, M. (2005) Correlation of proton MR spectroscopy and diffusion tensor brain MR imaging. Magn Reson Imaging 23(8), 851-858. [7] Kadota, T., Horinouchi, T. and Kuroda C. (2001) Devel- opment and aging of the cerebrum: assessment with pro- ton MR spectroscopy. American Journal of Neurora- diology, 22(1), 128-135. [8] Giroud, M., Walker, P., Guy, F., Lemesle, M., Lalande, A., Baudouin, N., Martin, D., Couveur, G. and Brunotte, F. (1999) Cerebral metabolism after transient ischemic at- tack. A 1H MR spectroscopy study. Neurological Re- search, 21(6), 563-565. |

