Journal of Cancer Therapy
Vol.3 No.5(2012), Article ID:23456,6 pages DOI:10.4236/jct.2012.35079
Management of Uterine Sarcomas
![]()
1Department of Radiotherapy and Oncologie University Center Hospital IBN ROCHD, Rue des Hôpitaux Casablanca, Casablanca, Morocco; 2Department of Anatomopathology University Center Hospital Center IBN ROCHD Casablanca, Casablanca, Morocco.
Email: *doc.a.naim@gmail.com
Received June 14th, 2012; revised July 18th, 2012; accepted July 29th, 2012
Keywords: Sarcoma; Uterus; Prognostic Factors
ABSTRACT
Uterine sarcomas are rare malignant tumors characterized by a great clinical and histopathological diversity. The aim of this work is to analyze the difficulties of diagnosis, therapeutic and prognosis posed by these tumors. Thirty-seven patients with uterine sarcoma, collected in the service Radiotherapy and Oncology, University Ibn Rochd of Casablanca between January 2000 and December 2007 were included in this study retrospective. The average patient age was 50 years (17 - 76). The bleeding was present in all patients, isolated in 54% of cases associated with pelvic pain in 24.6% and a mass abdomino-pelvic in seven patients. The average time of evolution was 10 months. The main histological type was found leiomyosarcoma. Twenty four patients in our series underwent total hysterectomy without annexial conservation. The surgery R0 was obtained in 43% of cases. The sarcomas were classified as stage IV in 51.4%. Adjuvant radiotherapy was indicated in 13 patients. After a mean of 20 months, half of patients evaluable presented a local relapse and/or metastatic, the third of cases were tumor progression while complete remission was maintained in 18.5% of cases. Uterine sarcomas are rare malignant mesenchymal tumor, which often occur in women after menopause. The main prognostic factors are hormonal status of the patient, stage clinical, histological type, histological grade and quality surgical excision. The management of uterine sarcomas is multidisciplinary, based mainly on surgery remains the only means of cure. Adjuvant radiotherapy allows decreased risk of local recurrence, with no impact on survival achieved at best 30% at 5 years. The role of chemotherapy remains confirm.
1. Introduction
Uterine sarcomas are a rare pathological entity representing 4% - 9% of all malignant tumors of the uterus [1]. They are characterized by high histological heterogeneity. Their diagnosis is usually retrospective as on one hand these tumors have no specific symptoms and on the other hand the contribution of both imaging and endometrial biopsy, in the preoperative diagnosis, is poor. Uterine sarcomas present also a therapeutic problem because of their rarity, the management is not codified. If it is accepted that the standard treatment for uterine sarcomas is surgical (hysterectomy with oophorectomy), the adjuvant therapies remain controversial. In fact, there have been few specific studies in the literature for the description of the terms of adjuvant therapy. Despite the various therapeutic methods used, the overall prognosis remains poor and presents many problems. Indeed, their prognosis since the five-year survival does not exceed 30% all stages confused [2-4]. Our study reports thirty seven cases of uterine sarcomas, in order to analyze their clinical and histopathological characteristics, and discuss the diagnostic and therapeutic difficulties associate with their therapeutic management compared to the literature data.
2. Patients and Methods
This is a retrospective study spread from January 2000 to December 2007, working for Therapeutic Radiology and Oncology University Hospital of Casablanca on 37 patients with histologically confirmed uterine sarcoma. For each patient, the following parameters were recorded: the context of appearance (age, parity, and hormonal status), clinical features and Para clinical, therapeutic management and clinical course.
3. Results
Thirty seven uterine sarcoma patients were enrolled in our study. Their age, fertility history and hormonal status are given in Table 1. Bleeding was present in all patients, isolated in 54% of cases associated with pelvic pain in 24.6% and an abdominopelvic mass in seven patients. The average time of evolution was 10 months (1 - 60). The main histological type was leiomyosarcoma found (54%), followed by endometrial sarcoma (22%), followed by carcinosarcoma (17%) and undifferentiated sarcoma (7%). The therapeutic management included: Surgery, Radiotherapy and Chemotherapy (Figure 1).
Thus, twenty four of the patients in our series underwent a total hysterectomy without conservation adnexal R0 surgery was obtained in 43% of cases (n = 10). Sarcomas were classified according of the FIGO 2009, as stage IV in 51.4% against 27% for stage I. Stages II and III represented respectively 16.2% and 5.4%.
In our series, chemotherapy was given, subject to status (PS ≤ 2), in the presence of one of these factors:
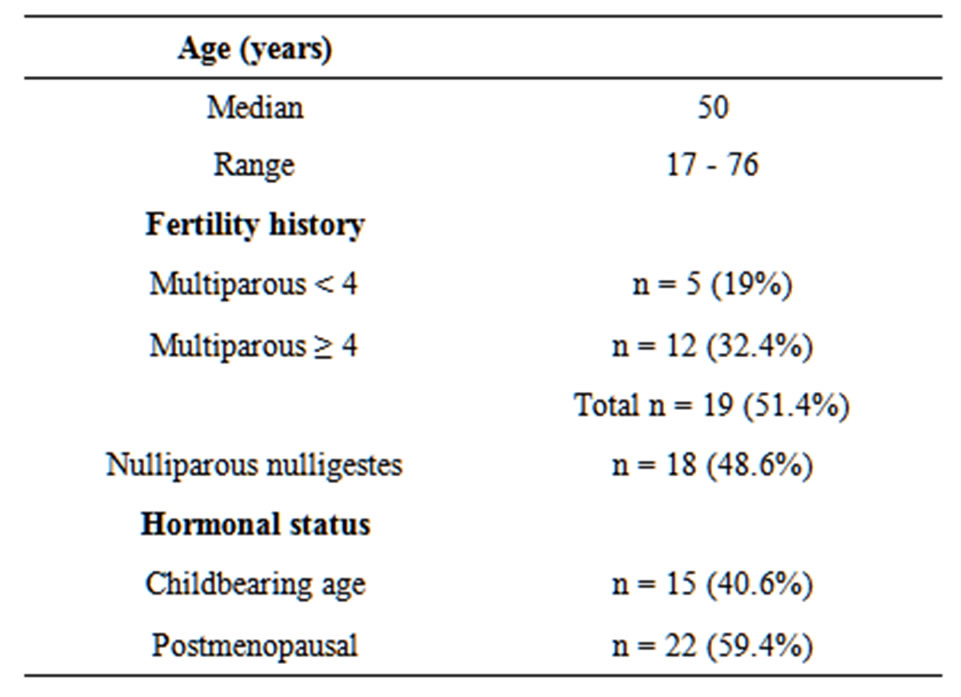
Table1.. Patient characteristics.
stage ≥ T3, lymph node involvement, histological grade 2 and 3, the presence of vascular emboli. It was recommended in 13 patients out, only seven patients have received the default way. The most widely used protocol was Adriamicyne/Cyclophosphamide. Adjuvant radiotherapy was indicated in 13 patients, combining between pelvic radiotherapy and brachytherapy. External radiotherapy in box technique by 4 beams (two beams antero posterior and two laterals) at a dose of 50 Gy with a Cobalt device 60. The vaginal brachytherapy with low dose rate (LDR) succeed externally in all cases except in one patient, who because of a vaginal synechia complement was done by external radiotherapy. The brachytherapy dose was 20 Gy.
After a mean of 20 months, 27 patients were evaluable only 48.2% of them experienced a local recurrence and/or metastatic, 33.3% of patients had tumor progression while in complete remission was maintained in 18.5% of cases.
4. Discussion
Uterine sarcomas are a relatively rare disease entity. The incidence appears increased in recent years. This is partly due to exposure to increasingly different predisposing factors such as pelvic irradiation and the use of tamoxifen in breast cancer [5,6] and partly to a better understanding of different histological features of uterine sarcoma, through the development of immunohistochemistry. Four main histological types:
*The Leiomyosarcoma (40%);
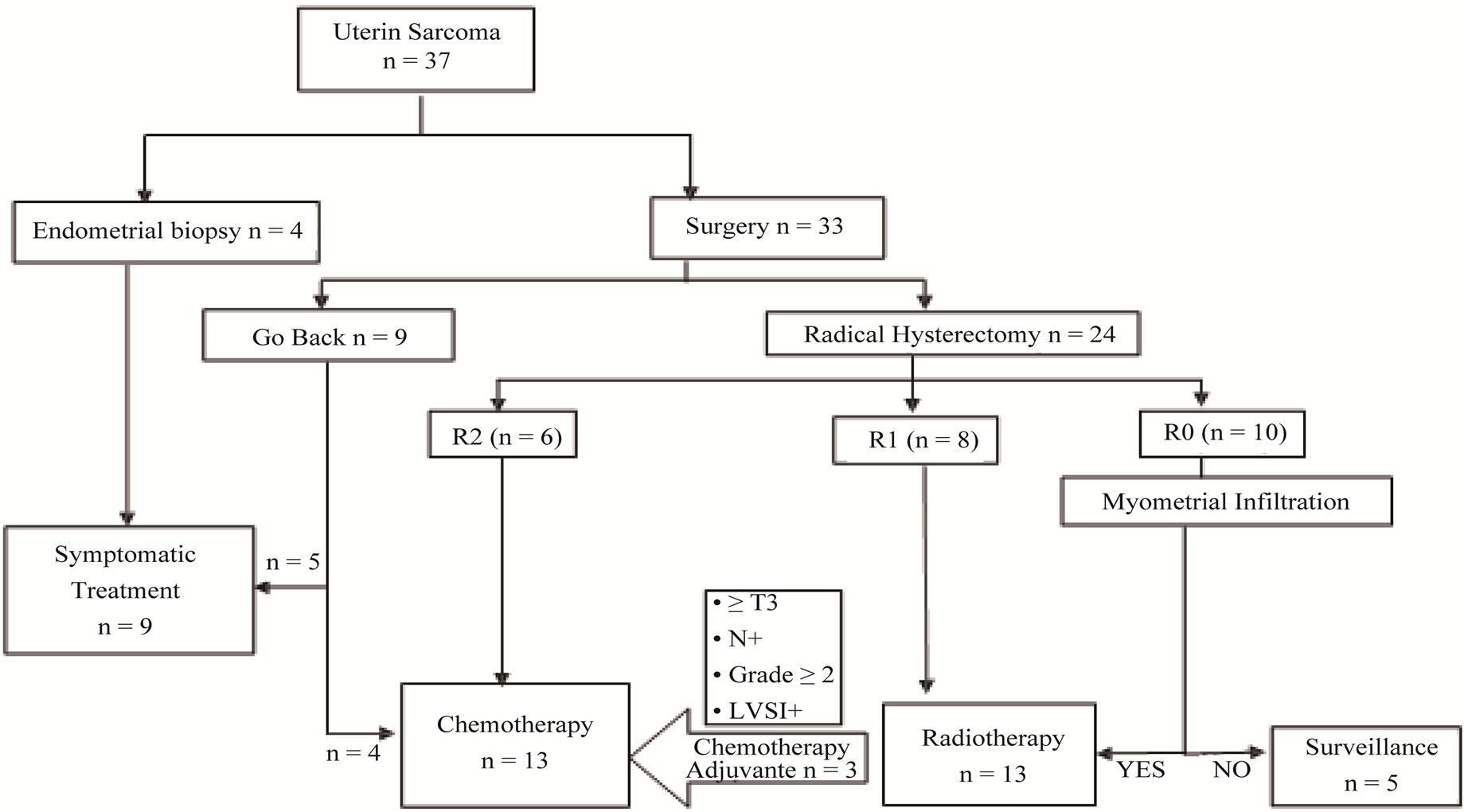
Figure 1. Organization chart summarizing the therapeutic management the patients of our series.
*The Carcinosarcoma or Mixed Mullerian Tumor Maligne” (40%);
*The endometrial stromal sarcoma (10% - 15%);
*The undifferentiated sarcomas (5% - 10%) [7].
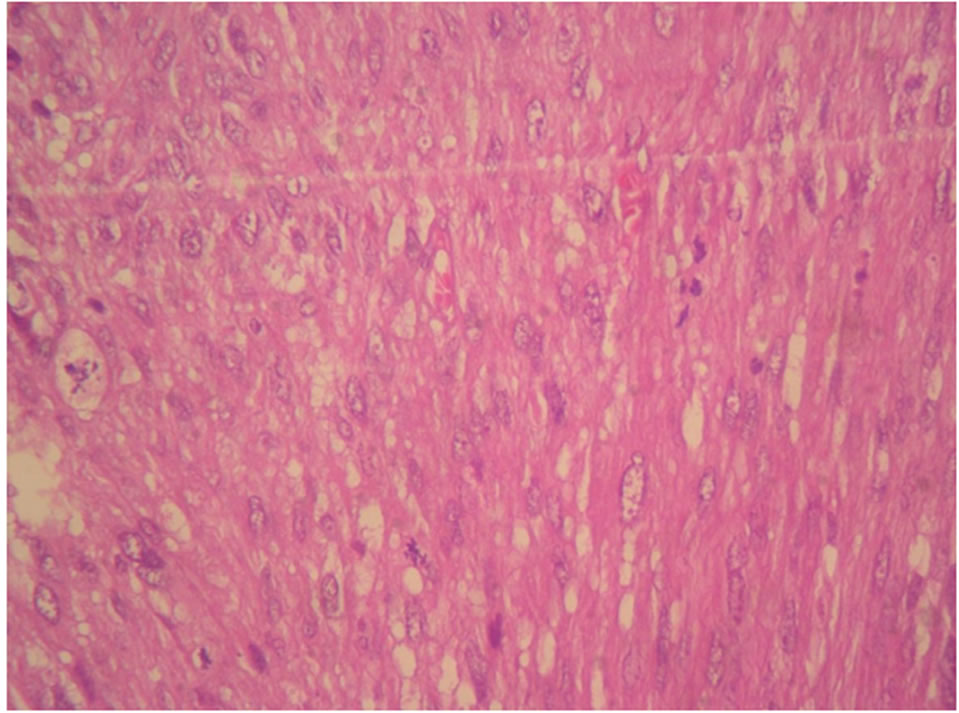
Our study joins the literature data for some histologic subtypes; namely leiomyosarcomas (Figures 2(a) and (b)) is predominant (54%) and undifferentiated sarcoma (7%). So it is distinguished by the low incidence of carcinosarcoma (17%) in favor of endometrial sarcoma reaching 22% in our study.
More common after menopause, uterine sarcoma can occur in women of the period of puberty to post-menopause. The average age of occurred varies in the literature 50 to 65 years with extremes ranging from 14 to 89 years [8,9]. In our study, the average age of occurred of uterine sarcomas was similar to most publications. The histological subtype of sarcoma would vary by age; indeed the leiomyosarcoma and endometrial stromal sarversely, the carcinosarcoma and Adénosarcome seem
 (a)
(a)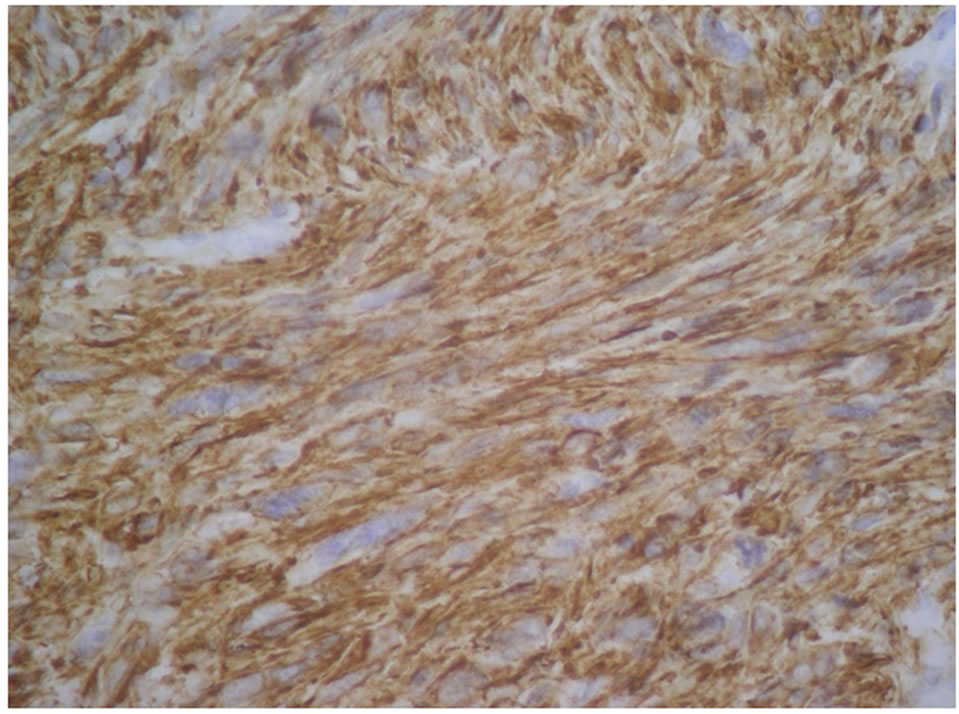 (b)
(b)
Figure 2. (a) H & E × 40: Leiomyosarcoma, atypical nuclei possess many abnormal mitoses; (b) Leiomyosarcoma, diffuse expression of H-caldesmon.
coma seems more interested with woman of fifty. Conoccur after 60 years [10]. According to the study of Gonzalez-Bosquet et al. [11], in a series on 93 cases of sarcoma, 47% of patients were symptomatic for more than 6 months before the final diagnosis with a mean period of clinical latency of 8 months. In our study, the time from onset of symptoms and diagnosis of sarcoma was higher with a median of 10 months (1 - 60 months). Uterine sarcomas are found most often by bleeding that are found in 45% - 86% of cases of pelvic pain related to tumor mass, found in 20% - 25% of cases. Similarly in our series, bleeding was the master symptom associated with pelvic pain in one quarter of cases. This nonspecific symptom explains the delay in their diagnosis mistaken for benign uterine pathologies, particularly leiomyoma. Hence, the importance of regular monitoring of any woman, with uterine fibroids [12]. The diagnosis of uterine sarcoma should be considered in any rapidly increasing volume of myoma especially in a menopausal patient. Indeed, 0.2 to 0.7% of lesions treated with preoperative diagnosis of fibroma turn out to be uterine sarcomas [13].
Imaging can help diagnose preoperatively because the search for anomalies that may be suspected sarcoma is essential before considering surgical treatment of uterine leiomyomata focused. Pelvic ultrasound did not reach pathognomonic echographic sign. However, Doppler can differentiate between fibroids and carcinosarcomas, according to the study of Aviram and all [14], which showed a significant difference in Doppler indices between these two types of lesions but there was no differences in Doppler indices between fibroids and leiomyosarcomas. Unlike CT, which is not specific, the magnetic resonance imaging may have an interest relative to point to a sarcomatous origin and make the best staging local and locoregional [15]. Some authors suggest that MRI in combination with Positron Emission Tomography with 18-fluoro-deoxyglucose (FDG-PET) could be valuable in the diagnosis of uterine sarcoma, especially in detecting lymph node metastasis and recurrence [16]. Given our socio economic status, the patients of our series were able to realize only ultrasound (n = 37) and CT (n = 33) (Figure 3).
The Endometrial biopsy doesn’t allow the diagnosis of uterine sarcoma that if the tumor reaches the endometrium outside leiomyosarcomas are usually covered with a normal endometrium and carcinosarcoma by necrotic tissue [17]. Thus, the preoperative diagnosis of uterine sarcoma seems not always easy, and therefore is most often based on histological analysis of the resected hysterectomy [18]. Surgery which is the major therapeutic weapon of uterine sarcomas should be to type of hysterectomy with bilateral salpingo-oophorectomy (Radical
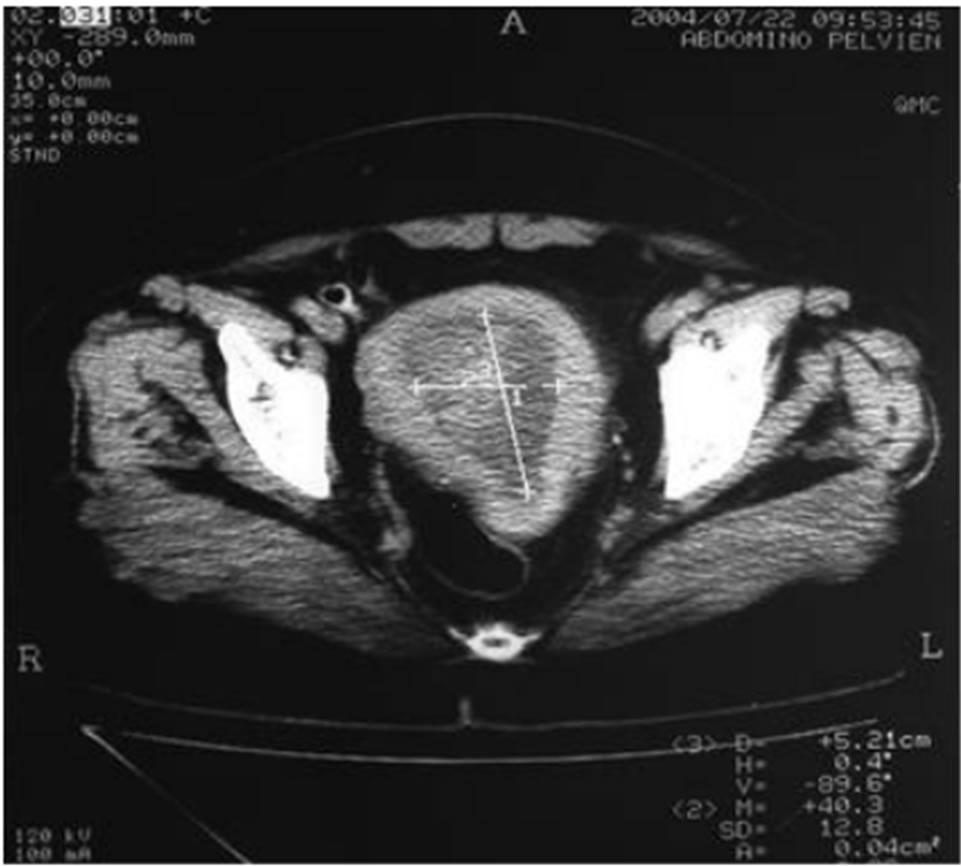
Figure 3. Scanner abdominopelvic objectifying an increased uterine size, site of a large tumor formation six centimeters dual component hypo dense, isodense, at its central part with the endometrial biopsy endometrial stromal sarcoma.
Hysterectomy) with peritoneal cytology. Supplemented by an omentectomy and pelvic lymphadenectomy in case of carcinosarcoma.
In our series, 33 patients underwent surgery; only 24 of them had a HTSCA and simple biopsy in 9 cases. The resection margins were microscopically negative after total hysterectomy “R0” in 10 cases, positive “R1” in 8 cases and macroscopically tumor “R2” in the other 6 cases.
The benefit of adjuvant radiotherapy to surgery has been demonstrated by several studies, with greater local control 85% versus 43% in five years, although the survival benefit remains uncertain [19-21]. Irradiation is either by delivering radiotherapy doses of 45-50 Gray, followed by vaginal brachytherapy 15 to 20 Gray; or by exclusive brachytherapy from 50 to 60 Gray [22]. For sarcomas of low histological grade, irradiation will be discussed in terms of histology (leiomyosarcoma and carcinosarcoma priority) and infiltration of the myometrium. It is a priori necessary in case of incomplete excision. It remains to determine the most appropriate way of irradiation. In our study, thirteen patients underwent adjuvant radiotherapy to surgery involving pelvic radiotherapy the mean dose of 50 Gy (18 × 2.5 Gy) and vaginal brachytherapy of 20 Gy, except in one patient, who because of synechia vaginally, received additional external radiotherapy.
The role of adjuvant chemotherapy remains controversial in uterine sarcomas. Several protocols were used: doxorubicin (Adriamycin) alone, VAC (Vincristine, Actimycine D and cyclophosphamide), Adriamycin, Cisplatin and Ifosfamide, Etoposide combination with hormonal therapy ···
Given the very low incidence of these tumors, the benefit of chemotherapy is uncertain and no standard is established. Currently, although we can not rely on previous studies, it seems nonetheless logical to propose adjuvant chemotherapy in case of unfavorable prognostic factors (high histological grade, high FIGO stage, presence of tumor emboli or lymphadenopathy) especially as the patient is young. In all cases, chemotherapy should not delay irradiation [23].
In our series, chemotherapy was indicated, subject to status (PS ≤ 2), in the presence of one of these factors: stage ≥ T3, lymph node involvement, histological grade 2 and 3, the presence of vascular emboli, but it was received only in seven patients, by default. The most widely used protocol was Adriamycin/cyclophosphamide.
Especially targeted therapies with anti-angiogenic remain good candidates for the treatment of sarcomas and are being evaluated in this disease; despite the negativity of the phase II trial of Thalidomide [24,25].
The prognosis of uterine sarcomas remains poor, with a five-year survival of 30 to 70% in localized stages who received an optimal therapeutic management. Where as in the metastatic stage, median survival is less than one year. Except, in the subgroup of endometrial stromal sarcoma of low grade, which has a relatively prolonged survival [26,27]. Recurrences are important; they vary from 50 to 70% depending on the series and occur in the first two years. Their frequency depends on the addition of prognostic factors and treatment of loco regional insufficient. Metastatic recurrences occur in 70% of cases and preferentially affect the lungs [28].
In our series, after a decline of 20 months, half of the patients developed recurrence, 55% of cases with metastatic lung and/or liver. Two of our patients have presented recurrence in the form of subcutaneous nodules.
The main prognostic factors accepted by most authors are hormonal status, tumor stage (survival at 5 years was 54% stage I versus 11% stage IV), histological type (The Carcinosarcomas have the worst prognosis), histological grade (high metastatic risk in grade 3 and low in grade 1) and the presence of residual tumor after surgery. Our series is characterized by a predominance of stage IV uterine sarcoma (51.4%), postmenopausal women in most cases (59.4%) and with residual tumor macro “R2” or microscopic “R1” in two thirds of cases.
5. Conclusion
Although uterine sarcomas are rare, it seems necessary to improve the standardization of their care through the development of larger studies and by encouraging patients to participate in randomized trials. Finally, only an early diagnosis and multidisciplinary collaboration are parked to perform relatively better.
REFERENCES
- S. E. Brooks, M. Zhan, T. Cote and C. R. Baquet, “Surveillance, Epidemiology, and End Results Analysis of 2677 Cases of Uterine Sarcoma 1989-1999,” Gynecologic Oncology, Vol. 93, No. 1, 2004, pp. 204-208. doi:10.1016/j.ygyno.2003.12.029
- A. Tinelli, “FIGO Staging for Uterine Sarcomas,” International Journal of Gynecology & Obstetrics, Vol. 104, No. 3, 2009, p. 179. doi:10.1016/j.ijgo.2008.12.009
- B. Harlow, N. Weiss and S. Lofton, “The Epidemiology of Sarcomas of the Uterus,” Journal of the National Cancer Institute, Vol. 76, No. 3, 1986, pp. 399-402.
- R. Nordal and S. Thoresen, “Uterine Sarcoma in Norway 1956-1992: Incidence, Survival and Mortality,” European Journal of Cancer, Vol. 33, No. 6, 1997, pp. 907-911. doi:10.1016/S0959-8049(97)00040-3
- N. S. Reed, “Uterine Sarcomas: The Biggest Challenge?’ Clinical Oncology, Vol. 14, No. 1, 2002, pp. 50-53. doi:10.1053/clon.2001.0043
- R. E. Curtis, D. M. Freedman, M. E. Sherman and J. F. Fraumeni Jr, “Risk of Malignant Mixed Mullerian Tumors after Tamoxifen Therapy for Breast Cancer,” Journal of the National Cancer Institute, Vol. 96, No. 1, 2004, pp. 70-74. doi:10.1093/jnci/djh007
- E. D’Angelo and J. Prat, “Uterine Sarcomas: A Review,” Gynecologic Oncology, Vol. 116, No. 1, 2009, pp. 3-6. doi:10.1016/j.ijgo.2008.12.008
- M. Abbes, B. Guillaume, J. Vershoore and M. Namer, “A Propos de 27cas de Forme Rare du Cancer du Corps de l’Utérus,” Revue Française de Gynécologie et d’Obstétrique, Vol. 79, No. 2, 1984, pp. 97-100.
- S. G. El Haloui, F. Bensaid, S. Nabil, R. Bezzad, S. El Fihri, M. T. Alaoui, “Les Sarcomes Utérins à Propos de 7cas Avec Revue de la Littérature,” Revue Française de Gynécologie et d’Obstétrique,Vol. 91, No. 3, 1996, pp. 94-100.
- E. Oliva, P. B. Clement and R. H. Young, “Mesenchymal Tumors of the Uterus: Selected Topics Emphasizing Diagnostic Pitfalls,” Current Diagnostic Pathology, Vol. 8, No. 4, 2002, pp. 282-286 doi:10.1054/cdip.2002.0126
- E. Gonzalez-Bosquet, J. M. Martinez-Palones, J. González-Bosquet, A. García Jiménez and Xercavins, “Uterine Sarcoma: A Clinicopathological Study of 93 Cases,” European Journal of Gynaecological Oncology, Vol. 18, No. 3, 1997, pp. 192-195.
- M. Nickie-Psikuta and K. Gawrychowski, “Different Types and Different Prognosis Study of 310 Uterine Sarcomas,” European Journal of Gynaecological Oncology, Vol. 14, 1993, pp. 105-113.
- S. Leibsohn, G. d’Albaing and S. Mishell, “Leiomyosarcoma in a Series of Hysterectomies Performed for Presumed Uterine Leiomyomas,” American Journal of Obstetrics and Gynecology, Vol. 162, No. 4, 1990, pp. 68-76.
- R. Aviram, Y. Ochshorn, O. Markovitch, A. Fishman, I. Cohen, M. M. Altaras, et al., “Uterine Sarcomas versus Leiomyomas: Gray-Scale and Doppler Sonographic Findings,” Journal of Clinical Ultrasound, Vol. 33, No. 1, 2005, pp.10-13. doi:10.1002/jcu.20075
- S. Taïeb, F. Narducci, A. Chevalier, M. C. Baranzelli, L. Ceugnart and E. Leblanc, “Imagerie des Sarcomes Uté- rins,” Imagerie de la Femme, Vol. 18, No. 4, 2008, pp. 229-235. doi:10.1016/S1776-9817(08)74626-1
- A. M. Maffione, M. Piva, C. S. Tsamita, C. Nanni, P. Castellucci, V. Ambrosini, et al., “Positron-Emission Tomography in Gynaecologic Malignancies,” Archives of Gynecology and Obstetrics, Vol. 280, No. 4, 2009, pp. 521-528. doi:10.1007/s00404-009-0979-2
- K. Oda, S. Okada, T. Nei, T. Shirai, M. Takahashi, Y. Sano, et al., “Cytodiagnostic Problems in Uterine Sarcoma. Analysis According to a Novel Classification of Tumor Growth Types,” Acta Cytologica, Vol. 48, No. 2, 2004, pp. 181-186. doi:10.1159/000326313
- M. Nickie-Psikuta and K. Gawrychowski, “Different Types and Different Prognosis Study of 310 Uterine Sarcomas,” European Journal of Gynaecological Oncology, Vol. 14, 1993, pp. 105-113.
- E. Deniaud-Alexandre, L. Chauveinc, A. de la Rochefordière, X. Sastre and K. B. Clough, “Intérêt des Traitements Adjuvants Dans les Sarcomes Utérins: Expérience de l’Institut Curie,” Cancer/Radiothérapie, Vol. 5, No. 6, 2001, pp. 743-749. doi:10.1016/S1278-3218(01)00133-0
- F. Ferrer, S. Sabater, B. Farrus, F. Guedea, A. Rovirosa and L. Anglada, “Impact of Radiotherapy on Local Control and Survival in Uterine Sarcomas: A Retrospective Study from the Group Oncologic Catala-Occita,” International Journal of Radiation Oncology, Biology, Physics, Vol. 4, No. 1, 1999, pp. 47-52. doi:10.1016/S0360-3016(98)00515-X
- G. Echt, J. Jepson, J. Steel, B. Langholz, G. Luxton and W. Hernandez, “Treatment of Uterine Sarcomas,” Cancer, Vol. 66, No. 1, 1990, pp. 35-39. doi:10.1002/1097-0142(19900701)66:1<35::AID-CNCR2820660108>3.0.CO;2-V
- B. Larson, C. Silfverswärd, B. Nilsson and F. Patterson, “Mixed Mullerian Tumours of the Uterus-Prognotic Factors: A Clinical and Histopathologic Study of 147 Cases,” Radiotherapy and Oncology, Vol. 17, No. 2, 1990, pp. 123-132. doi:10.1016/0167-8140(90)90100-B
- S. Kanjeekal, A. Chambers, M. F. Fung and S. Verma, “Systemic Therapy for Advanced Uterine Sarcoma: A Systemic Review of the Literature,” Gynecologic Oncology, Vol. 97, No. 2, 2005, pp. 624-637. doi:10.1016/j.ygyno.2005.01.041
- P. Pautier, “Sarcomes Utérins,” Oncologie, Vol. 9, No. 2, 2007, pp. 137-143. doi:10.1007/s10269-006-0545-5
- Y. S. Kuo, P. Timmins and S. V.Blank, “Phase II Trial of Thalidomide for Advanced and Recurrent Gynecologic Sarcoma: A Brief Communication from the New York Phase II Consortium,” Gynecologic Oncology, Vol. 100, No. 1, 2006, pp. 160-165. doi:10.1016/j.ygyno.2005.08.033
- S. E. Brooks, M. Zhan, T. Cote and C. R. Baquet, “Surveillance, Epidemiology, and End Results Analysis of 2677 Cases of Uterine Sarcoma 1989-1999,” Gynecologic Oncology, Vol. 93, No. 1, 2004, pp. 204-208. doi:10.1016/j.ygyno.2003.12.029
- L. Benoit, L. Arnould, N. Cheynel, S. Goui, F. Collin, J. Fraisse, et al., “The Role of Surgery and Treatment Trends in Uterine Sarcoma,” European Journal of Surgical Oncology, Vol. 31, No. 4, 2005, pp. 434-442. doi:10.1016/j.ejso.2005.01.010
- B. A. Goff, L. W. Rice, D. Fleischacker, H. G. Muntz, S. S. Falkenberry, N. Nikrui, et al., “Uterine Sarcoma and Endometrial Stromal Sarcoma: Lymph Node Metastases and Sites of Recurrence,” Gynecologic Oncology, Vol. 50, No. 1, 1993, pp. 105-109. doi:10.1006/gyno.1993.1172
NOTES
*Corresponding author.

