Paper Menu >>
Journal Menu >>
 American Journal of Analytical Chemistry, 2010, 1, 31-33 doi:10.4236/ajac.2010.11004 Published Online May 2010 (http://www.SciRP.org/journal/ajac) Copyright © 2010 SciRes. AJAC 31 Steroids from the Whole Plants of Leucaena Leucocephala Chung-Yi Chen*, Yau-Der Wang School of Medical and Heath Science, Fooyin University, Kaohuing , Taiwan, China E-mail: xx377@mail.fy.edu.tw Received January 10, 2009; revised February 21, 2009; accepted February 23, 2009 Abstract Four steroids, 5α,8α-epidioxy-(24ξ)-ergosta-6,22-dien-3β-ol (1), β-sitosterol (2), β-sitostenone (3), and stig- mastenone (4), along with 10 known compounds were isolated from the whole plants of Leucaena leuco- cephala (Leguminosae). 1, Lupeol (5), 1,3-dipalmitoyl-2-oleoylglycerol (6), methylparabene (8) and isova- nillic acid (9) were found for the first time from the species. The structure of these compounds were charac- terized and identified by spectra analyses. Keywords: Steroids, Leucaena Leucocephala, Leguminosae 1. Introduction Leucaena leucocephala (Leguminosae) is a small, legu- minous and native to tropical America, now widely dis- tributed in southern Asia and neighboring islands [1]. Previous studies have show that gallocatechin, epigallo- catechin, catechin and epicatechin, extracted from the roots of L. leucocephala was found to exhibit the nitrifi- cation inhibition bioassay against the bacterium Nitro- somonas europaea [2]. L. leucocephala was chosen for further phytochemical investigation. The MeOH extract of its plants were subjected to solvent partitioning and chromatographic separation to afford 14 pure substances. The chemical constituents in the plants of L. leuco- cephala were separated with column chromatography. Investigation on the MeOH extract of the plants has led to the isolation of 14 compounds, four steroids: 5α, 8α-epidioxy-(24ξ)-ergosta-6,22-dien-3β-ol (1) (Figure 1) [3], β-sitosterol (2) (Figure 2) [4], β-sitostenone (3) (Figure 3) and stigmastenone (4) (Figure 4) [5] ; one terpenoid: lupeol (5) [6]; one glyceride: 1,3-dipalmitoyl- 2-oleoylglycerol (6) [7]; one alkanoid: linoleic acid (7) [8]; two benzenoids: methylparabene (8) [9] and isova- nillic acid (9) [10]; and five chlorophylls: pheophytin-a (10) [11], pheophorbide a methyl ester (11) [12], methyl-132- hydroxy-(132-S)-pheophorbide-b (12) [13], 132-hydroxy-(132-S)-pheophytin-a (13) [14] and aristo- phyll-C (14) [15]. These compounds were obtained and characterized by the comparison of their physical and spectral data (UV, IR, NMR and MS) with values ob- tained in the literature. Among them, 1, 5, 6, 8 and 9 were found for the first time from the species. The specimen of L. leucocephala was collected from Pingtung County, Taiwan in December, 2008. A voucher specimen was characterized by Dr. Jin-Cherng Huang of Department of Forest Products Science and Furniture Engineering, National Chiayi University, Chiayi, Taiwan and deposited in the School of Medical and Health Sci- ences, Fooyin University, Kaohsiung County, Taiwan. The air-dried seeds of L. leucocephala (5.0 kg) were ex- tracted with MeOH (80 L × 6) at room temperature and the MeOH extract (162.5 g) was obtained upon concen- tration under reduced pressure. The MeOH extract was chromatographed over silica gel (800 g, 70-230 mesh) using n-hexane/acetone as eluent to produce 5 fractions. Part of fraction 1 (6.11 g) was subjected to Si gel chro- matography by eluting with n-hexane/acetone (40:1), HO O O Figure 1. Chemical structure of 5α,8α-epidioxy-(24ξ)-ergosta- 6,22-dien-3-ol (1). 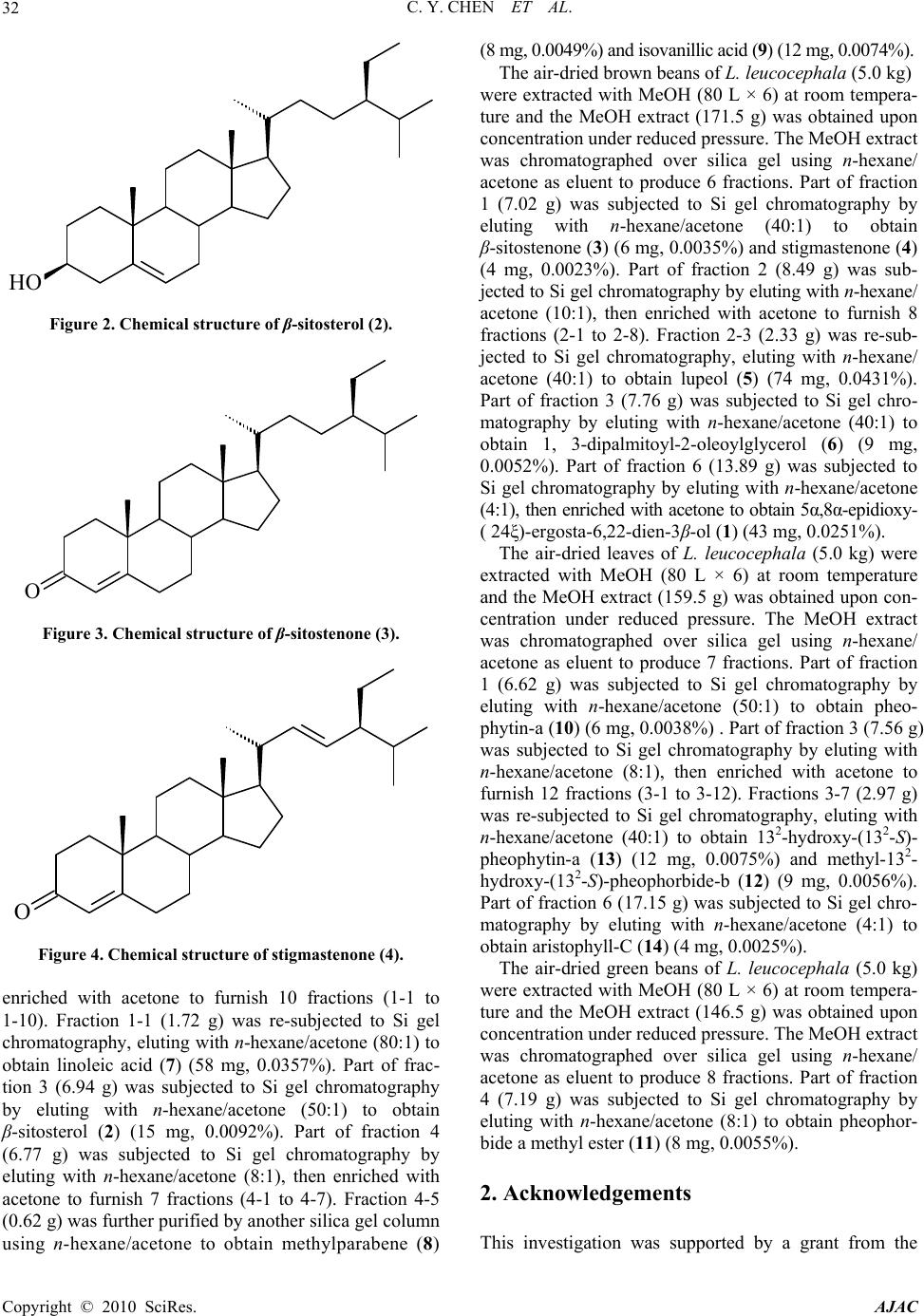 C. Y. CHEN ET AL. Copyright © 2010 SciRes. AJAC 32 HO Figure 2. Chemical structure of β-sitosterol (2). O Figure 3. Chemical structure of β-sitostenone (3). O Figure 4. Chemical structure of stigmastenone (4). enriched with acetone to furnish 10 fractions (1-1 to 1-10). Fraction 1-1 (1.72 g) was re-subjected to Si gel chromatography, eluting with n-hexane/acetone (80:1) to obtain linoleic acid (7) (58 mg, 0.0357%). Part of frac- tion 3 (6.94 g) was subjected to Si gel chromatography by eluting with n-hexane/acetone (50:1) to obtain β-sitosterol (2) (15 mg, 0.0092%). Part of fraction 4 (6.77 g) was subjected to Si gel chromatography by eluting with n-hexane/acetone (8:1), then enriched with acetone to furnish 7 fractions (4-1 to 4-7). Fraction 4-5 (0.62 g) was further purified by another silica gel column using n-hexane/acetone to obtain methylparabene (8) (8 mg, 0.0049%) and isovanillic acid (9) (12 mg, 0.0074%). The air-dried brown beans of L. leucocephala (5.0 kg) were extracted with MeOH (80 L × 6) at room tempera- ture and the MeOH extract (171.5 g) was obtained upon concentration under reduced pressure. The MeOH extract was chromatographed over silica gel using n-hexane/ acetone as eluent to produce 6 fractions. Part of fraction 1 (7.02 g) was subjected to Si gel chromatography by eluting with n-hexane/acetone (40:1) to obtain β-sitostenone (3) (6 mg, 0.0035%) and stigmastenone (4) (4 mg, 0.0023%). Part of fraction 2 (8.49 g) was sub- jected to Si gel chromatography by eluting with n-hexane/ acetone (10:1), then enriched with acetone to furnish 8 fractions (2-1 to 2-8). Fraction 2-3 (2.33 g) was re-sub- jected to Si gel chromatography, eluting with n-hexane/ acetone (40:1) to obtain lupeol (5) (74 mg, 0.0431%). Part of fraction 3 (7.76 g) was subjected to Si gel chro- matography by eluting with n-hexane/acetone (40:1) to obtain 1, 3-dipalmitoyl-2-oleoylglycerol (6) (9 mg, 0.0052%). Part of fraction 6 (13.89 g) was subjected to Si gel chromatography by eluting with n-hexane/acetone (4:1), then enriched with acetone to obtain 5α,8α-epidioxy- ( 24ξ)-ergosta-6,22-dien-3β-ol (1) (43 mg, 0.0251%). The air-dried leaves of L. leucocephala (5.0 kg) were extracted with MeOH (80 L × 6) at room temperature and the MeOH extract (159.5 g) was obtained upon con- centration under reduced pressure. The MeOH extract was chromatographed over silica gel using n-hexane/ acetone as eluent to produce 7 fractions. Part of fraction 1 (6.62 g) was subjected to Si gel chromatography by eluting with n-hexane/acetone (50:1) to obtain pheo- phytin-a (10) (6 mg, 0.0038%) . Part of fraction 3 (7.56 g) was subjected to Si gel chromatography by eluting with n-hexane/acetone (8:1), then enriched with acetone to furnish 12 fractions (3-1 to 3-12). Fractions 3-7 (2.97 g) was re-subjected to Si gel chromatography, eluting with n-hexane/acetone (40:1) to obtain 132-hydroxy-(132-S)- pheophytin-a (13) (12 mg, 0.0075%) and methyl-132- hydroxy-(13 2-S)-pheophorbide-b (12) (9 mg, 0.0056%). Part of fraction 6 (17.15 g) was subjected to Si gel chro- matography by eluting with n-hexane/acetone (4:1) to obtain aristophyll-C (14 ) (4 mg, 0.0025%). The air-dried green beans of L. leucocephala (5.0 kg) were extracted with MeOH (80 L × 6) at room tempera- ture and the MeOH extract (146.5 g) was obtained upon concentration under reduced pressure. The MeOH extract was chromatographed over silica gel using n-hexane/ acetone as eluent to produce 8 fractions. Part of fraction 4 (7.19 g) was subjected to Si gel chromatography by eluting with n-hexane/acetone (8:1) to obtain pheophor- bide a methyl ester (11) (8 mg, 0.0055%). 2. Acknowledgements This investigation was supported by a grant from the  C. Y. CHEN ET AL. Copyright © 2010 SciRes. AJAC 33 National Science Council of Republic of China (NSC 97-2320-B-242-002-MY3). 3. References [1] A. M. Gamal-Eldeen, H. Amer, W. A. Helmy, R. M. Talaat and H. Ragab, “Chemically-Modified Polysaccha- ride Extract Derived from Leucaena leucocephala Alters Raw 264.7 Murine Macrophage Functions,” International Immunopharmacology, Vol. 7, No. 6, March 2007, pp. 871-878. [2] A. J. Erickson, R. S. Ramsewak, A. J. Smucker and M. G. Nair, “Nitrification Inhibitors from the Roots of Leu- caena leucocephala,” Journal of Agricultural and Food Chemistry, Vol. 48, No. 12, December 2000, pp. 6174- 6177. [3] A. Gauvin, J. Smadja, M. Aknin, R. Faure and E. Gaydou, “Isolation of Bioactive 5α, 8α-Epidioxy-Sterols from the Marine Sponge Luffarie-lla cf. variabilis,” Canadian Journal of Chemistry, Vol. 78, July 2000, pp. 986-992. [4] L. Chen and W. Kang, “Chemical Constituents from Aes- chynanthus longicaullis,” China Journal of Chinese Ma- teria Medica, Vol. 34, No. 21, November 2009, pp. 2758-2760. [5] C. Y. Jiang, S. Z. Mu, B. Deng, Y. H. Ge, J. X. Zhang and X. J. Hao, “Studies on the Chemical Constituents from Euphorbia chrysocoma,” Journal of Chinese Me- dicinal Materials, Vol. 32, No. 9, September 2009, pp. 1390-1392. [6] C. Zhao, J. Shao and X. Li, “Chemical Constituents from Fruits of Ailanthus altissima,” China Journal of Chinese Materia Medica, Vol. 34, No. 17, September 2009, pp. 2197-2199. [7] T. Arishima, K. Sugimoto, R. Kiwata, H. Mori and K. Sato, “13C Cross-Polarization and Magic-Angle Spinning Nuclear Magnetic Resonance of Polymorphic Forms of Three Triacylglycerols,” Journal of the American Oil Chemists’ Society, Vol. 73, No. 10, May 1996, pp. 1231-1236. [8] I. L. Tsai, Y. F. Jeng, B. Jayaprakasam and I. S. Chen, “Cytotoxic Constituents from the Leaves of Litsea akoensis,” The Chinese Pharmaceutical Journal, Vol. 53, No. 6, December 2001, pp. 291-301. [9] H. K. Wang and K. H. Lee, “Plant-Derived Anticancer Agents and their Analogs Currently in Clinical Use or in Clinical Trials,” Botanical Bulletin of Academia Sinica, Vol. 38, No. 2, March 1997, pp. 225-235. [10] X. H. Yang, H. B. Lee, H. Chen, P. Li and B. P. Ye, “Chemical Constituents in the Leave of Rhizophora sty- losa L and their Biological Activities,” Acta Pharmaceu- tica Sinica, Vol. 43, No. 9, September 2008, pp. 974-978. [11] W. T. Li, H. W. Tsao, Y. Y. Chen, S. W. Cheng and Y. C. Hsu, “A Study on the Photodynamic Properties of Chlo- rophyll Derivatives Using Human Hepatocellular Carci- noma Cells,” Phytochemical and Phytobiological Sci- ences, Vol. 6, No. 12, December 2007, pp. 1341-1348. [12] M. C. Rho, M. Y. Chung, H. Y. Song, O. E. Kwon, S. W. Lee, J. A. Baek, K. H. Jeune, K. Kim, H. S. Lee and Y. K. Kim, “Pheophorbide A-Methyl Ester, Acyl-CoA: Cho- lesterol Acyltransferase Inhibitor from Diospyros kaki,” Archives of Pharmacal Research, Vol. 26, No. 9, Sep- tember 2003, pp. 716-718. [13] M. S. Buchanane, T. Hashimoto and Y. Asakawa, “Phytyl Esters and Phaeophytins from the Hornwort Megaceros flagellaris,” Phytochemistry, Vol. 41, No. 5, March 1996, pp. 1373-1376. [14] L. Ma and D. J. Dolphin, “Stereoselective Synthesis of New Chlorophyll a Related Antioxidants Isolated from Marine Organisms,” Journal of Organic Chemistry, Vol. 61, No. 7, April 1996, pp. 2501-2510. [15] Y. Y. Chan, Y. L. Leu and T. S. Wu, “The Constituents of the Leaves of Aristolochia heterophylla Hemsl.,” Chemical and Pharmaceutical Bulletin, Vol. 47, No. 6, June 1999, pp. 887-889. |

