Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2010, 3, 550-555 doi:10.4236/jbise.2010.36077 Published Online June 2010 (http://www.SciRP.org/journal/jbise/ JBiSE ). Published Online June 2010 in SciRes. http://www.scirp.org/journal/jbise Gene silencing of E-selectin block recruitment of endothelial progenitor cell to vascular endothelium under flow Sunil Sharma, Masayuki Yoshida Life Science and Bioethics Research Center, Tokyo Medical and Dental University, Tokyo, Japan. Email: masavasc@tmd.ac.jp Received 16 February 2010; revised 20 March 2010; accepted 25 March 2010. ABSTRACT Short interfering RNA (siRNA) is a powerful tech- nique that can suppress gene expression in a variety of cells including mammalian cells. Endothelial pro- genitor cells (EPCs) are bone marrow—derived haematopoietic progenitor cells that have been im- plicated in vasculogenesis. We demonstrated for the first time that gene silencing of endothelial E-selectin using siRNA transfection in human umbilical vein endothelial cells (HUVECs) causes inhibition of EPC adhesion under flow conditions. Fluorescence im- munobinding assay analysis showed that significant reduction of E-selectin surface expression in HU- VECs (activated with IL-1β (10 U/mL) for 4 h) transfected with siRNA against E-selectin, but not in HUVECs transfected with LacZ siRNA (control). An EPC adhesion assay under flow conditions (shear stress = 1.0 dyne/cm2) then demonstrated that HU- VECs transfected with E-selectin siRNA supported significantly less adhesion of EPCs than those HU- VECs treated with control siRNA and no siRNA after activation by IL-1β (p < 0.05). Our experiments have shown the importance of E-selectin in EPC adhesion to HUVECs and the potential utility of gene silencing of E-selectin in EPC recruitment. Keywords: Endothelial Progenitor Cell; E-Selectin; Rnai 1. INTRODUCTION The vascular endothelium is a vital border zone between the circulatory system and the tissues it supplies. It is a monolayer of endothelial cells (EC) that has pivotal roles in coagulation, inflammation, vasodilatation and vaso- constriction through substances such as nitric oxide and prostaglandins [1]. Recently identified vascular progenitor cells from bone marrow (BM) and non-BM origin have been shown to contribute to neovessel formation [2]. Upon inflam- matory conditions, the vascular endothelium was acti- vated to capture progenitor cells in regions where endo- thelium regeneration is needed. Though ischemia is believed to be the physiological stimulus for EPC mobilisation from the bone marrow, the mechanisms of EPC recruitment are less well under- stood. Little is understood concerning the molecular mecha- nisms of EPC adhesion to endothelial cells. In contrast, extensive studies have been conducted regarding leuko- cyte-endothelial interactions. Previous experiments have demonstrated that P-selectin and E-selectin are involved in leukocyte rolling, whilst intercellular adhesion mole- cule-1 (ICAM-1) is associated with leukocyte firm adhe- sion [3]. The molecular players involved in leukocyte transmigration have been found to be molecules such as platelet–endothelial-cell adhesion molecule-1 (PECAM-1), junctional adhesion molecules (JAMs), vascular endothe- lial-cadherin (VE-cadherin) and CD99 [4]. Chavakis et al had implicated roles for β2-integrins in the homing and revascularisation of EPCs [5]. Vajcokzy et al had carried out in vivo experiments with mice to demonstrate that E-selectin is involved in adhesion of embryonic EPCs [6]. We recently reported an important role of endothelial E-selectin in mediating endothelial progenitor cell (EPC) recruitment [7]. In this study, we used a flow chamber system to mimic the in vivo circulation. The major advantage of this set-up is that conditions can be much more tightly controlled, so that the effect of individual molecules can be better ascertained than with an in vivo procedure. RNA inhibition has allowed us to silence genes for a variety of purposes. Short interfering RNA (siRNA) is a useful technique to define the function of a given mole- cule [8]. In this study we look at the role of E-selectin in EPC adhesion to endothelial cells using double stranded short interfering RNA (siRNA), in conjunction with the in vitro flow assay system. Knowledge of this study will allow us to better understand the process of EPC re-  S. Sharma et al. / J. Biomedical Science and Engineering 3 (2010) 550-555 551 Copyright © 2010 SciRes. JBiSE cruitment to endothelial cells, which will give greater insight into the process of vasculogenesis and its clinical implications. 2. MATERIALS AND METHOD 2.1. Cell Culture and Reagents Human umbilical vein endothelial cells (HUVECs) were cultured on 0.1% gelatin-coated tissue culture dishes as described previously [9,10]. The following antibodies were used in this study; H18/7 [11] (anti-human E-selectin mAb), Hu5/3 [12] (anti-human ICAM-1 mAb). Recombinant human IL-1β was a gift from Bio- gen Inc (Cambridge, MA). The HL60 cell lines were obtained from the American Type Culture Collection and cultured in RPMI-1640 containing 10% FCS. For use in the flow-chamber apparatus, HUVECs were plated onto 22 mm fibronectin-coated glass coverslips as has been described previously [13,14]. Preparation of endothelial progenitor cells was previ- ously described in detail [7]. In brief, 25 mL of the blood was collected from hu- man volunteers, to which 15 mL Histopaque®-1077 so- lution (Sigma-Aldrich Japan, KK) was added. After cen- trifugation for 20 minutes at 1600 rpm, the upper layer was collected and diluted in Dulbecco’s phosphate buff- ered saline, then add 2 mM EDTA (DPBSE, Sigma) and centrifuged at 2000 rpm for 5 minutes. The residual pel- let was resuspended in 10 mL ammonium chloride solu- tion (Stem Cell Technologies Inc) to lyse red blood cells and centrifuged at 1000 rpm for 10 minutes. The pellet was resuspended in Endothelial Cell Basal Medium (EBM-2, Clonetics®) and cultured in each well of a C-6 plate (coated with fibronectin). Media exchange of the EPC culture was carried out after 4 days, and EPCs were used for flow assay after 7 days. All experimental pro- cedures were approved by the Experimental Research Review Committee of the Tokyo Medical and Dental University (No. 0090014). 2.2. Flow Assay The parallel-plate flow chamber that was used in this study has been described previously in more detail [15,16]. In summary, the chamber was composed of 2 aluminium steel plates separated by a 200 µm thick si- lastic gasket, and the flow channel was formed by re- moval of a 5 × 20 mm rectangular section from the gas- ket. Defined levels of flow were applied to the HUVEC monolayer by drawing the flow media (see above) through the channel with a syringe pump (model 44 Harvard Apparatus). A plastic heating plate (Tokai Hit Co) was attached to the stage of an inverted microscope (IX50, Olympus) to maintain the temperature at 37℃. Using this set-up, the channel flow could be modelled as a two-dimensional fully developed laminar flow with a simple parabolic velocity profile. HUVEC monolayers on coverslips were stimulated for 4 h using IL-1β (10 U/mL), mixed with RPMI and 1% FBS, then positioned in the flow chamber. The monolayers were perfused for 5 minutes with perfusion medium (flow media), then examined carefully to estab- lish the monolayer as confluent. HL60 cells or EPCs were then diluted in the flow media to 1 × 106 cells/mL. The cells were then drawn through the chamber at con- trolled flow rates to achieve wall shear stresses of 1.0 and 2.0 dyne/cm2 for 10 minutes. 2.3. Fluorescent Immunobinding Assay (FIA) HUVEC monolayers in 96-well plates were incubated on ice with the indicated primary antibody in RPMI/1% FCS at 10 μg/mL for 45 minutes. These wells were then washed three times with RPMI/1% FCS, after which they were incubated with a FITC-conjugated goat anti-mouse polyclonal F(ab’)2 antibody (Caltag Labora- tories) diluted 1:100 in DPBS on ice. After 45 minutes, the wells were washed with DPBS/20% FCS twice, fol- lowed by teo washes with DPBS only. Cells were lysed with 0.01% NaOH in 0.1% SDS. The fluorescence was then measured using a CytoFluor II (Perspective Bio- systems) fluorescent plate reader set at 485 (excitation)/ 535 (emission). 2.4. siRNA Transfection of E-Selectin Short interfering RNAs (siRNAs) were designed to tar- get the coding sequence of human E-selectin cDNA. The target sequences were directed as described previously8. Briefly, the sequences were targeted to the single-strand region consistent with the predicted secondary RNA structure and sequences of the form (AA/CA)N19 with GC contents of less than 70% were isolated from this region. Nineteen RNA nucleotides followed by TT/TG were selected, then chemically synthesised, and finally gel-purified. In order to anneal the single stock strands of RNAi (JbioS) 20 μL 50 μM sense, 20 μL 50 μM antisense and 10 μL 10 mM MgCl2 in DEPC-PBS was mixed together, giving a dsRNA concentration of 20 μM. This was then heated at 95℃ for 2 minutes, 70℃ for 1 minute, 20℃ for 30 minutes, and then 4℃ before being stored at –80℃. Solutions of 120 µL Optimem® (Invitrogen), 6 µL dou- ble-stranded siRNA for E-selectin (siE-01) (or control using LacZ (scE-01) or no siRNA (i.e. Lipofectin alone)), 120 µL Optimem® (Invitrogen) and 5 µL Lipofectin® (In- vitrogen) were prepared, and left for 30 minutes then combined and again left for 30 minutes. Optimem® was then added to make up a total volume of 1.2 mL. This solution was then added to HUVEC monolayers pre- washed with Optimem®, then incubated for 4 h at 37℃, 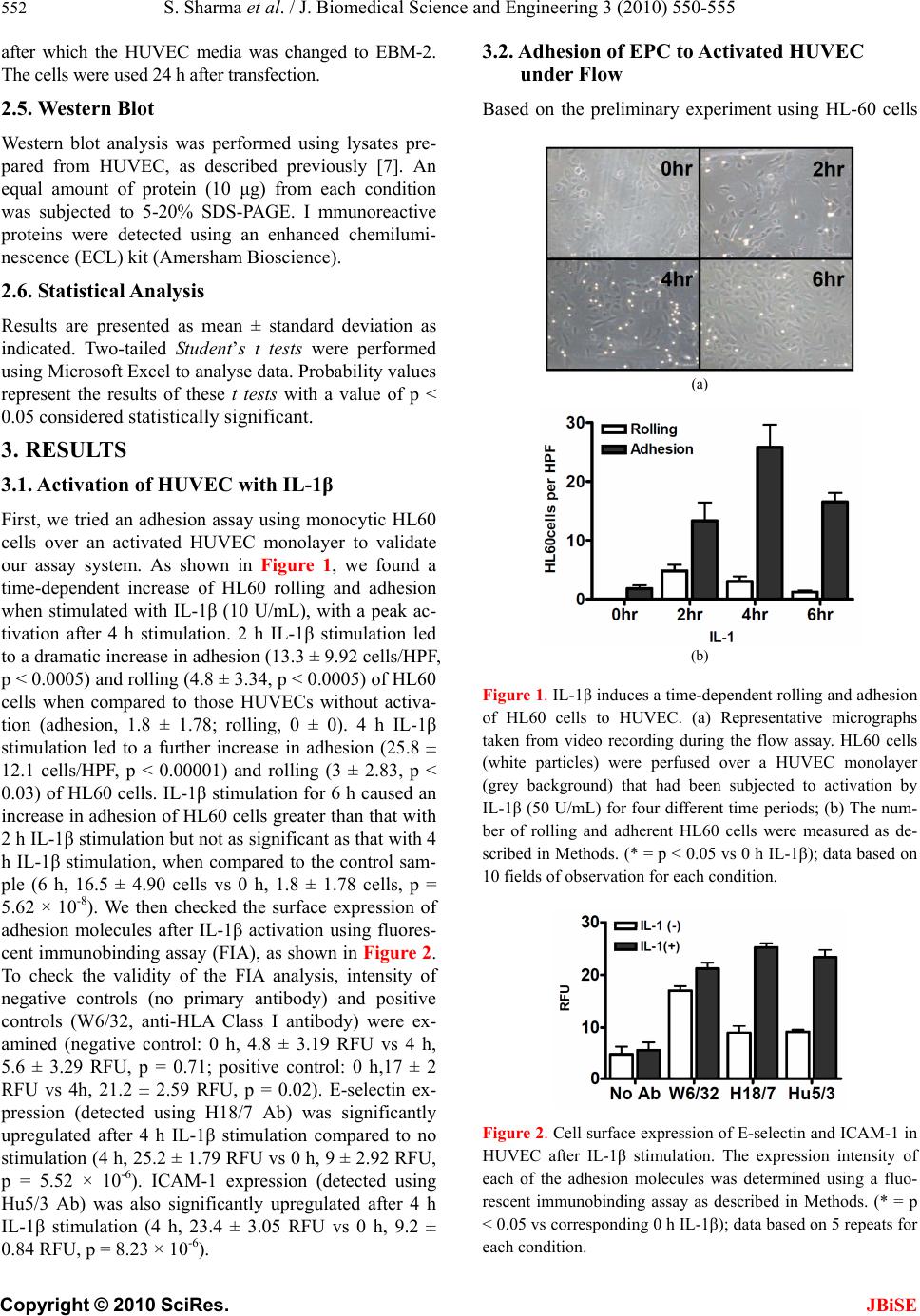 552 S. Sharma et al. / J. Biomedical Science and Engineering 3 (2010) 550-555 Copyright © 2010 SciRes. JBiSE after which the HUVEC media was changed to EBM-2. The cells were used 24 h after transfection. 2.5. Western Blot Western blot analysis was performed using lysates pre- pared from HUVEC, as described previously [7]. An equal amount of protein (10 μg) from each condition was subjected to 5-20% SDS-PAGE. I mmunoreactive proteins were detected using an enhanced chemilumi- nescence (ECL) kit (Amersham Bioscience). 2.6. Statistical Analysis Results are presented as mean ± standard deviation as indicated. Two-tailed Student’s t tests were performed using Microsoft Excel to analyse data. Probability values represent the results of these t tests with a value of p < 0.05 considered statistically significant. 3. RESULTS 3.1. Activation of HUVEC with IL-1β First, we tried an adhesion assay using monocytic HL60 cells over an activated HUVEC monolayer to validate our assay system. As shown in Figure 1, we found a time-dependent increase of HL60 rolling and adhesion when stimulated with IL-1β (10 U/mL), with a peak ac- tivation after 4 h stimulation. 2 h IL-1β stimulation led to a dramatic increase in adhesion (13.3 ± 9.92 cells/HPF, p < 0.0005) and rolling (4.8 ± 3.34, p < 0.0005) of HL60 cells when compared to those HUVECs without activa- tion (adhesion, 1.8 ± 1.78; rolling, 0 ± 0). 4 h IL-1β stimulation led to a further increase in adhesion (25.8 ± 12.1 cells/HPF, p < 0.00001) and rolling (3 ± 2.83, p < 0.03) of HL60 cells. IL-1β stimulation for 6 h caused an increase in adhesion of HL60 cells greater than that with 2 h IL-1β stimulation but not as significant as that with 4 h IL-1β stimulation, when compared to the control sam- ple (6 h, 16.5 ± 4.90 cells vs 0 h, 1.8 ± 1.78 cells, p = 5.62 × 10-8). We then checked the surface expression of adhesion molecules after IL-1β activation using fluores- cent immunobinding assay (FIA), as shown in Figure 2. To check the validity of the FIA analysis, intensity of negative controls (no primary antibody) and positive controls (W6/32, anti-HLA Class I antibody) were ex- amined (negative control: 0 h, 4.8 ± 3.19 RFU vs 4 h, 5.6 ± 3.29 RFU, p = 0.71; positive control: 0 h,17 ± 2 RFU vs 4h, 21.2 ± 2.59 RFU, p = 0.02). E-selectin ex- pression (detected using H18/7 Ab) was significantly upregulated after 4 h IL-1β stimulation compared to no stimulation (4 h, 25.2 ± 1.79 RFU vs 0 h, 9 ± 2.92 RFU, p = 5.52 × 10-6). ICAM-1 expression (detected using Hu5/3 Ab) was also significantly upregulated after 4 h IL-1β stimulation (4 h, 23.4 ± 3.05 RFU vs 0 h, 9.2 ± 0.84 RFU, p = 8.23 × 10-6). 3.2. Adhesion of EPC to Activated HUVEC under Flow Based on the preliminary experiment using HL-60 cells (a) (b) Figure 1. IL-1β induces a time-dependent rolling and adhesion of HL60 cells to HUVEC. (a) Representative micrographs taken from video recording during the flow assay. HL60 cells (white particles) were perfused over a HUVEC monolayer (grey background) that had been subjected to activation by IL-1β (50 U/mL) for four different time periods; (b) The num- ber of rolling and adherent HL60 cells were measured as de- scribed in Methods. (* = p < 0.05 vs 0 h IL-1β); data based on 10 fields of observation for each condition. Figure 2. Cell surface expression of E-selectin and ICAM-1 in HUVEC after IL-1β stimulation. The expression intensity of each of the adhesion molecules was determined using a fluo- rescent immunobinding assay as described in Methods. (* = p < 0.05 vs corresponding 0 h IL-1β); data based on 5 repeats for each condition.  S. Sharma et al. / J. Biomedical Science and Engineering 3 (2010) 550-555 553 Copyright © 2010 SciRes. JBiSE (Figure 1), we used a condition of IL-1β (10 U/mL) for 4 h in our following experiments. When EPCs, cultured ex vivo for 7 days, were perfused over activated HU- VEC monolayer, adhesion of EPCs was dramatically increased compared to the control HUVEC monolayer (4 h, 4.4 ± 1.50 cells vs 0 h, 1.3 ± 0.9 cells, p = 8.97 × 10-5), as shown in Figure 3. There was however no roll- ing of EPCs observed both in the control and 4 h IL-1β stimulation. 3.3. Gene Silencing of E-Selectin in HUVEC Having demonstrated that 4 h of IL-1β stimulation was optimal for adhesion, we looked at the effects of silenc- ing E-selectin, using siRNA transfection, on the rolling and adhesion of EPCs. siRNA transfection on HUVEC monolayers was carried out 24 h prior to the adhesion assay under flow conditions (the time point was deter- mined to optimize the effect of siRNAs in HUVECs, as previously described [8]). We utilized FIA assay to dem- onstrate the level of E-selectin in these cells. As demon- strated in Figure 4, positive controls (W6/32, anti-HLA Class I antibody) exhibited no significant difference be- tween siRNA treatment groups, enhancing the validity of these results. However, there was a significant reduction of E-selectin surface expression in the E-selectin siRNA —treated HUVECs when compared to the LacZ siRNA —treated HUVECs after 4 h IL-1β stimulation (E-selectin, 27.3 ± 5.40 RFU vs LacZ, 45.5 ± 7.37 RFU, p = 0.013) or no siRNA group (E-selectin, 27.3 ± 5.40 RFU vs no siRNA, 52.5 ± 10.4 RFU, p = 0.010). 3.4. Effect of Gene Silencing of E-Selectin on EPC Adhesion to HUVEC under Flow Figure 5 showed results of the flow assay using IL-1- activated HUVECs after siRNA transfection. There was Figure 3. The number of rolling and adherent EPC to ac- tivated HUVEC were measured as described in Methods. (* = p < 0.05 vs 0 h IL-1β); data based on 10 fields of observation for each condition. EPCs were perfused over a HUVEC monolayer (grey background) that had been subjected to activation by IL-1β (50 U/mL) for two dif- ferent time periods (0 h IL-1β and 4 h IL-1β). Figure 4. Cell surface expression of E-selectin (H18/7) and positive control (W6/32) in HUVEC after siRNA silencing. The expression intensity of each of the adhesion molecules was determined using a fluorescent immunobinding assay as de- scribed in Methods. (* = p < 0.05 vs corresponding 0hr IL-1β); data based on 5 repeats for each condition. Figure 5. The number of rolling and adherent EPC to activated HUVEC after siRNA transduction. Graph showing the effect of E-selectin siRNA transfection on rolling and adhesion of EPCs compared to control siRNA transfection and no siRNA treat- ment. (* = p < 0.05 vs control); data based on 10 fields of ob- servation for each condition. a significant decrease in EPC adhesion in HUVECs transfected with E-selectin siRNA compared to those treated with LacZ siRNA (controls) (E-selectin siRNA, 2.7 ± 0.64 cells vs LacZ siRNA, 5.1 ± 1.22 cells, p = 5.74 × 10-5). In comparison, there was no significant difference in rolling between those HUVECs transfected with E-selectin siRNA and the control HUVECs (E- selectin siRNA, 0.2 ± 0.4 cells vs LacZ siRNA, 0.5 ± 0.67 cells, p = 0.26). There was also a significant de- crease in EPC adhesion in HUVECs treated with E-selectin siRNA compared to those treated with no siRNA (Lipofectin® only) (E-selectin siRNA 2.7 ± 0.64 cells vs no siRNA, 7 ± 1.26 cells, p = 3.74 × 10-8). 4. DISCUSSION In this project, we have demonstrated that 1) 4 h of in-  554 S. Sharma et al. / J. Biomedical Science and Engineering 3 (2010) 550-555 Copyright © 2010 SciRes. JBiSE cubation with IL-1β induces adhesion of HL60 cells to HUVEC under flow; 2) 4 h of incubation with IL-1β induces expression of E-selectin and ICAM-1 in HU- VEC; 3) 4 h of incubation with IL-1β induces adhesion of EPCs to HUVEC under flow; 4) siRNA against E-selectin reduces endogenous expression of E-selectin in HUVEC; 5) gene silencing of E-selectin resulted in reduction of EPC adhesion to HUVEC. These lines of findings point a predominant role for this molecule in neovascularisation. In previous work from our labora- tory using siRNA transfection carried out by Nishiwaki et al (2003), we successfully silenced endogenous E- selectin expression in vascular endothelium using siRNA transfection, and demonstrated that this inhibition caused a significant reduction in leukocyte adhesion [8]. Current results compliment this work and emphasise the efficacy of siRNA in the field of vascular biology. We have also reinforced the work of Nishiwaki et al (using antibodies to downregulate E-selectin) and Va- jkoczy et al (using an in vivo method to silence E- selectin expression) in demonstrating that E-selectin is important in EPC adhesion by using a different method (siRNA transfection) [6,7]. Using siRNA transfection to inhibit E-selectin expres- sion, as demonstrated by our experiments, may have implications for its role in angiogenesis. Bischoff has reported that E-selectin is involved in angiogenesis through a series of experiments [17]. One of these ex- periments showed that antibodies directed against E- selectin or sialylated fucosylated oligosaccharides (stru- ctures to which E-selectin binds) inhibit the formation of capillary-like tubes in vitro [18]. The presence of E- selectin in human infantile haemangioma tumours (en- dothelial cell tumour of capillary blood vessels), as well as in tissues with on going growth of microvessels (such as human placenta and neonatal foreskin) has been shown in vivo [19]. Yu et al had also shown that endo- thelial progenitor cells are present in infantile haeman- gioma; 11 of 12 proliferating haemangioma specimens contained EPCs (determined by flow cytometry using CD133 and CD34 as markers for EPCs) [20]. This raises the possibility of using our method of silencing E- selectin expression in treating tumour angiogenesis, by preventing localised EPC adhesion to the endothelium, thus inhibiting angiogenesis in the tumour, reducing its growth. As described previously, we found that there was no significant reduction in rolling after E-selectin down-regulation. This could have been because of the low level of EPC rolling in general, as demonstrated in Figure 3. On the other hand, the binding characteristics of E-selectin (E-selectin has a greater role in adhesion than rolling) may have some effects. This speculation has been supported by the results from Mazo, et al.’s experiments [21]. They demonstrated that blocking E- selectin with antibodies resulted in a reduction of haema- topoietic progenitor cells (HPC) rolling of 32% com- pared to a reduction in HPC rolling of 58% when P-selectin was inhibited (measured using intravital mi- croscopy). Therefore the other selectins (P- and L- selectins) are probably more important for HPC rolling. A limitation of this experiment is that we have only demonstrated that gene silencing of E-selectin expres- sion reduces EPC adhesion in vitro, but not in vivo. Therefore future work could be aimed at seeing if siRNA transfection has similar effects in vivo. It would also be interesting to try similar experiments using siRNA trans- fection against other adhesion molecules such as ICAM- 1 or P-selectin, and look at the effects on EPC recruit- ment. In conclusion, we have demonstrated that E-selectin— short interfering RNA inhibits endothelial progenitor cell recruitment to vascular endothelium under flow condi- tions. 5. ACKNOWLEDGEMENTS Grateful thanks to Dr Naokazu Nakamura, Michiyo Deushi, Noriko Nitta and Mariko Tani for their experimental help and advice, Profes- sor Peter Sugden for his help and advice throughout the course of the project. REFERENCES [1] Cines, D.B., Pollak, E.S., Buck, C.A., Loscalzo, J, Zim- merman, G.A., McEver, R.P., Pober, J.S., Wick, T.M., Konkle, B.A., Schwartz, B.S., Barnathan, E.S., McCrae, K.R., Hug, B.A., Schmidt, A.M. and Stern, D.M. (1998) Endothelial cells in physiology and in the pathophysiol- ogy of vascular disorders. Blood, 91(10), 3527-3561. [2] Shi, Q., Rafii, S., Wu, M.H., Wijelath, E.S., Yu, C., Ishida, A., Fujita, Y., Kothari, S., Mohle, R., Sauvage, L.R., Moore, M.A., Storb, R.F. and Hammond, W.P. (1998) Evidence for circulating bone marrow-derived endothe- lial cells. Blood, 92(2), 362-367. [3] Kakkar, A.K. and Lefer, D.J. (2004) Leukocyte and en- dothelial adhesion molecule studies in knockout mice. Current Opinion in Pharmacology, 4(2), 154-158. [4] Muller, W.A. (2003) Leukocyte—endothelial-cell inter- actions in leukocyte transmigration and the inflammatory response. Trends in Immunology, 24(6), 326-333. [5] Chavakis, E., Aicher, A., Heeschen, C., Sasaki, K., Kaiser, R., El Makhfi, N., Urbich, C., Peters, T., Scharffetter- Kochanek, K., Zeiher, A.M., Chavakis, T. and Dimmeler, S. (2005) Role of beta2-integrins for homing and ne- ovascularization capacity of endothelial progenitor cells. Journal of Experimental Medicine, 201(1), 63-72. [6] Vajkoczy, P., Blum, S., Lamparter, M., Mailhammer, R., Erber, R., Engelhardt, B., Vestweber, D. and Hatzopoulos, A.K. (2003) Multistep nature of microvascular recruit- ment of ex vivo-expanded embryonic endothelial pro- genitor cells during tumor angiogenesis. Journal of Ex-  S. Sharma et al. / J. Biomedical Science and Engineering 3 (2010) 550-555 555 Copyright © 2010 SciRes. JBiSE perimental Medicine, 197(12), 1755-1765. [7] Nishiwaki, Y., Yoshida, M., Masuda, H. and Isobe, M. (2004) Recruitment of bone marrow-derived endothelial progenitor cells to vascular endothelium involves E-se- lectin dependent mechanism. Cardiovascular Pathology, 13(3), 171. [8] Nishiwaki, Y., Yokota, T., Hiraoka, M., Miyagishi, M., Taira, K., Isobe, M., Mizusawa, H. and Yoshida, M. (2003) Introduction of short interfering RNA to silence endogenous E-selectin in vascular endothelium leads to successful inhibition of leukocyte adhesion. Biochemical and Biophysical Research Communications, 310(4), 1062-1066. [9] Yoshida, M. and Gimbrone, M.A. Jr. (1997) Novel roles for E-selectin in endothelial-leukocyte adhesion. Annals of the New York Academic Sciences, 811, 493-497. [10] Yoshida, M., Szente, B.E., Kiely, J.M., Rosenzweig, A. and Gimbrone, M. Jr. (1998) Phosphorylation of the cy- toplasmic domain of E-selectin is regulated during leu- kocyte-endothelial adhesion. The Journal of Immunology, 161(2), 933-941. [11] Bevilacqua, M.P., Pober, J.S., Mendrick, D.L., Cotran, R.S. and Gimbrone, M.A. (1987) Identification of an in- ducible endothelial-leukocyte adhesion molecule. Pro- ceedings of the National Academy Sciences of USA, 84(24), 9238-9242. [12] Luscinskas, F.W., Cybulsky, M.I., Kiely, J.M., Peckins, C.S., Davis, V.M. and Gimbrone, M.A. (1991) Cytokine- activated human endothelial monolayers support en- hanced neutrophil transmigration via a mechanism in- volving both endothelial-leukocyte adhesion molecule-1 and intercellular adhesion molecule-1. The Journal of Immunology, 146(5), 1617-1625. [13] Gerszten, R.E., Garcia-Zepeda, E.A., Lim, Y.C., Yoshida, M., Ding, H.A., Gimbrone, M. Jr., Luster, A.D., Luscin- skas, F.W. and Rosenzweig, A. (1999) MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothe- lium under flow conditions. Nature, 398(6729), 718-723. [14] Kiely, J.M., Luscinkas, F.W. and Gimbrone, M. Jr. (1999) Leukocyte-endothelial monolayer adhesion assay (static conditions). Methods in Molecular Biology, 96, 131-136. [15] Gerszten, R.E., Luscinskas, F.W., Ding, H.T., Dichek, D.A., Stoolman, L.M., Gimbrone, M.A. Jr. and Rosenz- weig, A. (1996) Adhesion of memory lymphocytes to vascular cell adhesion molecule-1—transduced human vascular endothelial cells under simulated physiological flow conditions in vitro. Circulation Research, 79(6), 1205-1215. [16] Luscinskas, F.W., Kansas, G.S., Ding, H., Pizcueta, P., Schleiffenbaum, B.E., Tedder, T.F. and Gimbrone, M. Jr. (1994) Monocyte rolling, arrest and spreading on IL-4- activated vascular endothelium under flow is mediated via sequential action of L-selectin, β1-integrins, and β2-integrins. Journal of Cell Biology, 125(6), 1417-1427. [17] Bischoff, J. (1997) Cell adhesion and angiogenesis. Journal of Clinical Investigation, 99(3), 373-376. [18] Nguyen, M., Strubel, N.A. and Bischoff, J. (1993) A role for sialyl Lewis-X/A glycoconjugates in capillary mor- phogenesis. Nature, 365(6443), 267-269. [19] Kraling, B.M., Razon, M.J., Boon, L.M., Zurakowski, D., Seachord, C., Darveau, R.P., Mulliken, J.B., Corless, C.L. and Bischoff, J. (1996) E-selectin is present in prolifer- ating endothelial cells in human hemangiomas. American Journal of Pathology, 148(4), 1181-1191. [20] Yu, Y., Flint, A.F., Mulliken, J.B., Wu, J.K. and Bischoff, J. (2004) Endothelial progenitor cells in infantile heman- gioma. Blood, 103(4), 1373-1375. [21] Mazo, I.B., Gutierrez-Ramos, J.C., Frenette, P.S., Hynes, R.O., Wagner, D.D. and von Andrian, U.H. (1998) He- matopoietic progenitor cell rolling in bone marrow mi- crovessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. Journal of Ex- perimental Medicine, 188(3), 465-474. |

