 Materials Sciences and Applications, 2012, 3, 272-280 http://dx.doi.org/10.4236/msa.2012.35040 Published Online May 2012 (http://www.SciRP.org/journal/msa) SnO2 Dense Ceramic Microwave Sintered with Low Resistivity Leinig Antonio Perazolli, Gisane Gasparotto, Natalia Jaomaci, Miguel Ruiz, Maria Aparecida Zaghete Bertochi, Cesar Renato Foschini, Ederson Carlos Aguiar, José Arara Varela Interdisciplinary Laboratory of Electrochemistry and Ceramics—LIEC, Institute of Chemistry, Paulista State University “Júlio Mesquita Filho” (UNESP), São Paulo, Brazil. Email: leinigp@iq.unesp.br Received December 19th, 2011; revised February 25th, 2012; accepted March 22nd, 2012 ABSTRACT The Hall-Héroult process is used for alumina reduction by the use of graphite anodes even though it involves a high emission of carbon dioxide (CO2) and several other organic compounds. Proposals have been made aiming at substitut- ing graphite for a single-phase SnO2-based ceramic with low resistivity and chemical resistance to cryolite, which is characterized as an inconsumable anode, reducing pollutant emissions. To this end, a wide range of studies were carried out on SnO2-based ceramics modified with ZnO as a densification aid doped with the promoters of electrical conduc- tivity such as Nb2O5, Al2O3 and Sb2O3 through a mixture of oxides and hybrid sintering in a microwave oven. The pressed pellets were sintered in a microwave oven up to 1050˚C under a constant heating rate of 10˚C/min. After sin- tering, the density was determined by the Archimedes method, the phases were then characterized by X-ray diffraction, the microstructure and chemical composition resulting from the sintered SnO2-based ceramics were also investigated by field emission scanning electron microscopy (FE-SEM) coupled with an energy-dispersive X-ray spectroscopy (EDS) and the electrical properties were determined by the measurements of the electric field × current density. A single-phase ceramic was obtained with a relative density of above 90% and electrical resistivity of 6.1 ·cm at room temperature. The ceramics obtained in this study could be a potential candidate as an inconsumable anode to replace the current fused coke used in the reduction of alumina. Keywords: Tin Oxide; Anodic Electrode; Inconsumable Anode; Microwave Sintering; Electrical Resistivity 1. Introduction To obtain the primary aluminum, the industries use the reduction process which is constituted by the extraction of metal (aluminum) from its oxide (alumina). The alu- mina is placed into an electrolytic vat (Hall-Héroult cell) basically formed by a carbon electrode and an electrolyte of cryolite (Na3AlF6-sodium hexafluoroaluminate), a melted mineral at a temperature of approximately 1000˚C, which is used as a solvent for aluminum. However, the side effect has to do with the release of CO2 in the at- mosphere, one of the gases responsible for the green- house effect. In order to reduce this effect, Clark [1], Alder [2,3] and Hansey [4] patented the inconsumable anodic SnO2- based electrode which eliminates the consumption of graphite or coke. However, it was found out that these electrodes have a high concentration of additives, such as CuO and Sb2O3, which lead to the formation of segre- gates and/or precipitates at the grain boundaries, reduce- ing their chemical resistance to cryolite. These precipi- tated additives at the grain boundaries can lead to an in- creased rate of corrosion of the electrode, thereby reduce- ing its useful life. In addition, SnO2 electrodes are subject to corrosion by hydrogen from the molten cryolite bath and by ceramic compounds such as perovskite or spinel formed in the bath. Therefore, it is believed that the de- velopment of a single phase of SnO2 ceramics would increase the corrosion resistance, leading to an improve- ment in the electrolytic process. Electrodes of similar composition to those proposed were patented by Citti et al. [5] and used for melting glass. Tin oxide SnO2 is an n-type semiconductor whose crystal structure is similar to the tetragonal rutile. The use of ceramic oxide, when pure, is found to be limited due to its low densification during sintering because of the high surface diffusion at low temperatures and the high partial pressure of this oxide at high temperatures [6]. So it was necessary to add densification agents to obtain densities greater than 90% during sintering. The densification of SnO2 became feasible by the ad- Copyright © 2012 SciRes. MSA  SnO2 Dense Ceramic Microwave Sintered with Low Resistivity 273 dition of densifying agents such as ZnO [7] and CoO [8] taking the process of oxygen vacancies generation into consideration, leading to diffusion when in solid solution, as shown in Equations (1) and (2): 2 SnO x Sn 00 ZnOZnV O (1) 2 SnO x Sn 00 CoOCoVO (2) The single-phase ceramic with high density greater than 95% becomes interesting to prevent aluminum infil- tration, when dissolved in cryolite at 1000˚C, this infil- tration of both aluminum and cryolite can cause cracks in the electrode or the reaction of these materials with seg- regates and/or precipitates at the grain boundaries, thus weakening the electrode. According to Pianaro et al. [9], doping with Nb2O5 causes an increase in the ceramic conductivity. The addition of metals with charges higher than that of Sn4+ leads to an excess of electrons or cations vacancies in the crystal structure. On the other hand, the addition of metals with charges lower than that of Sn4+ causes electron acceptor defects [10] as shown in Equations (3) and (4): 2 Sn SnO x 25Sn o 2NbO4NbV10O (3) 2 SnO x 23Sn 0 2Sb O4SbV10O (4) Oliveira et al. [11] found out that the antimony oxide acts as an electron donor, increasing the conductivity of the SnO2-based ceramics. Although this oxide is trivalent and contains fewer valence electrons than tin, studies on SnO2-based varistors indicate the coexistence of Sb5+ and Sb3+ and that the ratio between them depends on both the temperature as well as the oxygen concentration in the environment [12]. In order to explain the electrical be- havior of SnO2 doped with Sb2O3, Mishra et al. [13] car- ried out quantum-mechanical calculations for the SnO2- Sb2O3 system and showed that antimony ions form an impurity band partially filled by free electrons within the band gap of tin oxide. Coleto Júnior [14] studied the action of Al2O3, which decreases the resistivity of SnO2 ceramics and this can be duly confirmed from the results obtained by Kovalevsky et al. [15]. Once Al2O3 is introduced in the SnO2 lattice, the Al3+ replaces the Sn4+, creating electron acceptors defects, as shown in Equation (5), with similar effect to that of Sb2O3: 2 SnO 23Sn00 Al O2AlV3O (5) According to Ivanova et al. [16], working with ytria stabilized zirconia (YSZ), the addition of Al2O3 reduced the resistivity of the grain boundary of this ceramic, and thus removing the silica. This “cleaning effect” occurs because the Al2O3 forms a liquid phase (silicon aluminate) once it is in contact with silica, moving to the triple point and cleaning the grain boundary. Clark [1] obtained a resistivity of 0.0025 ·cm at 975˚C, while Citti et al. [5] obtained 0.1 ·cm at room temperature, these data were used as comparison para- meters with the results obtained in the present work. Gisane Gasparotto et al. [17] verified the possibility of obtaining low resistivity SnO2-based ceramics using microwave sintering. The ceramics obtained showed uniform microstructure with little presence of intra or intergranular pores, and they neither observed the pre- sence of precipitates and/or segregates at the grain boundaries nor triple points. In contrast, a different result was obtained when conventional sintering was used, for purposes of comparison, see Figure 1. The ceramic with 99.00% mol of SnO2, 0.95% mol of ZnO and 0.05% mol of Nb2O5, sintered by microwave, showed an ohmic behavior with a resistivity of 1.3 ohm.cm and 93% of density. Thus, the objective of this work was to investigate the influence of the Al2O3 and the Sb2O3 in different compositions on the electrical resisitivity of SnO2 doped with 0.95% mol of ZnO and 0.05% mol of Nb2O5, sintered in conventional oven and microwave, aiming at (a) (b) Figure 1. Micrographies of fractured samples of the compo- sition of 99.00% mol SnO2 + 0.95% mol de ZnO + 0.05% mol de Nb2O5, fractured obtained by conve ntional (a) mi cr o- wave and (b) sintering [17]. Copyright © 2012 SciRes. MSA 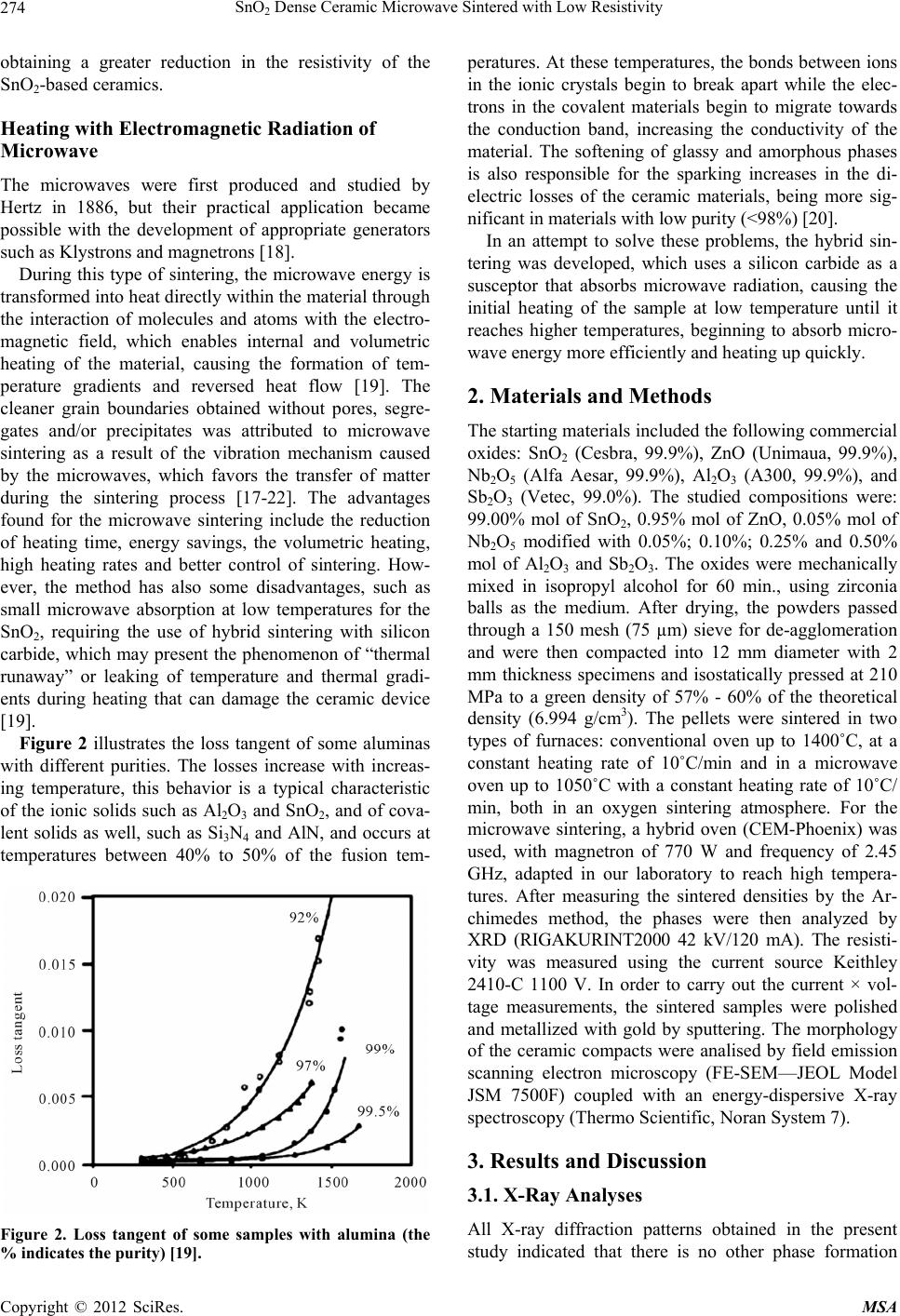 SnO2 Dense Ceramic Microwave Sintered with Low Resistivity 274 obtaining a greater reduction in the resistivity of the SnO2-based ceramics. Heating with Electromagnetic Radiation of Microwave The microwaves were first produced and studied by Hertz in 1886, but their practical application became possible with the development of appropriate generators such as Klystrons and magnetrons [18]. During this type of sintering, the microwave energy is transformed into heat directly within the material through the interaction of molecules and atoms with the electro- magnetic field, which enables internal and volumetric heating of the material, causing the formation of tem- perature gradients and reversed heat flow [19]. The cleaner grain boundaries obtained without pores, segre- gates and/or precipitates was attributed to microwave sintering as a result of the vibration mechanism caused by the microwaves, which favors the transfer of matter during the sintering process [17-22]. The advantages found for the microwave sintering include the reduction of heating time, energy savings, the volumetric heating, high heating rates and better control of sintering. How- ever, the method has also some disadvantages, such as small microwave absorption at low temperatures for the SnO2, requiring the use of hybrid sintering with silicon carbide, which may present the phenomenon of “thermal runaway” or leaking of temperature and thermal gradi- ents during heating that can damage the ceramic device [19]. Figure 2 illustrates the loss tangent of some aluminas with different purities. The losses increase with increas- ing temperature, this behavior is a typical characteristic of the ionic solids such as Al2O3 and SnO2, and of cova- lent solids as well, such as Si3N4 and AlN, and occurs at temperatures between 40% to 50% of the fusion tem- Figure 2. Loss tangent of some samples with alumina (the % indicates the purity) [19]. peratures. At these temperatures, the bonds between ions in the ionic crystals begin to break apart while the elec- trons in the covalent materials begin to migrate towards the conduction band, increasing the conductivity of the material. The softening of glassy and amorphous phases is also responsible for the sparking increases in the di- electric losses of the ceramic materials, being more sig- nificant in materials with low purity (<98%) [20]. In an attempt to solve these problems, the hybrid sin- tering was developed, which uses a silicon carbide as a susceptor that absorbs microwave radiation, causing the initial heating of the sample at low temperature until it reaches higher temperatures, beginning to absorb micro- wave energy more efficiently and heating up quickly. 2. Materials and Methods The starting materials included the following commercial oxides: SnO2 (Cesbra, 99.9%), ZnO (Unimaua, 99.9%), Nb2O5 (Alfa Aesar, 99.9%), Al2O3 (A300, 99.9%), and Sb2O3 (Vetec, 99.0%). The studied compositions were: 99.00% mol of SnO2, 0.95% mol of ZnO, 0.05% mol of Nb2O5 modified with 0.05%; 0.10%; 0.25% and 0.50% mol of Al2O3 and Sb2O3. The oxides were mechanically mixed in isopropyl alcohol for 60 min., using zirconia balls as the medium. After drying, the powders passed through a 150 mesh (75 µm) sieve for de-agglomeration and were then compacted into 12 mm diameter with 2 mm thickness specimens and isostatically pressed at 210 MPa to a green density of 57% - 60% of the theoretical density (6.994 g/cm3). The pellets were sintered in two types of furnaces: conventional oven up to 1400˚C, at a constant heating rate of 10˚C/min and in a microwave oven up to 1050˚C with a constant heating rate of 10˚C/ min, both in an oxygen sintering atmosphere. For the microwave sintering, a hybrid oven (CEM-Phoenix) was used, with magnetron of 770 W and frequency of 2.45 GHz, adapted in our laboratory to reach high tempera- tures. After measuring the sintered densities by the Ar- chimedes method, the phases were then analyzed by XRD (RIGAKURINT2000 42 kV/120 mA). The resisti- vity was measured using the current source Keithley 2410-C 1100 V. In order to carry out the current × vol- tage measurements, the sintered samples were polished and metallized with gold by sputtering. The morphology of the ceramic compacts were analised by field emission scanning electron microscopy (FE-SEM—JEOL Model JSM 7500F) coupled with an energy-dispersive X-ray spectroscopy (Thermo Scientific, Noran System 7). 3. Results and Discussion 3.1. X-Ray Analyses All X-ray diffraction patterns obtained in the present study indicated that there is no other phase formation Copyright © 2012 SciRes. MSA 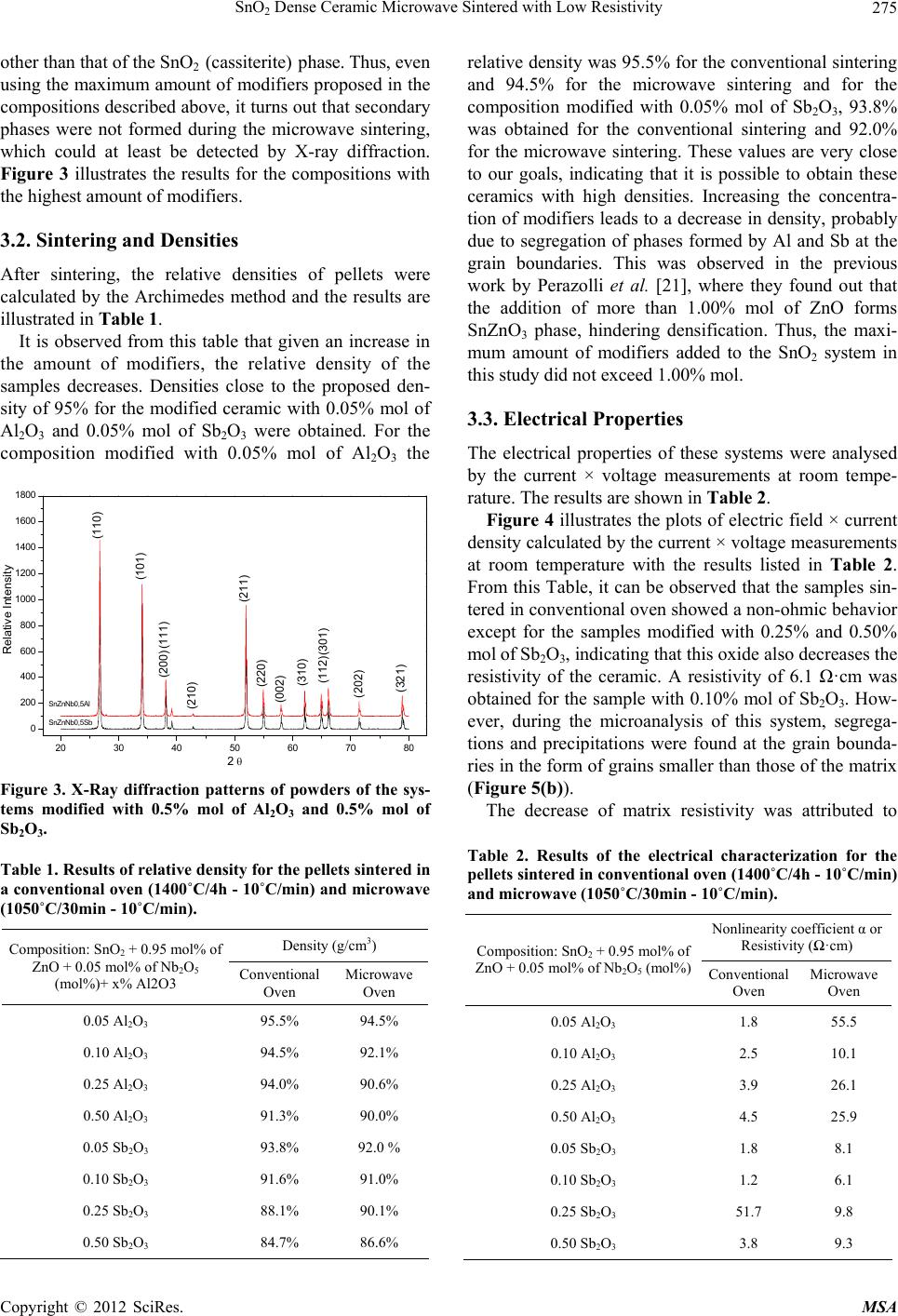 SnO2 Dense Ceramic Microwave Sintered with Low Resistivity 275 other than that of the SnO2 (cassiterite) phase. Thus, even using the maximum amount of modifiers proposed in the compositions described above, it turns out that secondary phases were not formed during the microwave sintering, which could at least be detected by X-ray diffraction. Figure 3 illustrates the results for the compositions with the highest amount of modifiers. 3.2. Sintering and Densities After sintering, the relative densities of pellets were calculated by the Archimedes method and the results are illustrated in Table 1. It is observed from this table that given an increase in the amount of modifiers, the relative density of the samples decreases. Densities close to the proposed den- sity of 95% for the modified ceramic with 0.05% mol of Al2O3 and 0.05% mol of Sb2O3 were obtained. For the composition modified with 0.05% mol of Al2O3 the 20 30 40 50 60 70 80 0 200 400 600 800 1000 1200 1400 1600 1800 Relative Intensity 2 SnZnNb0,5Al SnZnNb0,5Sb (110) (101) (200) (211) (220) (111) (210) (002) (310) (112)(301) (202) (321) Figure 3. X-Ray diffraction patterns of powders of the sys- tems modified with 0.5% mol of Al2O3 and 0.5% mol of Sb2O3. Table 1. Results of relative density for the pellets sintered in a conventional oven (1400˚C/4h - 10˚C/min) and microwave (1050˚C/30min - 10˚C/min). Density (g/cm3) Composition: SnO2 + 0.95 mol% of ZnO + 0.05 mol% of Nb2O5 (mol%)+ x% Al2O3 Conventional Oven Microwave Oven 0.05 Al2O3 95.5% 94.5% 0.10 Al2O3 94.5% 92.1% 0.25 Al2O3 94.0% 90.6% 0.50 Al2O3 91.3% 90.0% 0.05 Sb2O3 93.8% 92.0 % 0.10 Sb2O3 91.6% 91.0% 0.25 Sb2O3 88.1% 90.1% 0.50 Sb2O3 84.7% 86.6% relative density was 95.5% for the conventional sintering and 94.5% for the microwave sintering and for the composition modified with 0.05% mol of Sb2O3, 93.8% was obtained for the conventional sintering and 92.0% for the microwave sintering. These values are very close to our goals, indicating that it is possible to obtain these ceramics with high densities. Increasing the concentra- tion of modifiers leads to a decrease in density, probably due to segregation of phases formed by Al and Sb at the grain boundaries. This was observed in the previous work by Perazolli et al. [21], where they found out that the addition of more than 1.00% mol of ZnO forms SnZnO3 phase, hindering densification. Thus, the maxi- mum amount of modifiers added to the SnO2 system in this study did not exceed 1.00% mol. 3.3. Electrical Properties The electrical properties of these systems were analysed by the current × voltage measurements at room tempe- rature. The results are shown in Table 2. Figure 4 illustrates the plots of electric field × current density calculated by the current × voltage measurements at room temperature with the results listed in Table 2. From this Table, it can be observed that the samples sin- tered in conventional oven showed a non-ohmic behavior except for the samples modified with 0.25% and 0.50% mol of Sb2O3, indicating that this oxide also decreases the resistivity of the ceramic. A resistivity of 6.1 ·cm was obtained for the sample with 0.10% mol of Sb2O3. How- ever, during the microanalysis of this system, segrega- tions and precipitations were found at the grain bounda- ries in the form of grains smaller than those of the matrix (Figure 5(b)). The decrease of matrix resistivity was attributed to Table 2. Results of the electrical characterization for the pellets sintered in conventional oven (1400˚C/4h - 10˚C/min) and microwave (1050˚C/30min - 10˚C/min). Nonlinearity coefficient or Resistivity (·cm) Composition: SnO2 + 0.95 mol% of ZnO + 0.05 mol% of Nb2O5 (mol%) Conventional Oven Microwave Oven 0.05 Al2O3 1.8 55.5 0.10 Al2O3 2.5 10.1 0.25 Al2O3 3.9 26.1 0.50 Al2O3 4.5 25.9 0.05 Sb2O3 1.8 8.1 0.10 Sb2O3 1.2 6.1 0.25 Sb2O3 51.7 9.8 0.50 Sb2O3 3.8 9.3 Copyright © 2012 SciRes. MSA  SnO2 Dense Ceramic Microwave Sintered with Low Resistivity Copyright © 2012 SciRes. MSA 276 (a) (b) (c) (d) Figure 4. Electric field × current density measurements at room temperature of the samples sintered in conventional oven: (a) modified with 0.05% mol of Sb2O3; (b) modified with 0.50% mol of Sb2O3 in microwave oven; (c) modified with 0.05% mol of Al2O3 and (d) modified with 0.50% mol of Al2O3. these formations, which consist of conductive phases, and also to the formation of Sb5+ during heating, leading to the formation of free electrons by the generation of metal vacancies as shown in Equation (6). 3.4. Scanning Electronic Microscopy In order to verify the action of the modifiers (Sb and Al) on the crystal structure of SnO2, samples with large excess of cations were produced. From Figure 5, it can be ob- served as expected that the Al formed a liquid phase and was segregated at the grain boundaries. The triple point is formed at the grain boundaries by the joining together of three grains with three dimensions instead of 2 as seen on the grain boundary. This action may be positive provided the goal is to eliminate contaminations, responsible for increasing the ceramic resistivity at the grain boundaries (Figure 5(a)). Further addition of Sb resulted in segre- gated phases at the grain boundaries forming precipitates in the form of small grains observable in Figure 5(b). All the compositions with modifiers sintered in mi- crowave oven presented an ohmic behavior, however the behavior was opposite to that presented by the samples sintered in conventional oven. For samples modified with Sb2O3, it was found out that the amount of Sb did not significantly affect the value of resistivity, with the re- sults obtained being equivalent to the lowest value ob- tained by conventional sintering 6.1 ·cm. Thus, it can be concluded that during the microwave sintering, Sb tends to reduce the resistivity of the system regardless of the concentration, due to a larger extent to a greater dif- fusion of Sb in SnO2. The influence of the segregated phases at the grain boundaries, in this case, should not be considered once the microwave sintering produces ce- ramic with cleaner grain boundaries. The addition of Al2O3 in the samples sintered in con- ventional oven leads to an increase in non-ohmic beha- vior with the increase in the value of α, or an increase in  SnO2 Dense Ceramic Microwave Sintered with Low Resistivity 277 (a) (b) Figure 5. Micrographs of the ceramics with large amounts of modifiers (1.00% mol): (a) Al and (b) Sb. resistivity, due to the probable segregation of phases formed with aluminum excess at the grain boundaries, as can be observed by FE-SEM, Figures 5 and 6. The addition of Al did not contribute to the decrease in resistivity. Indeed for a further addition of modifiers, the obtained resistivity 10.1 ·cm is found to be equivalent to the resistivity obtained for ceramics without Al [17] 1.3 ·cm. The aluminum has only the cationic form Al3+ with a tendency to form aluminates and segregates at the grain boundaries, moving to the triple points [15,16], cleaning the boundaries (Figure 5(a)) and thus justifying the ob- served decrease in resistivity with the increase in the ad- dition of Al. The effect of sintering on the microstructure can be seen in Figure 6, which presents the microstructure of samples with the same composition sintered in con- ventional oven as well as in microwave oven. The micro- graphs illustrate that the samples sintered in microwave oven showed little or no pore formation and segregations at the grain boundaries as compared to the samples sintered in conventional oven, leading to the formation of a cleaner microstructure as a result of the diffusion caused by the microwave heating. 3.5. Cations Diffusion During the microwave sintering, there is a greater diffu- sion of cations and anions of the SnO2-based ceramics, due to the vibrational characteristics of the microwave heating. Hence, Sb diffuses in the SnO2 matrix, leading to a decrease in resistivity, as shown in Table 2. However, as the concentration of Sb increases the value of the resis- tivity does not decrease, this could be attributed to another mechanism. Further investigation is therefore being con- ducted in order to arrive at a flawless explanation. Nonetheless, due to the shorter time and lower tem- perature for the microwave sintering, it can be observed that the average size of the grains obtained is smaller to that obtained by conventional sintering. Moreover, given an increase in the sintering temperature coupled with a reduction in the heating rate and/or an increase in the isothermal sintering, it is possible to increase the average size of the grains with a probable reduction in resistivity. The analysis of the compositions at the grain bounda- ries was performed by means of FE-SEM/EDS and the presence of Al (Figure 7) and Sb (Figure 8) modifiers can be observed at the grain boundaries of SnO2-based ceramics. However, as can be seen on the micrographs of the ceramics containing Sb, the peaks in keV for the Sn have values close to those of the Sb and the presence of Sb was determined by the change in the peaks, which showed the formation of an irregularity in the form of shoulders. After analyzing all the points shown in Figure 7, only points 1, 3, and 5 showed peaks of Al in 1.52 keV. In Figure 8(b), the vertical bars that are clearly observed indicate the position of the peaks in keV for Sb, the for- mation of irregularities can also be seen in the peaks in the form of shoulders, which are attributed to the presence of Sb at the grain boundaries. 4. Conclusions Single-phase SnO2 based-ceramics were obtained with high density and low resistivity by the conventional mixed oxide method, doped with Zn+2, Nb5+, Al3+and Sb3+ using microwave sintering. The addition of modifiers in SnO2, with total concentrations above 1.00% mol leads to the formation of segregates at the grain boundaries in both sintering methods. The microwave sintering is ndicated for the production i Copyright © 2012 SciRes. MSA 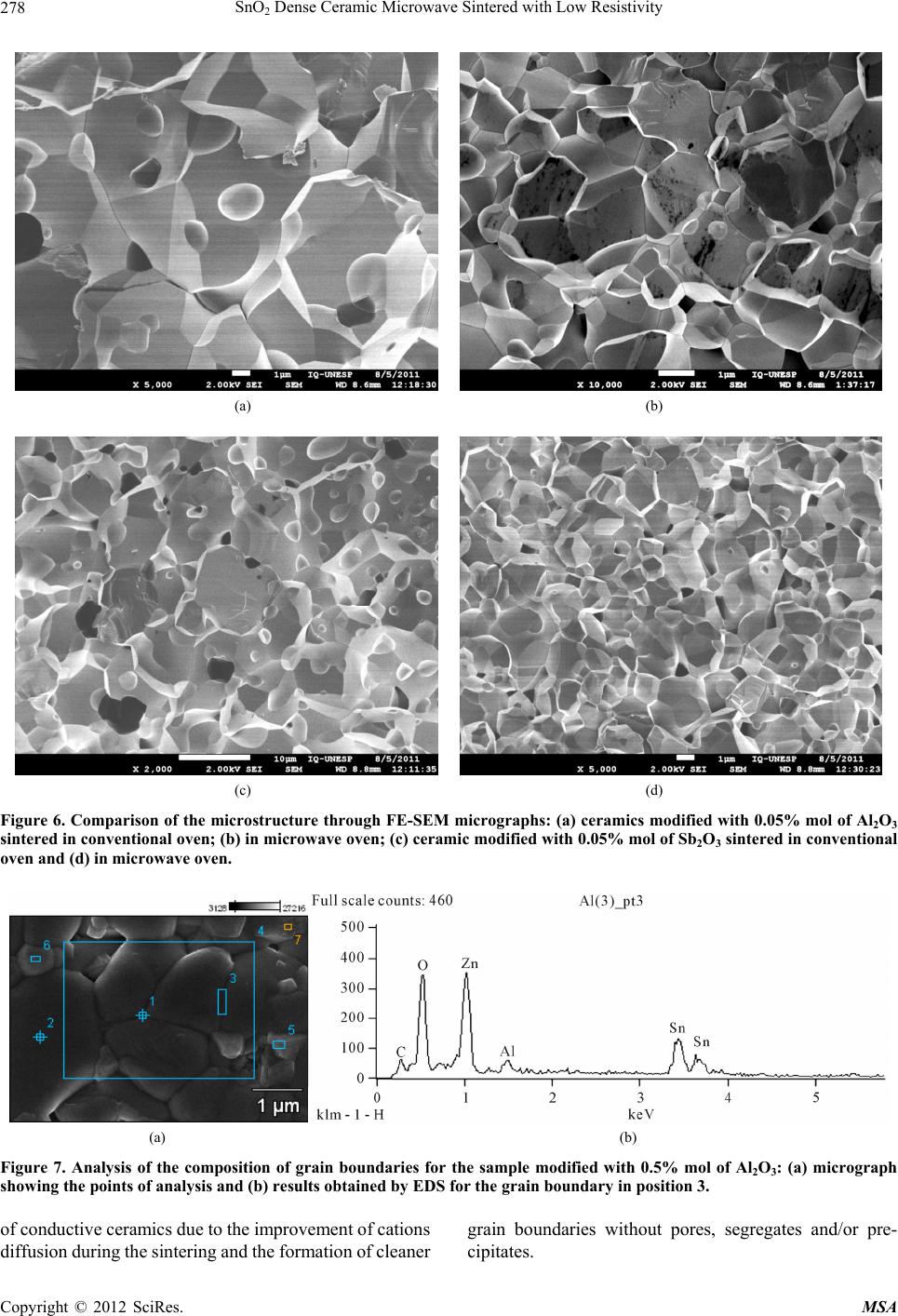 SnO2 Dense Ceramic Microwave Sintered with Low Resistivity 278 (a) (b) (c) (d) Figure 6. Comparison of the microstructure through FE-SEM micrographs: (a) ceramics modified with 0.05% mol of Al2O3 sintered in conventional oven; (b) in microwave oven; (c) ceramic modified with 0.05% mol of Sb2O3 sintered in conventional oven and (d) in microwave oven. (a) (b) Figure 7. Analysis of the composition of grain boundaries for the sample modified with 0.5% mol of Al2O3: (a) micrograph showing the points of analysis and (b) results obtaine d by EDS for the grain boundary in position 3. of conductive ceramics due to the improvement of cations diffusion during the sintering and the formation of cleaner grain boundaries without pores, segregates and/or pre- cipitates. Copyright © 2012 SciRes. MSA  SnO2 Dense Ceramic Microwave Sintered with Low Resistivity 279 (a) (b) Figure 8. Analysis of the composition of grain boundaries for the sample modified with 0.5% mol of Sb2O3: (a) micrograph showing the points of analysis and (b) results obtaine d by EDS for the grain boundary in position 3. The addition of Sb decreased the resistivity of the ce- ramic and the increasing of Sb concentration resulted in no further reduction in resistivity for the microwave sin- tering method. The addition of Al did not lead to the re- duction of resistivity. 5. Acknowledgements The authors gratefully acknowledge the financial support granted by the Brazilian research funding institutions CNPq, CAPES and FAPESP. REFERENCES [1] J. M. Clark and D. R. Secrist, “Method of Manufacturing Aluminum in a Hall-Heroult Cell,” US Patent No. 4379033, 1981. [2] H. Alder, “Process for the Electrolysis of a Molten Charge Using Inconsumable Bipolar Electrodes,” US Patent No. 3930967, 1976. [3] H. Alder, “Electrolysis of a Molten Charge Using In- comsumable Electrodes,” US Patent No. 3960678, 1976. [4] D. E. Hansey and L. I. Grindstaff, “Electrode Composi- tion,” US Patent No. 4233148, 1980. [5] O. Citti, C. McGarry and Y. Boussant-Roux, “Tin Ox- ide-Based Electrodes Having Improved Corrosion Resis- tance,” US Patent No. 018662, 2005. [6] Z. M. Jarzebski and J. P. Marton, “Physical Properties of SnO2 Materials II: Electrical Properties,” Journal of the Electrochemical Society, Vol. 123, No. 9, 1976, pp. 299C- 310C. doi:10.1149/1.2133090 [7] J. A. Varela, L. Perazolli, E. R. Cerri, E. Leite and E. Longo, “Sintering of Tin Oxide and Its Applications in Electronics and Processing of High Purity Optical Glasses,” Ceram, Vol. 47, No. 302, 2001, pp. 117-123. doi:10.1590/S0366-69132001000200010 [8] J. A. Cerri, E. R. Leite, D. Gouvea, E. Longo and J. A. Varela, “Effect of Cobalt (II) Oxide and Manganese (IV) Oxide on Sintering of Tin (IV) Oxide,” Journal of the American Ceramic Society, Vol. 79, No. 3, 1996, pp. 799- 804. doi:10.1111/j.1151-2916.1996.tb07949.x [9] S. A. Pianaro, P. R. Bueno, P. Olivi, E. Longo and J. A. Varela, “Electrical Properties of the SnO2-Based Varis- tor,” Journal of Materials Science: Materials in Electron- ics, Vol. 9, No. 2, 1998, pp. 159-165. doi:10.1023/A:1008821808693 [10] M. M. Oliveira; P. R. Bueno, J. A. Varela and E. Longo, “Influence of La2O3, Pr2O3 and CeO2 on the Nonlinear Properties of SnO2 Multicomponent Varistors,” Materials Chemistry and Physics, Vol. 74, No. 2, 2002, pp. 150-153. doi:10.1016/S0254-0584(01)00458-8 [11] M. M. Oliveira, J. H. G. Rangel, V. C. Souza, E. Longo and R. N. R. Filho, “Review—Effect of Donor Metals on the Electrical and Microstructural Properties of SnO2- Based Ceramic Varistors,” Cerâmica, Vol. 54, No. 331, 2008, pp. 296-302. doi:10.1590/S0366-69132008000300005 [12] A. Ovenston, D. Spînceanã, J. R. Walls and M. Cãldãraru, “Effect of Frequency on the Electrical Characteristics of Tin-Antimony Oxide Mixtures,” Journal of Materials Sci- ence, Vol. 29, No. 19, 1994, pp. 4946-4952. doi:10.1007/BF01151083 [13] K. C. Mishra, K. H. Johnson and P. C. Schmidt, “Elec- tronic Struture of Antimony-Doped Tin Oxide,” Physical Review B, Vol. 51, No. 20, 1995, pp. 13972-13976. doi:10.1103/PhysRevB.51.13972 [14] F. M. Filho, A. Z. Simões, A. Ries, E. C. Souza, L. Perazolli, M. Cilense, E. Longo and J. A. Varela, “Inves- tigation of Electrical Properties of Tantalum Doped SnO2 Varistor System,” Ceramics International, Vol. 31, No. 3, 2005, pp. 399-404. doi:10.1016/j.ceramint.2004.06.004 [15] A. V. Kovalevsky, F. M. B. Marques, V. V. Kharton, F. Maxim and J. R. Frade, “Silica-Scavenging Effect in Zir- conia Electrolytes: Assessment of Lanthanum Silicate Formation,” Ionics, Vol. 12, No. 3, 2006, pp. 179-184. doi:10.1007/s11581-006-0031-5 [16] D. Ivanova, A. Kovalevsky, V. V. Kharton and F. M. B. Marques, “Silica-Scavenging Effects in Ceria-Based Solid Electrolytes,” Boletines Sociedad de Cerámica y Vidrio, Vol. 47, No. 4, 2008, pp. 201-206. [17] M. A. N. Bordignon, C. R. Foschini, G. Gasparotto, E. C. Aguiar, M. A. Zaghete and L. Perazolli, “SnO2 Ceramic with Low Electrical Resistivity Obtained by Microwave Copyright © 2012 SciRes. MSA  SnO2 Dense Ceramic Microwave Sintered with Low Resistivity 280 Sintering,” Journal of Advanced Microscopy Research, Vol. 6, No. 3, 2011, pp. 193-200. [18] A. C. R. N. Barbosa, C. V. M. S. Cruz, M. B. Graziani, M. C. F. Lorenzetti and E. Sabadini, “Heating in Microwave Ovens/Developing of Basic Concepts,” Química Nova, Vol. 24, No. 6, 2001, pp. 901-904. [19] R. R. Menezes, P. M. Souto and R. H. G. A. Kiminami, “Microwave Sintering of Ceramics. Part I: Fundamental Aspects,” Cerâmica, Vol. 53, No. 325, 2007, pp. 1-10. doi:10.1590/S0366-69132007000100002 [20] W. W. Ho, “High-Temperature Dielectric Properties of Polycrystalline Ceramics,” In: W. H. Sutton, M. H. Brooks and I. J. Chabinsky, Eds., Microwave Processing of Ma- terials, Materials Research Society, Pittsburgh, 1988, pp. 137-148. [21] C. R. Foschini, L. Perazolli and J. A. Varela, “Sintering of Tin Oxide Using Zinc Oxide as a Densification Aid,” Journal of Materials Science, Vol. 39, No. 18, 2004, pp. 5825-5830. [22] W. D. Callister Jr., “Fundamentals of Materials Science and Engineering,” 5th Edidion, John Wiley & Sons Inc., New York, 2001. Copyright © 2012 SciRes. MSA
|