 World Journal of Neuroscience, 2012, 2, 68-73 WJNS http://dx.doi.org/10.4236/wjns.2012.22010 Published Online May 2012 (http://www.SciRP.org/journal/wjns/) Hypertension effects on p73 expression in the rat circumventricular organs and cerebrospinal fluid Emilia M. Carmona-Calero1,2, Ibrahim González-Marrero1,2, Manuela Castañeyra-Martin2, Juan M. González-Toledo1, Leandro Castañeyra-Ruiz2, Héctor de Paz-Carmona1, Agustín Castañeyra-Ruiz2, Lidia Ruiz-Mayor1, Agustín Castañeyra-Perdomo1,2* 1Departamento de Anatomía, Facultad de Medicina, Universidad de La Laguna, Tenerife, Islas Canarias, Spain 2Departamento de Biotecnología, Instituto de Investigación y Ciencias de Puerto del Rosario, Fuerteventura, Islas Canarias, Spain Email: *acastane@ull.es Received 14 December 2011; revised 30 January 2012; accepted 16 February 2012 ABSTRACT It has been reported that spontaneously hypertensive rats (SHR) show ventricular dilation, changes in CSF proteins and variations in the circumventricular organs (CVO) such as: the subcommissural organ (SCO), the subfornical organ (SFO) and the area postrema (AP) which are located in the walls of the third and fourth ventricles. On the other hand, p73 proteins are pre- sent in cells of the central nervous system (CNS) such as circumventricular structures and the neuroepithe- lium which are altered in ventricular dilation. The purpose of the present work is to study the TAp73 isoform expression in the circumventricular organs (CVO) and their variations in ventricular dilatation and arterial hypertension. Brains and cerebrospinal fluid (CSF) from control Wistar-Kyoto rats (WKY) and SHR were used. The paraffin sections containing the CVO were immunohistochemically processed with anti-TAp73 and by western blot, p73 bands in the CSF and circumventricular organ extract were also identi- fied. The western blot study showed bands marked with p73 in the CSF and CVO, the p73 band expres- sion was bigger in the SHR than in the WKY rats. We also found stronger markings in the SFO, SCO and AP of the hypertensive rats than in the WKY rats. It could be concluded that hypertension in the SHR produces alterations in the relationship between the p73 protein, circumventricular structures and CSF. Keywords: Circumventricular Organs; p73; CSF; Hypertensive Rats 1. INTRODUCTION Ritter and Dinh [1] described that spontaneously hyper- tensive rats present a progressive increase in ventricular size from 4 to 56 weeks of age, and in some SHRs ven- tricle size increased to 270% of control and such ven- tricular dilation could be produced by a loss of grey and white matter similar to what occurs in hydrocephalus, for this reason, certain systemic and behavioural signs which are concomitant with hypertension in the SHR may be attributable to hydrocephalus and its neuropathological correlates [1]. It has also been observed in previous studies [2,3] that the increase in ventricular size can be qualitatively observed after 15 weeks of postnatal age. On the other hand, alterations of the subcommissural organ, subfornical organ in the hypertensive and hydrocephalic rats were also observed [4-7]. The subcommissural organ (SCO) is a cerebral struc- ture associated with the circulation and composition of the CSF, which secretes glycoprotein into the CSF where its greater part is condensed and forms Reissner’s fibre (RF), and the other minor part remains soluble in the CSF. The human SCO has a great development during the foetal life [8,9], variations in the CSF and SCO have been reported in different kinds of hydrocephalus [4,10, 11]. Alterations of the SCO in hydrocephalic mice and rats have been described, Takeuchi et al. [12] de- scribes agenesis of the SCO and the posterior commissure (PC) in hydrocephalic mice, Irigoin et al. [11], Rodriguez et al. [4] and Carmona-Calero et al. [7] found alterations in the secretion of the SCO in induced and spontaneously hy- drocephalic rats. In addition, alterations in the secretions of cerebrospinal fluid proteins from the SCO have been described in spontaneously hypertensive rats [6]. The Subfornical Organ (SFO) is a circumventricular organ located below the commissure fornici, entering the rostral wall of the third ventricle [13], and is a neuroglio- vascular structure containing neurons, glia and plexus of fenestrated capillaries. The SFO is characterized by the absence of a blood-brain barrier [14]. The SFO has con- nections with the brain regions involved in the central regulation of drinking, salt appetite, blood pressure and *Corresponding author. OPEN ACCESS  E. M. Carmona-Calero et al. / World Journal of Neuroscience 2 (2012) 68-73 69 cardiovascular function, such a region is the anteroven- tral region of the third ventricle which is involved in the control of drinking behaviour [15,16]. The area postrema located at end of the fourth ventri- cle under the obex [13] also contains neurons, glia and fenestrated capillaries, and does not have a blood-brain barrier. The AP functions are related with cardiovascular regulation and the catecholaminergic system as well as with the anteroventral region of the third ventricle [17,18]. The role of p73 is significant in the central nervous system development, since the p73 knock-out mice pre- sent hippocampal dysgenesis, cortical hypoplasia and hy- drocephalus, meaning that p73 plays an important role in the different parameters regulating brain development [19]. The transactivating isoforms of p73 (TAp73) are similar to p53 acting as transcription factors inducing cellular apoptosis; on the other hand, the N-terminal truncated isoforms (ΔNp73) can inhibit the transcript- tional function of p53 and TAp73 [20]. It has recently been reported that TAp73 is present in the SCO and CSF where it could play an important role in the maintenance of the ventricular wall and in the development of neu- roepithelium proliferation [21]. Bearing in mind that SHRs present ventricular dilation, alterations in the circumventricular organs and CSF pro- teins variations, the purpose of the present work is to study the expression of p73 in the CSF, SCO, SFO and the AP in arterial hypertension and ventricular dilation. 2. MATERIAL AND METHODS Brains and cerebrospinal fluid (CSF) from 16 control Wistar-Kyoto rats (WKY) and 16 spontaneously hyper- tensive rats (SHR) from Charles River Laboratories España S.A. (Barcelona, Spain) of 12 months of age were used. The rats were anesthetized with chloral hydrate (200 l/100g of body weight at 160 mg/ml) and 100 l of CSF from the cistern magna of each animal was ex- tracted before sacrifice. Extracts of circumventricular or- gans and CSF were prepared from 12 rats from each group which were processed by protein electrophoresis accord- ing to Laemmli [22] (sodium docecyl sulfate-poly- acrylamide gel electrophoresis SDS-PAGE, 5% - 15% gradient). Four rats from each group were fixed by in- tracardiac perfusion with Bruin’s fluid, dehydrated and embedded in paraffin under standard conditions. Brains were cut into four serial coronal and sagittal sections of 10 μm thickness. One of the serial coronal sections was stained by the Klüver-Barrera method. The polyclonal antibody against TAp73 (Ab 14430, Abcam, Cambridge, UK) was used as the primary anti- body. In each rat brain of each group, the sections at the level of the SCO, SFO and AP from the WKY and SHR were simultaneously incubated in the same coupling jar and each jar contained: anti-p73 1:1000. Incubation was for 24 h at room temperature, followed by “DAKO” StreptABCcomplex/HRP Duet, Mouse/Rabbit procedure. The peroxidase reaction product was visualized using diaminobenzidine reaction. Western blots of CSF protein and SCO, SFO and AP extract protein were performed. The membranes with the blotted proteins were incubated in tris-saline (TBS) non-fat milk 5% for 60 minutes and then incubated in the primary antibody anti-p73 1:1000 overnight. Anti-mouse IgG labelled with peroxidase (PIERCE) was used as the secondary antibody at a dilution of 1:80,000 for 1.45 h at room temperature. The peroxidase reaction products from western blot were visualized by quimioluminis- cence (PIERCE). The primary antibody was omited to validate the control method specificity. The immunohis- tochemistry slides were converted to digital images by using an LEICA DMRB photomicroscope with an LEICA DC 300 F camera (Gemany). Image analysis was completed in Image J (v. 1.43 u, NIH, Bethesda, MD, USA). The “Mean Gray Value” was measured from the selected nuclei for all stained tissue and membranes. This value gives the average stain intensity in grayscale units for all threshold pixels. A single-factor analysis of vari- ance (ANOVA) was used for the immunohistochemistry statistical study, which was conducted using the IBM SPSS statistic 19 software. 3. RESULTS Hydrocephalus was clearly observed at 12 months of age in the SHRs by comparing the ventricular size of these SHRs with the normal ventricular size observed in WKYs of 12 months of age (Figu r e s 2 (K), (L)). 3.1. Immunohistochemistry The expression of the TAp73 in the SCO of WKY rats was present in apical perinuclear parts of ependymal and hypendymal cells and some marks were even observed in the ventricle (Figures 1(A), (C); 2(I), (J)). A significant increase of TAp73-ir (F1-6 = 134.975 P < 0.05) was ob- served in the different parts of the ependymal and hypendymal cells in the SCO of the SHR (Figures 1(B), (E); 2(M), (N); 4). The cytoplasm of cells in the ependyma layer was marked with TAp73-ir and this was also found in several body neurons and in perivascular spaces in the SFO of the WKY rats (Figures 1(A), (D); 2(A), (B)). A note- worthy increase of TAp73 expression (F1-6 = 224.615 P < 0.05) in the parenchyma and neurons was found in the SFO hypertensive rats (Figures 1 (B), (F); 2(E), (F); 4). The AP showed immunoreactive material in several neurons and perivascular spaces in the WKY rats (Figures 2(C), (D)) and a slight and not significant increase in the immunoreaction intensity was observed in neurons and ependyma in AP of the SHRs, (Figures 2(G), (H); 4). Copyright © 2012 SciRes. OPEN ACCESS 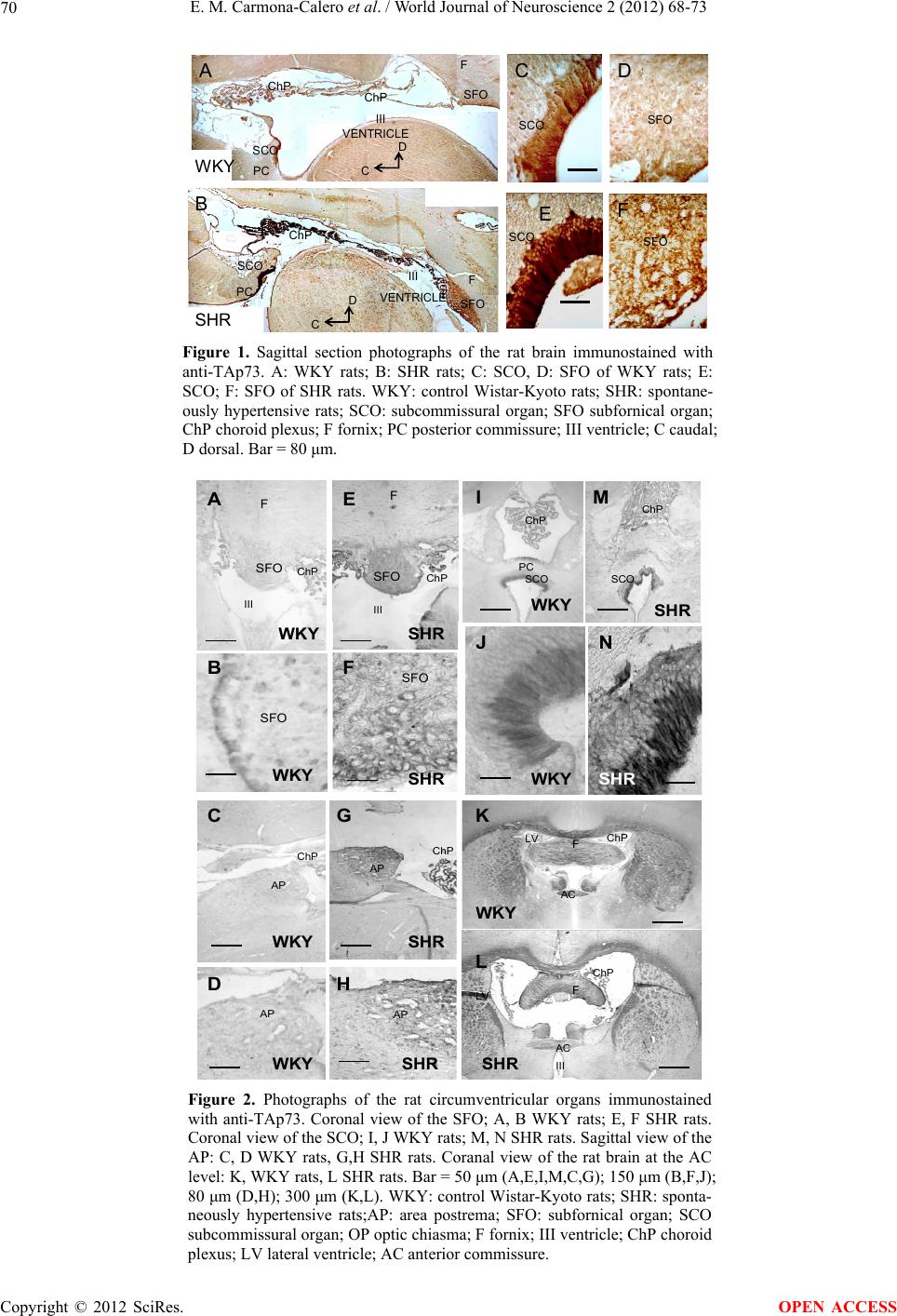 E. M. Carmona-Calero et al. / World Journal of Neuroscience 2 (2012) 68-73 Copyright © 2012 SciRes. 70 ChP SCO SFO SFO III VENTRICLE ChP D C PC PC F F ChP III VENTRICLE D C WKY SHR SCO A B SCO SCO SFO F C E D SFO Figure 1. Sagittal section photographs of the rat brain immunostained with anti-TAp73. A: WKY rats; B: SHR rats; C: SCO, D: SFO of WKY rats; E: SCO; F: SFO of SHR rats. WKY: control Wistar-Kyoto rats; SHR: spontane- ously hypertensive rats; SCO: subcommissural organ; SFO subfornical organ; ChP choroid plexus; F fornix; PC posterior commissure; III ventricle; C caudal; D dorsal. Bar = 80 μm. SHR SFO FSFO F SFO F AP AP AP AP ChP SCO SCO PC ChP III III I J M N A B D C E F G H WKY ChP K L F F AC AC SHR WKY WKY WKY WKY WKY WKYSHR SHR SHR SHR SHR SHR ChP III LV LV ChP ChP ChP SFO SFO ChP Figure 2. Photographs of the rat circumventricular organs immunostained with anti-TAp73. Coronal view of the SFO; A, B WKY rats; E, F SHR rats. Coronal view of the SCO; I, J WKY rats; M, N SHR rats. Sagittal view of the AP: C, D WKY rats, G,H SHR rats. Coranal view of the rat brain at the AC level: K, WKY rats, L SHR rats. Bar = 50 μm (A,E,I,M,C,G); 150 μm (B,F,J); 80 μm (D,H); 300 μm (K,L). WKY: control Wistar-Kyoto rats; SHR: sponta- neously hypertensive rats;AP: area postrema; SFO: subfornical organ; SCO subcommissural organ; OP optic chiasma; F fornix; III ventricle; ChP choroid plexus; LV lateral ventricle; AC anterior commissure. OPEN ACCESS 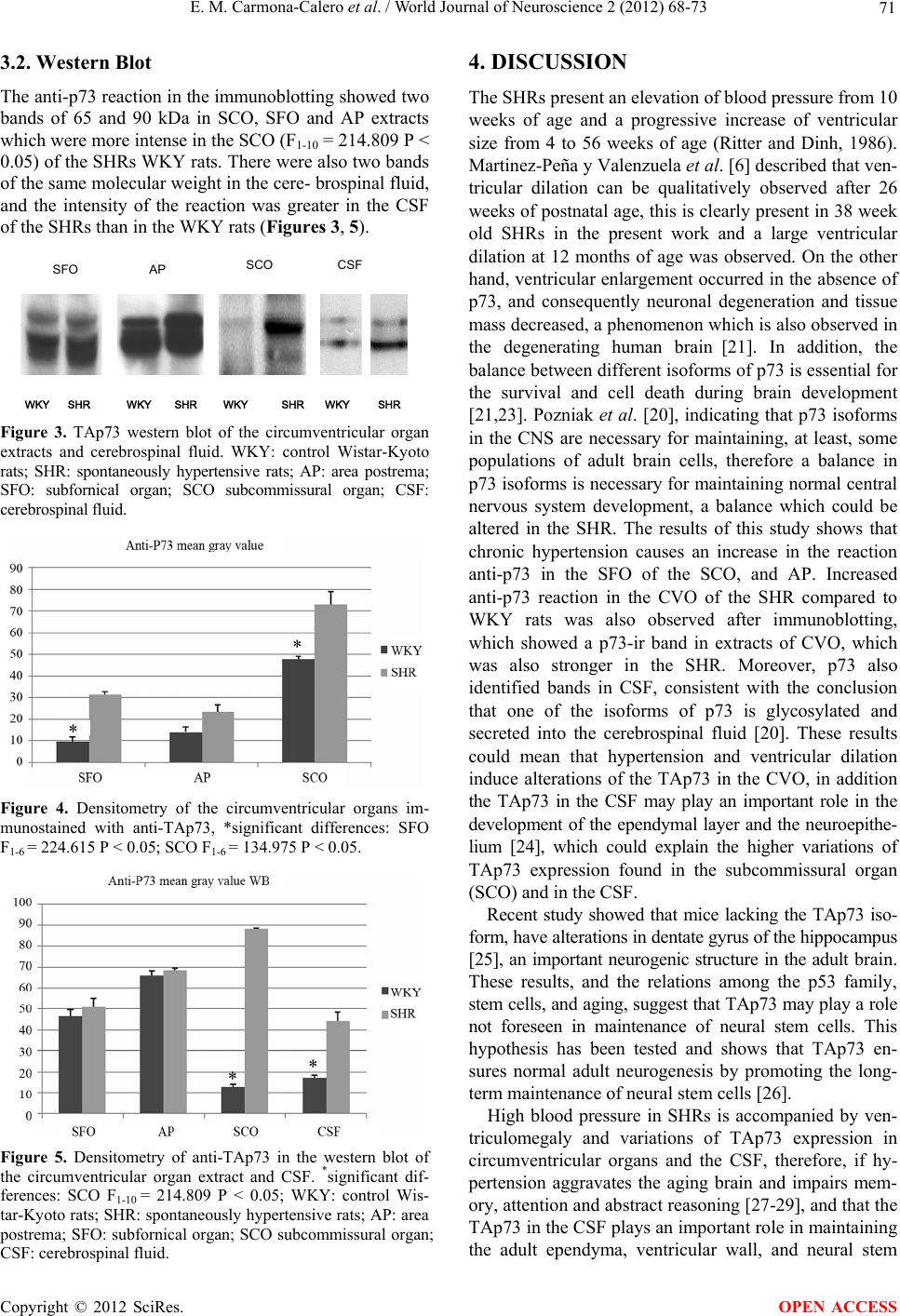 E. M. Carmona-Calero et al. / World Journal of Neuroscience 2 (2012) 68-73 71 3.2. Western Blot The anti-p73 reaction in the immunoblotting showed two bands of 65 and 90 kDa in SCO, SFO and AP extracts which were more intense in the SCO (F1-10 = 214.809 P < 0.05) of the SHRs WKY rats. There were also two bands of the same molecular weight in the cere- brospinal fluid, and the intensity of the reaction was greater in the CSF of the SHRs than in the WKY rats (Figures 3, 5). CSF SHRWKY SFO APSCO WKYWKY WKYSHR SHRSHR Figure 3. TAp73 western blot of the circumventricular organ extracts and cerebrospinal fluid. WKY: control Wistar-Kyoto rats; SHR: spontaneously hypertensive rats; AP: area postrema; SFO: subfornical organ; SCO subcommissural organ; CSF: cerebrospinal fluid. * * Figure 4. Densitometry of the circumventricular organs im- munostained with anti-TAp73, *significant differences: SFO F1- = 224.615 P < 0.05; SCO F1-6 = 134.975 P < 0.05. 6 ** Figure 5. Densitometry of anti-TAp73 in the western blot of the circumventricular organ extract and CSF. *significant dif- ferences: SCO F1-10 = 214.809 P < 0.05; WKY: control Wis- tar-Kyoto rats; SHR: spontaneously hypertensive rats; AP: area postrema; SFO: subfornical organ; SCO subcommissural organ; CSF: cerebrospinal fluid. 4. DISCUSSION The SHRs present an elevation of blood pressure from 10 weeks of age and a progressive increase of ventricular size from 4 to 56 weeks of age (Ritter and Dinh, 1986). Martinez-Peña y Valenzuela et al. [6] described that ven- tricular dilation can be qualitatively observed after 26 weeks of postnatal age, this is clearly present in 38 week old SHRs in the present work and a large ventricular dilation at 12 months of age was observed. On the other hand, ventricular enlargement occurred in the absence of p73, and consequently neuronal degeneration and tissue mass decreased, a phenomenon which is also observed in the degenerating human brain [21]. In addition, the balance between different isoforms of p73 is essential for the survival and cell death during brain development [21,23]. Pozniak et al. [20], indicating that p73 isoforms in the CNS are necessary for maintaining, at least, some populations of adult brain cells, therefore a balance in p73 isoforms is necessary for maintaining normal central nervous system development, a balance which could be altered in the SHR. The results of this study shows that chronic hypertension causes an increase in the reaction anti-p73 in the SFO of the SCO, and AP. Increased anti-p73 reaction in the CVO of the SHR compared to WKY rats was also observed after immunoblotting, which showed a p73-ir band in extracts of CVO, which was also stronger in the SHR. Moreover, p73 also identified bands in CSF, consistent with the conclusion that one of the isoforms of p73 is glycosylated and secreted into the cerebrospinal fluid [20]. These results could mean that hypertension and ventricular dilation induce alterations of the TAp73 in the CVO, in addition the TAp73 in the CSF may play an important role in the development of the ependymal layer and the neuroepithe- lium [24], which could explain the higher variations of TAp73 expression found in the subcommissural organ (SCO) and in the CSF. Recent study showed that mice lacking the TAp73 iso- form, have alterations in dentate gyrus of the hippocampus [25], an important neurogenic structure in the adult brain. These results, and the relations among the p53 family, stem cells, and aging, suggest that TAp73 may play a role not foreseen in maintenance of neural stem cells. This hypothesis has been tested and shows that TAp73 en- sures normal adult neurogenesis by promoting the long- term maintenance of neural stem cells [26]. High blood pressure in SHRs is accompanied by ven- triculomegaly and variations of TAp73 expression in circumventricular organs and the CSF, therefore, if hy- pertension aggravates the aging brain and impairs mem- ory, attention and abstract reasoning [27-29], and that the TAp73 in the CSF plays an important role in maintaining the adult ependyma, ventricular wall, and neural stem Copyright © 2012 SciRes. OPEN ACCESS  E. M. Carmona-Calero et al. / World Journal of Neuroscience 2 (2012) 68-73 72 cells, one might conclude that TAp73 appears to play a role in maintaining the ventricular system and circum- ventricular structures, and that alterations of TAp73 CSF levels in hypertension may be an important factor that should be taken into account in the development of pre- mature aging and cognitive disorders. 5. ACKNOWLEDGEMENTS This work was supported by the Fundación Canaria de Instituto de Investigacion y Ciencias de Puerto del Rosario (INIPRO) project n 01/08. REFERENCES [1] Ritter, S. and Dinh, T.T. (1986) Progressive postnatal dilation of brain ventricles in spontaneously hypertensive rats. Brain Research, 370, 327-332. doi:10.1016/0006-8993(86)90488-9 [2] Carmona-Calero, E., Pérez-González, H., Martínez-Peña y Valenzuela, I., González-Marrero, I., Pérez-García, C.G., Marrero-Gordillo, N., Ormazabal-Ramos, C., Cas- tañeyra-Perdomo, A. and Ferres-Torres, R. (2005) Effect of the arterial hypertension and captopril treatment on the angiotensin II content in the subfornical organ. A study in SHR rats. Histology and Histopathology, 20, 135-138. [3] González-Marrero, I., Carmona-Calero, E.M., Fernández- Rodríguez, P., Pérez-González, H., Ormazabal-Ramos, C., Castañeyra-Ruiz, L., Pérez-García, C.G., Martínez-Peña- Valenzuela, I., Castañeyra-Ruiz, A., Castañeyra-Perdomo, A. and Ferres-Torres, R. (2007) Expression of certain proteins in the subfornical organ and cerebrospinal fluid of spontaneously hypertensive rats. Histology and Histo- pathology, 22, 1371-1378. [4] Rodríguez E.M., Oksche, A., Hein, S. and Yulis, C.R. (1992) Cell biology of the subcommissural organ. Inter- national Review of Cytology, 2, 39-121. [5] Castañeyra-Perdomo, A., Carmona-Calero, E., Meyer, G., Perez-Gonzalez, H., Pérez-Delgado, M.M., Marrero- Gordillo, N., Rodríguez, S. and Rodríguez, E.M. (1998) Changes in the secretory activity of the subcommissural organ of spontaneously hypertensive rats. Neuroscience Letters, 246,133-136. doi:10.1016/S0304-3940(98)00252-3 [6] Martínez-Peña y Valenzuela, I., Carmona-Calero, E.M., Pérez-González, H., Ormazabal-Ramos, C., Fernández- Rodríguez, P., González-Marrero, I., Castañeyra-Perdo- mo, A. and Ferres-Torrer, R. (2006) Alterations of the cerebrospinal fluid proteins and subcommissural organ secretion in the arterial hypertension and ventricular dila- tation. A study in SHR rats. Histology and Histopathol- ogy, 21, 179-185. [7] Carmona-Calero, E.M., González-Marrero, I., González- Toledo, J.M., Castañeyra-Ruiz, A., De Paz-Carmona, H., Castañeyra-Ruiz, L., Fernández-Rodríguez, P., Ruiz- Mayor, M.L. and Castañeyra-Perdomo, A. (2009) Reiss- ner’s fibre proteins and p73 variations in the cerebrospi- nal fluid and subcommissural organ of hydrocephalic rats. Anatomia, Histologia, Embryologia, 38, 282-285. doi:10.1111/j.1439-0264.2009.00939.x [8] Castañeyra-Perdomo, A., Meyer, G. and Ferres-Torres,R. (1985) The early development of the human subcom- missural organ. Journal of Anatomy, 143, 195-200. [9] Castañeyra-Perdomo, A., Carmona-Calero, E.M, Pérez- Gonzáles, H., Martínez-Peña y Valenzuela, I., Plaza- Moreno, P., Ormazabal-Ramos. C. and González- Marrero, I., Trujillano-Dorado, A. and Ferres-Torres, R. (2004) Ontogenic development of the human subcom- missural organ. European Journal of Anatomy, 8, 107- 120. [10] Castañeyra-Perdomo, A., Meyer, G.,. Carmona-Calero, E, Bañuelos-Pineda, J., Méndez-Medina, R., Ormazabal- Ramos, C. and Ferres-Torres, R. (1994) Alterations of the subcommissural organ in the hydrocephalic human fetal brain. Developmental Brain Research, 79, 316-320. doi:10.1016/0165-3806(94)90138-4 [11] Irigoin, C., Rodriguez, E.M., Heinrichs, M., Frese, K., Herzog, S., Oksche, A. and Rott, R. (1990) Immunocyto- chemical study of the subcommissural organ of rats with induced postnatal hydrocephalus. Experimental Brain Research, 82, 384-392. doi:10.1007/BF00231257 [12] Takeuchi, I.K., Kimura, R., Matsuda, M. and Shoji, R. (1987) Absence of subcommissural organ in the cerebral aqueduct of congenital hydrocephalus spontaneously oc- curring in MT/HokIdr mice. Acta Neuropathologica, 73, 320-322. doi:10.1007/BF00688253 [13] Castañeyra-Perdomo, A., Meyer, G. and Heylings, D.J. (1992) Early development of the human area postrema and subfornical organ. The Anatomical Record, 232, 612- 619. doi:10.1002/ar.1092320416 [14] Akert, K. and Steiner, F.A. (1970) The ganglion psalterii. A brief review of anatomical and physiological aspects of the subfornical organ in mammals. Bibliotheca Psychiat- rica, 10, 1-14. [15] Lenkei, Z., Corvol, P. and Llorenz-Cortes, C. (1995) The angiotensin receptor subtype AT1A predominates in rat forebrain areas involved in blood pressure, body fluid homeostasis and neuroendocrine control. Molecular Brain Research, 30, 53-60. doi:10.1016/0169-328X(94)00272-G [16] Ushigome, A., Nomura, M. and Tanaka, J. (2004). Modulation of noradrenaline release in the median preop- tic area by GABAergic inputs from the organum vascu- losum of the lamina terminalis in the rat. Neurochemistry International, 44, 139-144. doi:10.1016/S0197-0186(03)00134-7 [17] Brody, M.J. (1988) Central nervous system and mecha- nisms of hypertension. Clinical Physiology and Bio- chemistry, 6, 230-239. [18] Saper, C.B., Reis, D. and Joh, T. (1983) Medullary cate- cholamine inputs to the anteroventral third ventricular cardiovascular regulatory region in the rat. Neuroscience Letters, 42, 285-291. doi:10.1016/0304-3940(83)90276-8 [19] Meyer, G., Cabrera-Socorro, A., Perez-Garcia, C.G., Martinez-Millan, L., Walker, N. and Caput, D. (2004) Developmental roles of p73 in Cajal-Retzius cells and Copyright © 2012 SciRes. OPEN ACCESS  E. M. Carmona-Calero et al. / World Journal of Neuroscience 2 (2012) 68-73 Copyright © 2012 SciRes. 73 OPEN ACCESS cortical patterning. Neuroscience, 24, 9878-9887. doi:10.1523/JNEUROSCI.3060-04.2004 [20] Pozniak, C.D., Radinovic, S., Yang, A., McKeon, F., Kaplan, D.R. and Miller, F.D. (2000) An anti-apoptotic role for the p53 family member p73, during develop- mental neuron death. Science, 289, 304-306. doi:10.1126/science.289.5477.304 [21] Cabrera-Socorro, A., Pueyo Morlans, M., Suarez Sola, M., González Delgado, F.J., Castañeyra Perdomo, A., Marín, M.C. and Meyer, G. (2006) Multiple isoforms of the tu- mor protein p73 are expresssed in the adult human telen- cephalon and choroid plexus and present in the cerebro- spinal fluid. European Journal of Neuroscience, 23, 2109-2118. doi:10.1111/j.1460-9568.2006.04750.x [22] Laemmli, U.K., (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680-685. doi:10.1038/227680a0 [23] Wilson, C., Henry, S., Smith, M.A. and Bowser, R. (2004) The p53 homologue p73 accumulates in the nucleus and localizes to neurites and neurofibrillary tangles in Alz- heimer disease brain. Neuropathology and Applied Neu- robiology, 30, 19-29. doi:10.1046/j.0305-1846.2003.00496.x [24] Cabrera-Socorro, A., Hernandez-Acosta, N.C., González- Gomez, M. and Meyer, G. (2007) Comparative aspects of p73 and Reelin expression in Cajal-Retzius cells and the cortical hem in lizard, mouse and human. Brain Research, 1132, 59-70. doi:10.1016/j.brainres.2006.11.015 [25] Tomasini, R., Tsuchihara, K., Wilhelm, M., Fujitani, M., Rufini, A., Cheung, C.C., Khan, F., Itie-Youten, A., Wakeham, A., Tsao, M.S., Iovanna, J.L., Squire, J., Jurisica, I., Kaplan, D., Melino, G., Jurisicova, A. and Mak, T.W. (2008) TAp73 knockout shows genomic in- stability with infertility and tumor suppressor functions. Genes & Development, 22, 2677-2691. doi:10.1101/gad.1695308 [26] Fujitani, M., Cancino, G.I., Dugani, C.B., Weaver, I.C., Gauthier-Fisher, A., Paquin, A., Mak, T.W., Wojtowicz, M.J., Miller, F.D. and Kaplan, D.R. (2010) TAp73 acts via the bHLH Hey2 to promote long-term maintenance of neural precursors. Current Biology, 20, 2058-2065. doi:10.1016/j.cub.2010.10.029 [27] Jennings, J.R. and Zanstra, Y. (2009) Is the brain the essential in hypertension? NeuroImage, 47, 914-921. doi:10.1016/j.neuroimage.2009.04.072 [28] Raz, N. and Rodrigue, K.M. (2006) Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience & Biobehavioral Reviews, 30, 730-748. doi:10.1016/j.neubiorev.2006.07.001 [29] Waldstein, S.R., Giggey, P.P., Thayer, J.F. and Zonder- man, A.B. (2005) Nonlinear relations of blood pressure to cognitive function: The Baltimore longitudinal study of aging. Hypertension, 45, 374-379. doi:10.1161/01.HYP.0000156744.44218.74
|