American Journal of Plant Sciences
Vol.5 No.5(2014), Article ID:43786,13 pages DOI:10.4236/ajps.2014.55084
Interactive Effects of Elevated [CO2] and Soil Water Stress on Leaf Morphological and Anatomical Characteristic of Paper Birch Populations
Anjala Pyakurel, Jian R. Wang
Faculty of Natural Resources Management, Lakehead University, Thunder Bay, Canada
Email: apyakure@lakeheadu.ca
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 8 January 2014; revised 14 February 2014; accepted 5 March 2014
ABSTRACT
The leaf morphological and stomatal characteristics of four paper birch (Betula papyrifera Marsh) populations, grown at four treatment conditions of carbon dioxide [CO2] and soil water levels were investigated to determine whether future increases in atmospheric [CO2] and water deficit affected the leaf characteristics. The populations from Cussion Lake, Little Oliver, Skimikin and Wayerton were grown for 12 weeks under ambient (360 ppm) and elevated (720 ppm) [CO2] at both high and low water levels. The populations significantly differed in leaf area and stomatal characteristics due to the interaction effects of [CO2], water levels and population differences. Most leaf morphological characteristics and stomatal density varied due to the effects of [CO2] and/or populations, but not due to the effect of water levels. Although elevated [CO2] alone barely affected stomatal area of the birch populations, simultaneous elevated [CO2] at both water levels had stimulated stomatal characteristics within and among the populations. Overall, elevated [CO2] reduced leaf area and increased stomatal density; and low water level resulted in smaller stomatal area, pore area and guard cell width. However, the populations responded differently to an increase in [CO2] and water levels. All populations showed plastic responses with respect to [CO2] and water levels either by decreasing stomatal area under low water level or by increasing stomatal density under elevated [CO2]. Hence, integration between and within leaf characteristics had helped paper birch populations maintain balance between [CO2] gain and water loss.
Keywords:Carbon Dioxide Levels; Plasticity; Leaf Area; Stomatal Area; Stomatal Density; Pore Area and Guard Cell Width

1. Introduction
Atmospheric carbon dioxide [CO2] concentration has increased from pre-industrial level of 280 ppm to more than 390 ppm and is predicted to increase almost two-folds, reaching 730 ppm by the end of 2100 [1] [2] . As consequence, the rise in [CO2] together with other greenhouse gases could increase the average global temperature by 0.6˚C - 4.0˚C resulting uncertainty in both magnitude and degree of precipitation [1] [3] . Moreover, elevated [CO2], together with rising temperatures, may increase the rate and depth of evaporation, resulting soil water reduction [4] [5] . Atmospheric [CO2] and soil water availability are key resources for plant growth, structure and function. Hence, it is essential to understand the effects of predicted [CO2] and reduced soil water levels on plant structure, such as morphology and anatomy [6] .
The effects of elevated [CO2] and soil water levels on plants have been reported in numerous studies [7] -[9] . These studies suggest that in elevated [CO2] and soil water levels, plants modify their leaf morphology and anatomy, often referred to as plasticity, which enables them to thrive well under environmental stress [10] . Many studies suggest that elevated [CO2] enhances leaf size [11] [12] and decreases specific leaf area (SLA) [13] . But, under drought conditions, leaf area decreases whereas petiole area, foliar tissue density and stomatal pore area increase, acting as mechanical support to promote leaf cooling [14] , resistance to physical damage by desiccation [15] , and inducing efficient water use and lower evapo-transpiration [16] -[18] .
While there is no doubt that elevated [CO2] and drought have affected leaf area, stomatal area and stomatal density, there is no general consensus among studies concerning the increase or decrease in these leaf characteristics. For example, in response to the main effects of elevated [CO2] or decrease in soil water, some studies report increases in leaf area [19] [20] , stomatal area [21] and stomatal density; whereas others report decreases in leaf area [13] [22] , stomatal area [23] [24] and stomatal density [25] . But, relatively few studies have addressed the consequences of elevated [CO2] and low soil water levels on leaf area, SLA, stomatal area and stomatal density [23] [26] . Moreover, most studies that focused on the responses of leaf morphological and anatomical characteristics to environmental stress are at multispecies-specific and focused either of these leaf characteristics [21] [23] [27] Thus, integrating both leaf anatomy and morphology of species in elevated [CO2] and low soil water level are required to understand the effect of environmental stresses at intraspecific level.
Studies on pioneer species, including paper birch (Betula papyrifera Marsh) that inhabits a wide climatic gradient, have shown remarkable leaf morphological and anatomical variations [28] -[31] . Paper birch adapts to a wide range of climatic regimes in North America and the species is significantly gaining ecological and economic importance [32] . However, less is known about how such widely distributed species respond to an environmental stress, such as elevated [CO2] and low soil water level, with respect to variations in leaf characteristics.
In this study, by combining [CO2] and soil water levels (hereafter water levels) with morphological and anatomical information, we aimed at identifying the influences of these climatic variables and their interactions on the paper birch. The major objectives of this study are to examine the individual effects of elevated [CO2] and water levels, as well as their interaction, on the leaf characteristics and to explore the ability of different birch populations to adapt to predicted environmental stress. We hypothesized that: 1) interaction and main effects of [CO2], water levels and different populations would result in significant leaf morphological and anatomical variations among the paper birch population; 2) interaction or main effect of elevated [CO2] and high water level would increase leaf area and leaf dry mass, but decrease SLA, petiole area and foliar tissue density; 3) the interaction or main effect of elevated [CO2] and low water level would decrease stomatal density, stomatal area, pore area and guard cell width; and 4) significant correlations exit among stomatal density, stomatal area, leaf area, foliar tissue density, petiole area and SLA.
2. Material and Methods
2.1. Plant Material
Seeds were collected from paper birch populations originating from four different habitats across Canada: Wayerton (47.22N, 65.93W), Skimikin (50.43N, 120.25W), Cussion Lake (52.53N, 122.24W) and Little Oliver Lake (54.48N, 128.16W). Annual precipitation of the population origins ranged from 279 - 1322 mm, and growing season precipitation ranged from 29.55 - 90.90 mm.
Seeds were initially germinated in petri dishes for 15 days in greenhouses at Lakehead University, Thunder Bay. Seeds were germinated in the same greenhouses in which the experiment was conducted to ensure that seedlings were growing in their appropriate experimental conditions from the moment of emergence. The seedlings were transferred and grown in a 2:1 (v/v) mixture of peat moss and vermiculite. A total of 80 seedlings (5 seedlings per population, per treatment combination) were grown into plastic containers of 21 - 25 cm (upper circle) diameter. The experiment was conducted for 12 weeks (February to April, 2012).
The experiment followed a split-split plot design, with atmospheric [CO2] (ambient = 360 ppm; elevated = 720 ppm) as the whole plots and two water levels (well-watered and water-stressed) as sub-plots on four paper birch populations as sub-sub plots. The [CO2] was achieved using Argus [CO2] generators and monitored by an Argus control system (Argus, Vancouver, Canada). Water levels (soil) were nested under [CO2] levels and controlled experimentally by varying the frequency and quantity of watering [4] [33] [34] . In well-watered treatment, seedlings were watered every three days and all containers freely drained. Whereas in water-stressed treatment, seedling were watered every four days, with limited water in order to eliminate free draining of containers. Seedlings were fertilized once a week with 20-20-20 NPK water soluble fertilizer which was scheduled on watering days.
During the entire experiment, air temperature in the greenhouses were maintained at 20˚C - 26˚C during the day and at 15˚C - 19˚C overnight. The relative humidity was 50% ± 5% for the entire experiment period. The supplemental light system was programmed between 5:00 hours and 21:00 hours on a cloudy day, defined as when light levels fell below 200 µmol·m−2·s−1.
2.2. Sampling and Data Collection
In May 2012, five well developed leaves from each seedling were randomly harvested from each treatment for leaf morphological and anatomical measurements. The samples collected were weighted for fresh mass and stored immediately in sealed plastic bags and kept at 4˚C in the dark for 24 hours. Leaf morphological data, such as leaf area (LA), perimeter (P), blade length (BL), petiole length (PL), petiole area (PA), maximum width (MW), position of maximum width (PMW), horizontal width (HW) and aspect ratio (AR) were measured using Win Folia software (Regent Instrument Inc. Quebec, Canada). Stomatal data were collected by obtaining stomatal impressions from the middle section of the leaves using clear nail varnish [35] . While collecting stomatal impressions leaf veins were avoided as much as possible. We used electronic microscope and Motic Images Plus 2.0 software (Motic Instruments Inc., Richmond, Canada) to obtain photos of stomata. Stomatal density (number of stomata per 0.1 mm2 i.e., 100,000 µm2), length, width, pore size and guard cell width per leaf [36] were estimated on the JPEG (Joint Photographic Experts Group) image, acquired through the Motic Images Plus. After morphological and anatomical measurements, the sample leaves were oven-dried in paper bags at 70˚C for at least 2 days and dry masses were weighted. The equations used for leaf characteristics [35] -[38] are listed in Table 1.
2.3. Data Analysis
Assumptions of normality and homogeneity were checked for all leaf characteristics with Shapiro-Wilk’s Test and Levene test, respectively. Split-split plot analysis of variance (ANOVA) was used to analyze differences in leaf characteristics of the populations. [CO2] concentration, water levels, populations and their interactions were treated as independent variables of experiment and considered significant at p ≤ 0.05. Tukey’s honest significant difference test was used for pair-wise means comparison when ANOVA results were significant for any given characteristics. Pearson correlation was used to analyze the correlation within and between leaf morphological and anatomical characteristics. All statistical analysis was conducted using IBM SPSS Statistics-21 (SPSS, Chicago, IL, USA) and R- 2.12.1 (R Development Core team, 2011).
3. Results
3.1. Leaf Morphology
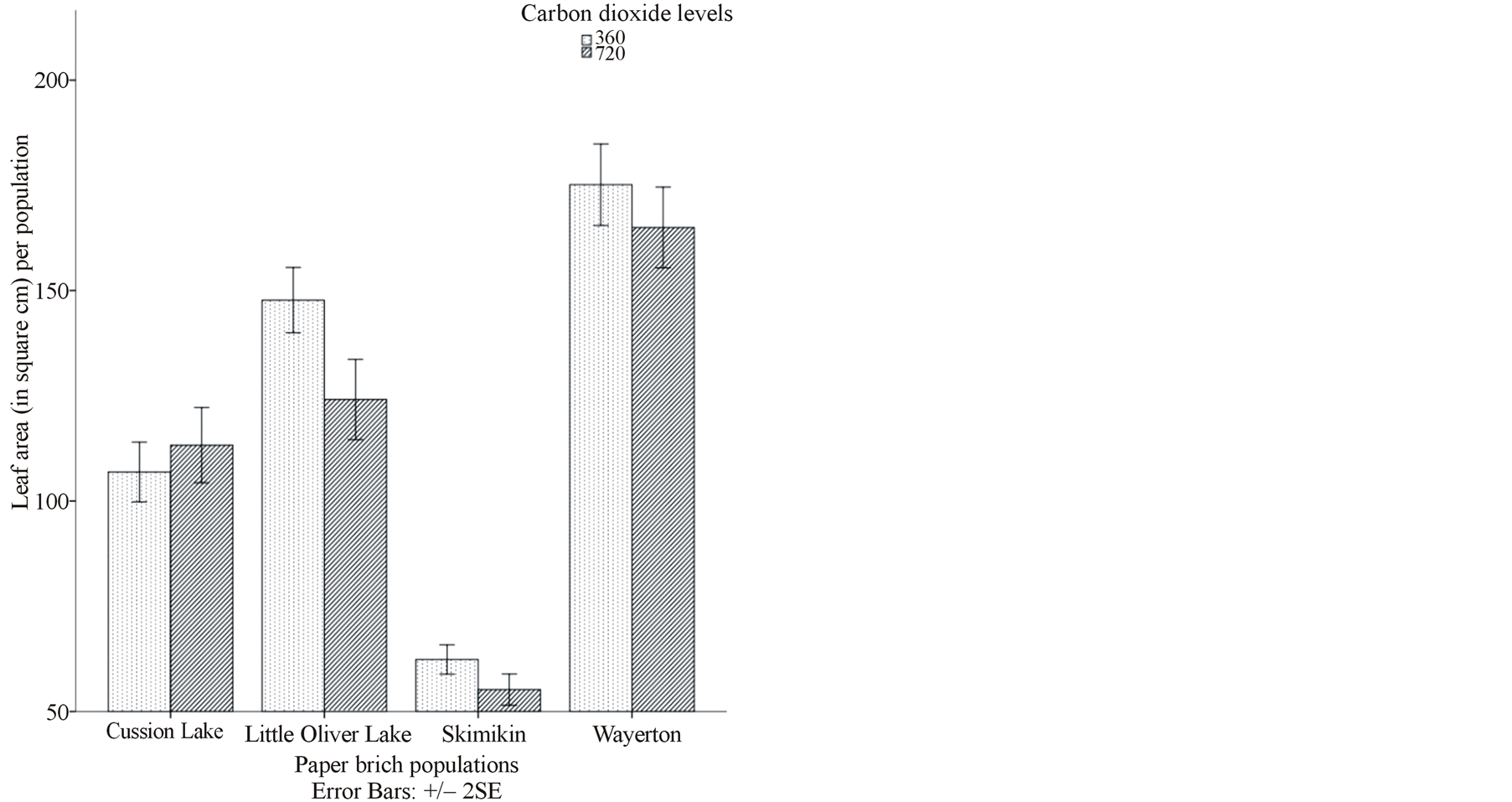
Leaf area was significantly affected by the interaction and also main effects of [CO2], water levels and populations (Table 2, Figure 1(a)). Under both ambient and elevated [CO2], and at both water levels, populations from Cussion Lake, Little Oliver Lake, Skimikin and Wayerton significantly differed from each other (Figure 1(a)). Wayerton had significantly larger average leaf area in both [CO2] and at both water levels while Skimikin had significantly smaller leaf area (Figure 1(a)). Under elevated [CO2] and lower water treatment, Little Oliver had
Table 1. Equations used for leaf morphological and anatomical characteristics (Chrs.) of paper birch populations.
Here, PEI—petiole intensity, PL—petiole length (cm), LL—leaf length (cm), S:leaf succulence (gH2Ocm−2), LFM—leaf fresh mass (gm), LDM—leaf dry mass (gm), LA—leaf area (cm2), SLA—specific leaf area (cm−2·gm−1), FTD—foliar tissue density, SA—stomatal area (µm2), SL—stomatal length (µm), SW—stomatal width (µm), SD—stomatal density, ED—epidermal cell density, PA—stomatal pore area (µm2), PL—pore length (µm), PW—pore width (µm), SI—stomatal intensity, SHC— stomatal shape coefficient.
Table 2. Analysis of variance (ANOVA) with p-values for the main and interaction effects of carbon dioxide levels [CO2], soil water levels (W) and populations (P) on leaf morphology and anatomy (Chrs.). DF denotes degrees of freedom.
Here, LA—leaf area (cm2), AR—aspect ratio, PEA—petiole area (cm2), PEI— petiole intensity, SLA—specific leaf area (cm2·g−1), S—succulence (gH2Ocm−2), FTD—foliar tissue density, SD—stomatal density, SA—stomata area (µm2), PA—pore area (µm2), GCW—guard cell width (µm), SHC—stomatal shape coefficient.
significantly smaller leaf area (98.52 cm2) per population that differed among Little Oliver population treated under both elevated and ambient [CO2] at different water levels (Figure 1(a)). Interactions between elevated [CO2] and high water level and ambient [CO2] and low soil moisture level resulted in larger (115.54 cm2) and smaller (103.94 cm2) leaf areas (average per population), respectively, in Cussion Lake population that differed significantly from Little Oliver, Skimikin and Wayerton populations. However, there were no significant changes in SLA, petiole area and aspect ratio due to interaction effects of [CO2], water levels and populations (Table 2).
Interactions of elevated [CO2] and low water level generally had the smallest average leaf area (108.96 cm2), petiole area (4.20 cm2), aspect ratio (0.64) and petiole intensity (30.76) in the birch populations (Table 3, Figures 1(a) and (b)). Average leaf dry mass under elevated [CO2] and high water level was significantly larger (2.89 gm) compared to that produced in the interaction of ambient [CO2] and water levels (Tables 2 and 3). There was no significant effect of the interaction between [CO2] and water levels on average SLA (Table 2).
The interaction effect of water levels and populations showed that an increase in water level had significantly increased average leaf area per population in Cussion Lake (112.72 cm2) and Little Oliver (148.23 cm2) whereas the leaf area decreased in Skimikin (57.61 cm2) and Wayerton (163.31 cm2) (Table 3). Under high water level, petiole area per population significantly decreased in Cussion Lake (4.52 cm2) and Wayerton (6.51 cm2) whereas significantly increased in Little Oliver (7.13 cm2). Although there were no significant main effects of water levels and populations or an interaction effects of [CO2], water levels and populations on SLA, foliar tissue density and leaf dry mass, a significant effect of [CO2] levels were resulted on these characteristics (Table 2).
Paper birch populations under elevated [CO2] had a significant reduction in leaf area (114.39 cm2), width (6.60 cm), blade length (14.89 cm), aspect ratio (0.70), petiole area (5.07 cm2), and SLA (45.46 cm2·g−1) compared with ambient [CO2] (Table 3). Nevertheless, leaf dry weight (2.61 gm) and foliar tissue density (0.30) increased significantly under elevated [CO2] (Table 3). Apart from petiole intensity, SLA and foliar tissue density, majority of leaf morphological characteristics significantly differed between the populations. For illustration, Skimikin had significantly smaller leaf area (58.78 cm2), petiole area (3.23 cm2), dry mass (1.20 gm) and succulence (0.05 gH2O cm2) whereas these leaf characteristics were significantly larger in Wayerton (leaf area—170 cm2, petiole area—7.57 cm2, fresh mass—13.38 gm, dry mass—3.36 gm and succulence—0.06 gH2O cm2 respectively) (Table 3). There was no significant effect of water levels on leaf morphology except for leaf blade length (Table 2). Thus, either [CO2] or populations had a significant effect on leaf morphological characteristics.
3.2. Leaf Anatomy
There was significant interaction effect of [CO2], water and population on stomatal characteristics such as stomatal density, area, pore area, guard cell width, stomatal intensity and stomatal shape coefficient (Table 2).
Table 3. Mean values of leaf characteristics for each paper birch populations-P treated under two carbon dioxide concentrations-[CO2] (360 and 720 ppm) and two soil water level-W ( lowwater stress and high-well watered).
Here, LA—leaf area (cm2), PEA—petiole area (cm2), SLA—specific leaf area (cm2·g−1), LDM—leaf dry mass (gm), SD—stomatal density, SA—stomata area (µm2), PA—pore area (µm2) and GCW—guard cell width (µm), SHC—stomatal shape coefficient.
Skimikin population treated under elevated [CO2] and high water level had significantly more stomatal density (20.32) and smaller stomatal area (286.81 µm2) per population compared to other paper birch populations treated under both [CO2] and at both water levels (Figures 1(c) and (d)). At the same time, Cussion lake seedlings under ambient [CO2] and high water level had significantly low stomatal density per population that differed significantly from Cussion Lake seedlings treated under elevated [CO2] and low water level (Table 3). Under both ambient and elevated [CO2], high water treatment significantly increased average stomatal area, pore area and guard cell width in compare to low water treatment in the birch populations (Table 3, Figures 1(d) and (e)). For example, under high water treatments at both ambient and elevated [CO2] mean stomatal areas in Cussion Lake were 562.75 µm2 and 656.58 µm2, Little Oliver were 639.69 µm2 and 539.04 µm2, and Wayerton were 510.52 µm2 and 537.06 µm2 respectively (Table 3). Whereas, under low water treatment at both ambient and elevated [CO2] mean stomatal areas per population in Cussion Lake were 516.91 µm2 and 500.95 µm2, , Little Oliver were 503.73 µm2 and 492.41 µm2, and Wayerton were 417.54 µm2 and 530.27 µm2, respectively (Table 3). Under elevated [CO2], high water treatment had significantly decreased mean stomatal area per population in Skimikin (286.81 µm2) than at low water level, while mean pore area (103.39 µm2) and mean guard cell width (4.38 µm) were comparatively larger. Little Oliver showed significant decrease in average stomatal pore area at decreased water levels under both ambient and elevated [CO2] which differed within the birch populations (Table 3). Population from Skimikin had signify cantly rounded stomata (i.e., larger stomatal shape coefficient) under low water treatments at both ambient and elevated [CO2] with mean value 71.49% and 65.60% respectively, despite the fact that more rounded stomata were observed in Wayerton (69.10%), and Cussion Lake (69.24%) for the interactions between high water levels to ambient and elevated [CO2] respectively (Table 3).
Under both ambient and elevated [CO2], high water treatment had significantly larger mean stomatal area (529.32 µm2 and 504.87 µm2 respectively) (Figure 1(d)) and guard cell width (5.87 µm2 and 6.18 µm2 respectively) (Figure 1(e)). Similarly, interaction between elevated [CO2] and different paper birch populations showed significant stomatal density variations (Table 2) where, Skimikin had higher and Little Oliver had less stomatal density (Table 3, Figure 1(c)). Average stomatal area per population was significantly larger in Cussion Lake (578.77 µm2) and smaller in Skimikin (289.16 µm2) when treated under elevated [CO2] (Table 3, Figure 1(d)). Paper birch populations treated under low water level showed significant decrease in mean stomatal area (Figure 1(d)), pore area, guard cell width and shape coefficient (except for Skimikin) when compared to well watered seedlings (Table 3). Under low water treatment, seedlings from Cussion Lake had increased average stomatal density unlike in Skimikin and Wayerton (Table 3).
Water treatment in paper birch populations had significant effect on average stomatal area, pore area, guard cell width and shape coefficient (p < 0.001) (Table 2). Under increased water treatment, paper birch populations showed significant increases in stomatal area (517.10 µm2), pore area (172.05 µm2), guard cell width (6.03 µm) and shape coefficient (66.44%) (Figure 1(f)). There was significant main effect of paper birch populations on all stomatal characteristics measured (p < 0.001) (Table 2). Seedlings from Skimikin had significantly higher stomatal density (16.38) and shape coefficient (67.56%) but lower stomatal area (310.94 µm2), pore area (113.23 µm2) and guard cell width (4.36 µm) (Table 3). The lowest stomatal density (11.43) and wider guard cell (6.28 µm) were noticed in Little Oliver population. The largest stomatal and pore area were noticed in Cussion Lake with mean values 559.30 µm2 and 190.30 µm2, respectively. Under elevated [CO2], average stomatal density (14.05) and guard cell width (5.92 µm) significantly increased whereas, pore area (150.72 µm2) and shape coefficient (64.98%) (Figure 1(f)) significantly decreased under ambient [CO2] (Table 3). However, no significant difference was observed in average stomatal area between ambient and elevated [CO2] levels. Thus, the majority of stomatal characteristics are altered by water and population levels.
3.3. Correlation between Leaf Morphological and Stomatal Characteristics
Leaf with larger stomatal area had lower stomatal density (r = −0.49, p < 0.001) (Table 4). Within leaf morphological characteristics larger leaf area had significantly larger petiole area (r = 0.56, p < 0.001), and wider aspect ratio (r = 0.30, p < 0.001) (Table 4); while none of these leaf characteristics were correlated with SLA (Table 4). Comparing leaf morphological and stomatal characteristics, the results showed larger leaf area had significantly larger stomatal area, pore area and guard cell width (Table 4) but had low stomatal density (Figure 2). However, stomatal density, stomatal area, pore area and guard cell width were not significantly correlated with SLA and aspect ratio (Table 4).
4. Discussion
Leaf morphological and anatomical characteristics are sensitive to environmental changes such as rising [CO2] and reduced water availability for plants [9] [24] [39] . Supporting our hypothesis, the result showed that leaf area and stomatal characteristics differed as a result of an interactive effect of [CO2], water levels and paper birch populations. However, the interaction had no significant effect on leaf morphological characteristics such as shape (aspect ratio), petiole area, SLA, foliar tissue density, succulent and leaf dry mass. This indicated that stomatal characteristics are more sensitive to water stress in compare to [CO2] levels. For different species, both an increase and a decrease in leaf area have been reported as an effect of elevated [CO2] [9] [13] [19] [20] [22] . Partly rejecting our second hypothesis, this study showed reduction in leaf area of paper birch populations from Little Oliver, Skimikin and Wayerton either under elevated [CO2] or the interaction between elevated [CO2] and water levels. Comparable to the present study, leaf area were reduced in elevated [CO2] in sweet chestnuts,
 (a)
(a)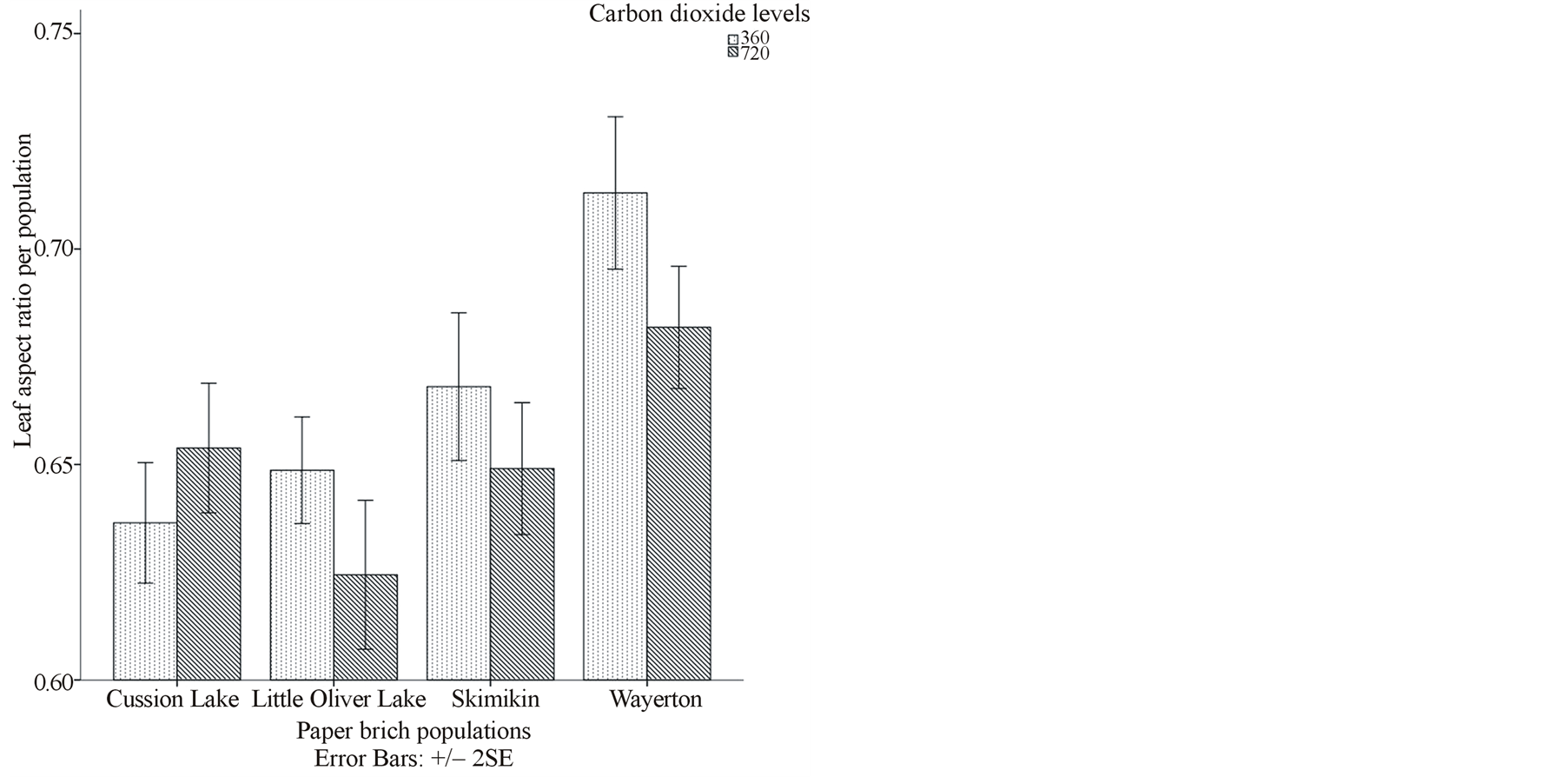 (b)
(b)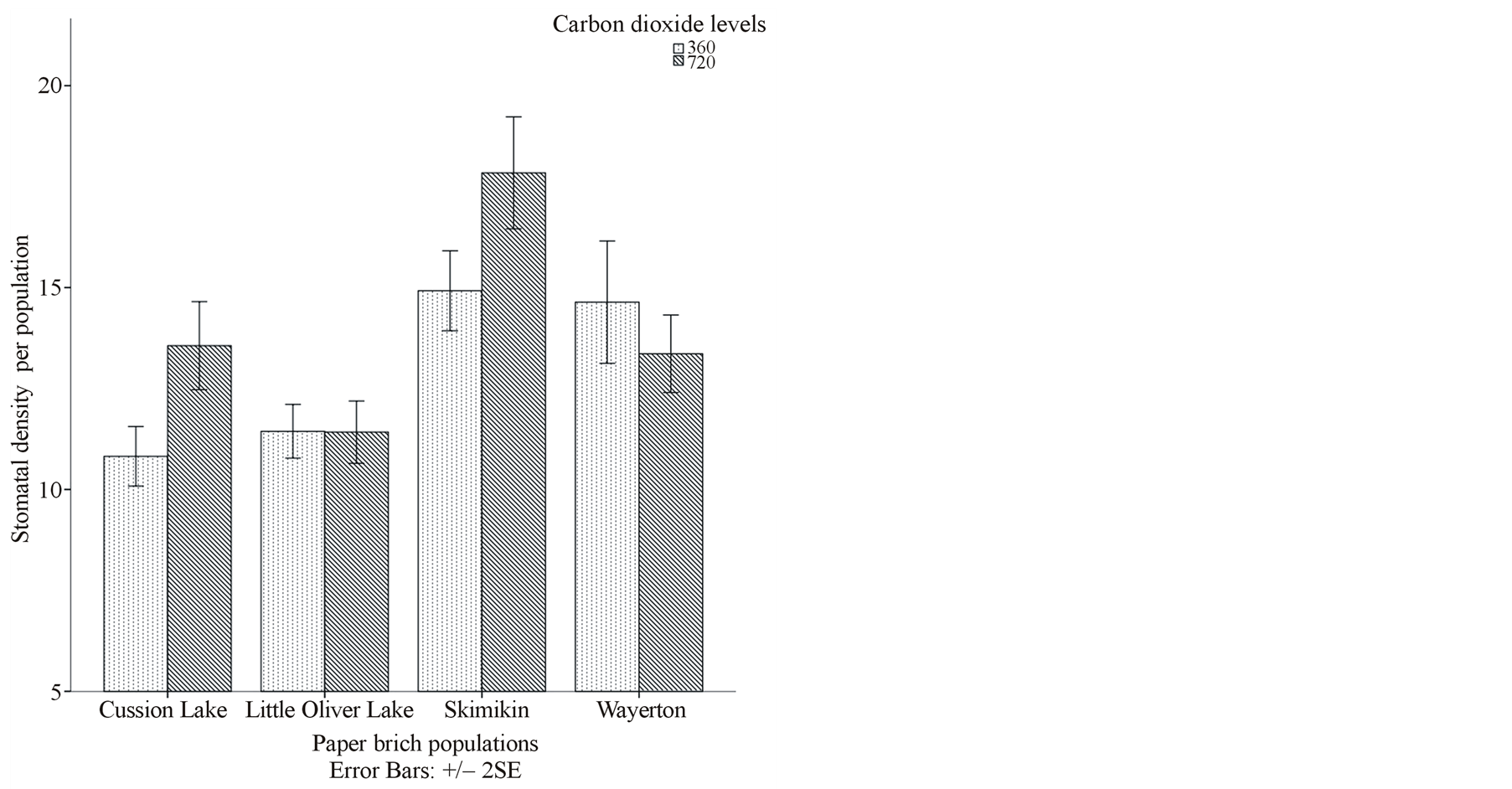 (c)
(c)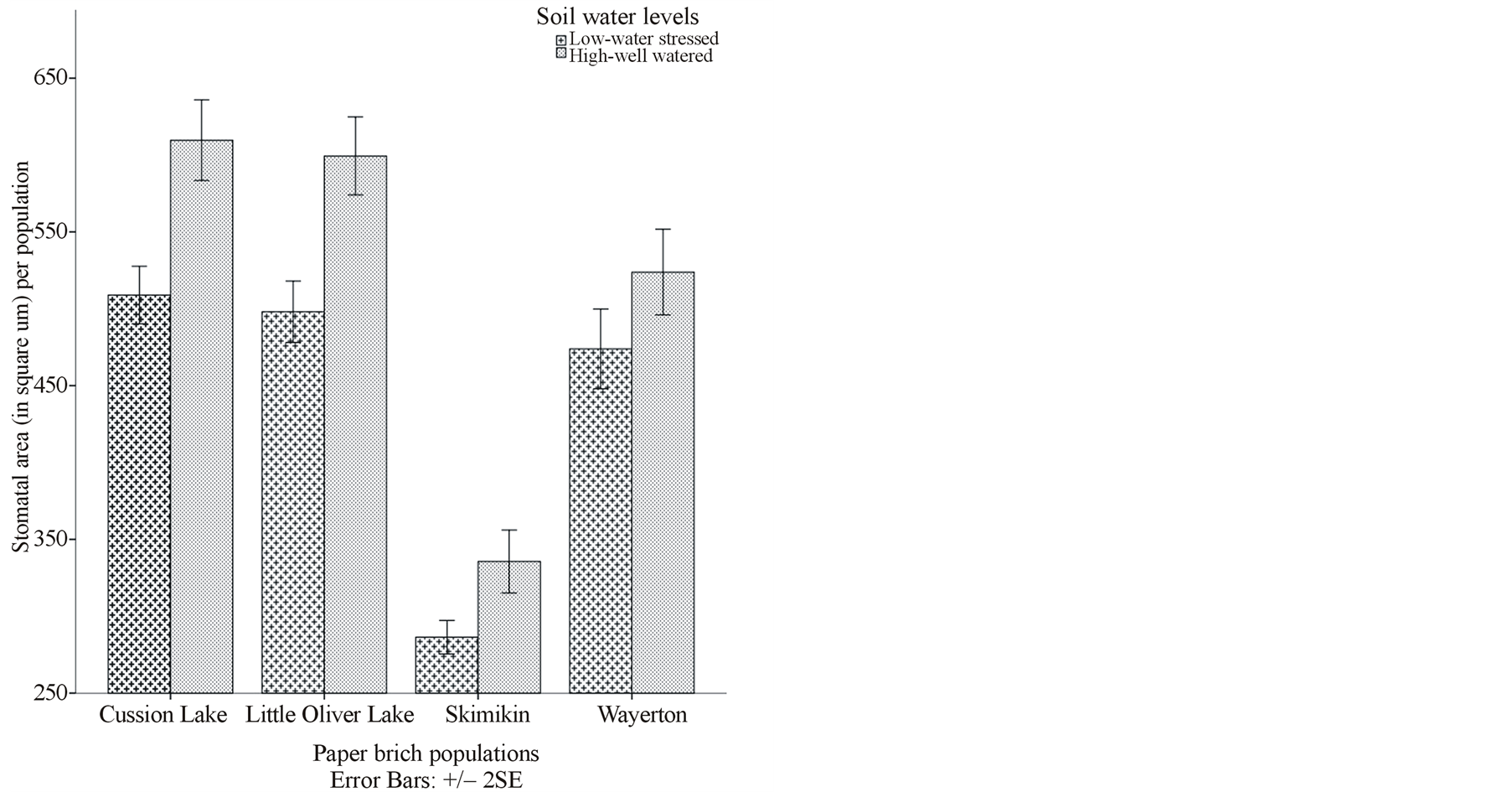 (d)
(d)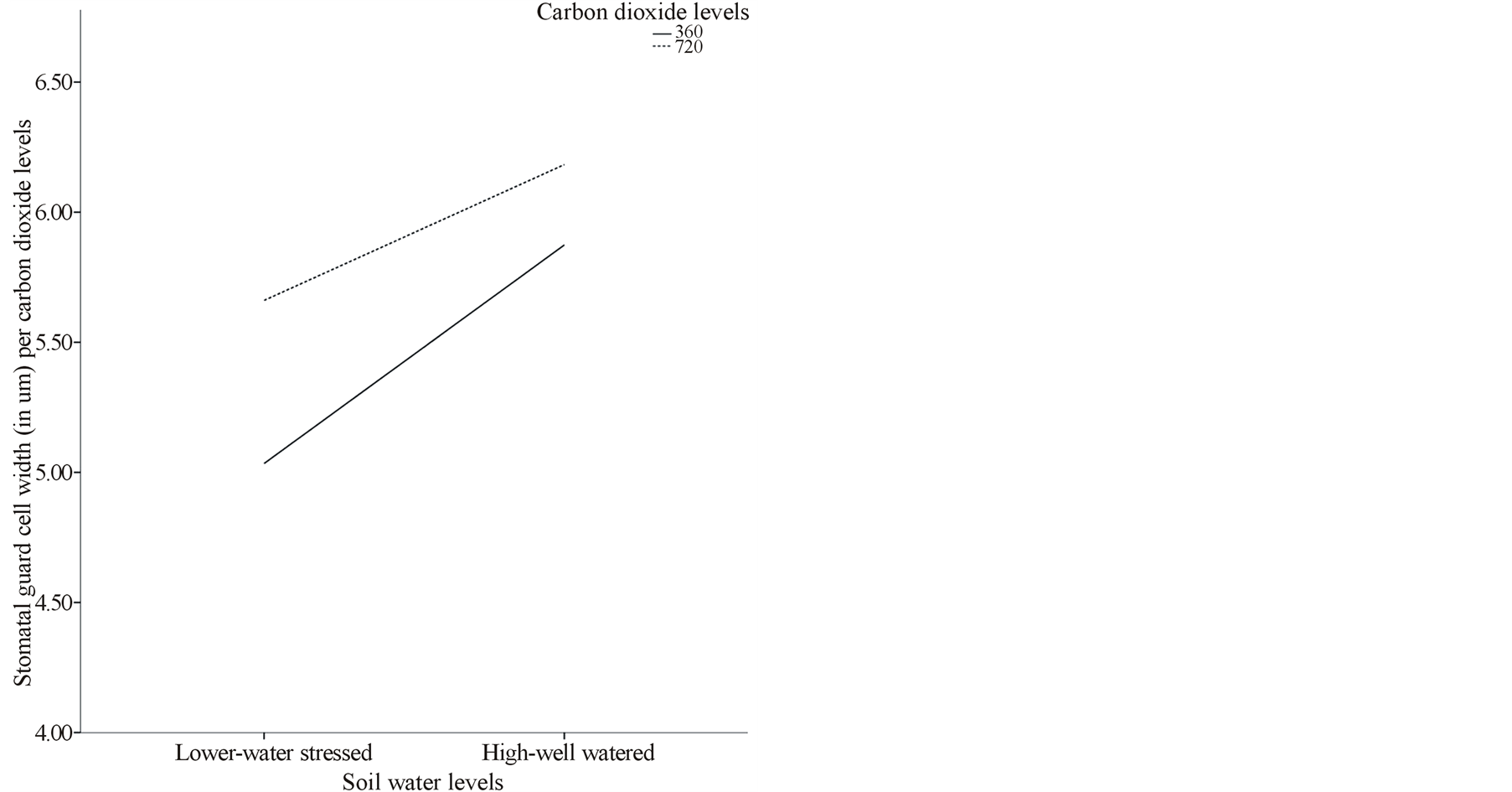 (e)
(e)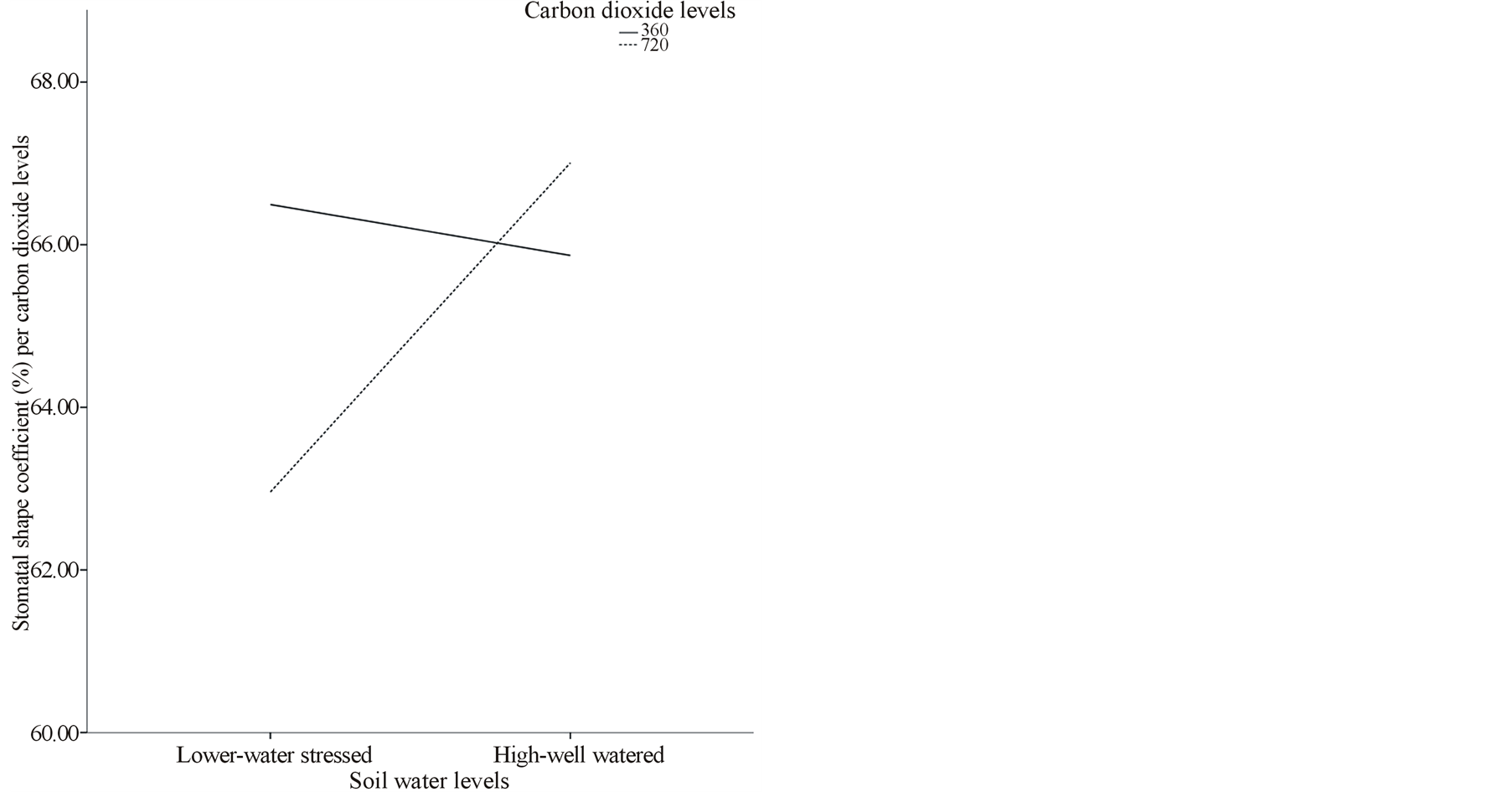 (f)
(f)
Figure 1. (a), (b) and (c) Effects of carbon dioxide [CO2] levels (360 ppm and 720 ppm) and paper birch populations on average leaf area, aspect ratio (ratio of leaf width to leaf length) and stomatal density respectively. (d) Effects of soil water levels (high and low) and paper birch populations on stomatal area (in µm2) per population. The stomatal area is average value per population. (e) Effects of carbon dioxide [CO2] levels (360 ppm and 720 ppm) and soil water levels (high-well watered and low-water stressed) on stomatal guard cell width in µm. The guard cell width is average value per [CO2] levels. (f) Effects of carbon dioxide [CO2] levels (360 ppm and 720 ppm) and soil water levels (high-well watered and low-water stressed) on stomatal shape coefficient in percentage. The shape coefficient is average value per [CO2] across populations.
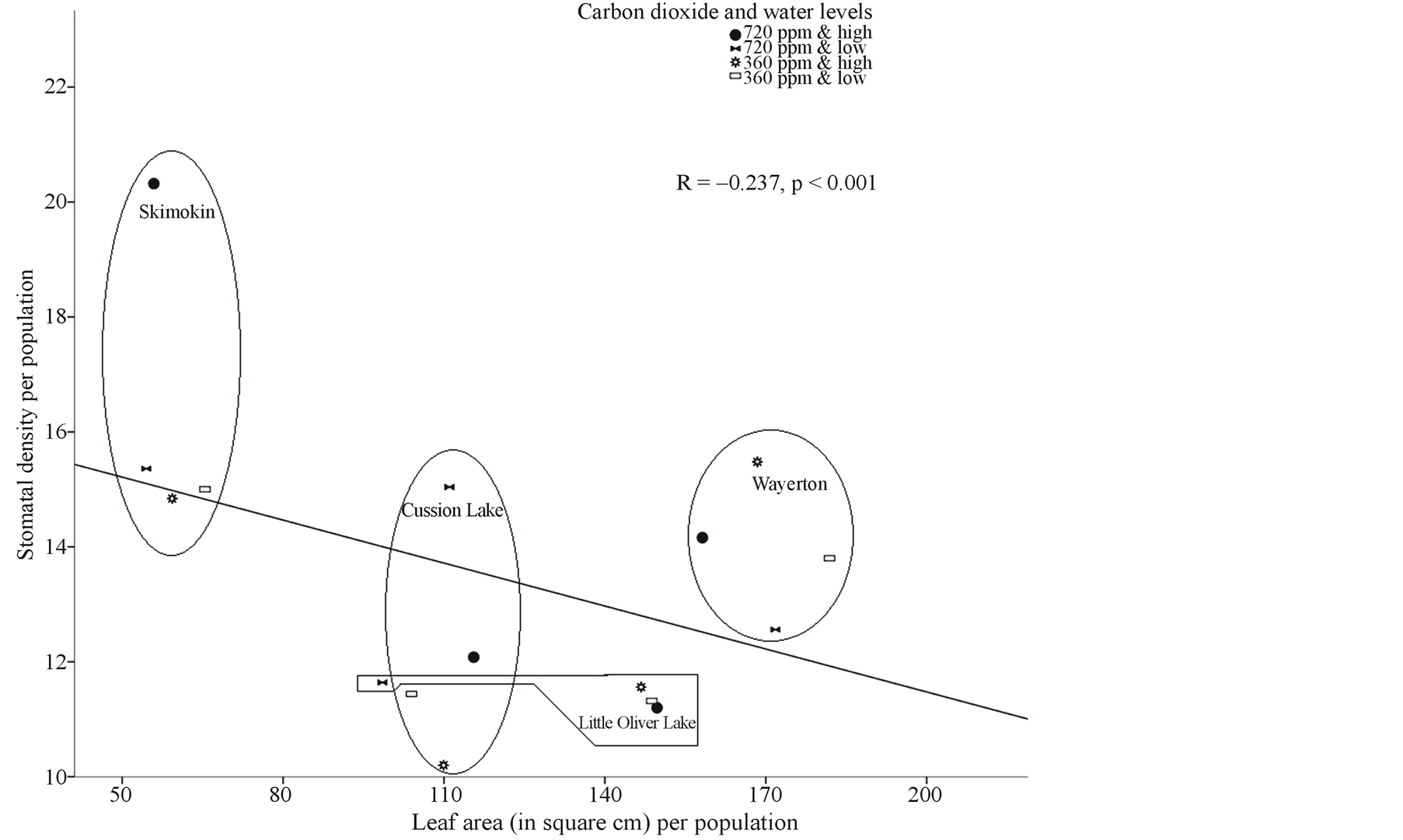
Figure 2. Correlation between average leaf area (in cm2) and average stomatal density (number of stomata per unit square area) of paper birch populations treated under carbon dioxide [CO2] (720 ppm and 360 ppm) and soil water levels (high-well watered and low-water stressed).
Table 4. Pearson correlation between leaf morphological and stomatal characteristics (Chrs.) of paper birch populations. The leaf characteristics used in Pearson correlation are an average per seedling per population (N = 80). Values are correlation coefficient (r) with p values.
Here, *, **, ** is significant at p < 0.05, p < 0.01, p < 0.001 respectively and ns is not significant. SD— stomatal density, SA—stomata area (µm2), PA—pore area (µm2), GCW—guard cell width (µm), LA—leaf area (cm2), AR—aspect ratio, PEA—petiole area (cm2), SLA— specific leaf area (cm2·g−1) and S—succulence (g H2O cm2).
yellow poplar seedlings, and silver birch [13] [22] [23] [40] . These species reported much larger effect of elevated [CO2] on root growth rather than shoot or leaf growth which might be true in the case of paper birch seedling. Cussion Lake population had larger average leaf area in elevated [CO2] irrespective of water levels, similar to a study on Phaseolus vulgaris which also found increased leaf area by CO2 enrichment [9] [41] . However, leaf area decreased in Little Oliver population treated under elevated [CO2] and low soil moisture. And this result is consistent to a study on P. interamericana, P. euramercana and P. trichocarpa [42] which reported smaller but more leaves per seedling under elevated [CO2]. Although number of leaves and leaf, shoot and root biomass per seedling are not reported in this paper, there was a trade-off between leaf area and number of leaf per seedling. For example, Skimikin and Cussion Lake comparatively had more branches, and small and numerous leaves per seedling whereas Wayerton and Little Oliver had less branch, and larger and fewer leaves per seedling. Thus, we concluded that increase or decrease in leaf area in paper birch is not only related to [CO2] but also population differences, as is the case of Populus genotypes [43] and a trade -off between leaf morphological characteristics as in the cases of P. interamericana, P. euramercana and P. trichocarpa [40] . And these morphological characteristics together with stomatal characteristics must have strongly influenced water use efficiency in the species [44] [45] . The present study has confirmed that stomatal density in paper birch varied according to main effects of [CO2] levels and population difference, and interaction of [CO2], water levels and populations, but not due to water manipulations alone. Previously, studies had reported that stomatal characteristics are affected by [CO2] [24] [46] [47] , water levels [48] and population differences [49] . Differing from our hypothesis, stomatal density was significantly higher in elevated [CO2] and higher water level than under ambient [CO2] and at both water levels in the birch populations. Furthermore, stomatal density significantly differed within Cussion Lake and Skimikin under [CO2] treatments. The birch populations responded differently to the treatments and the results are consistent with previous studies of stomatal responses to [CO2] where individual, population or species responded differently from large reduction, no change, to large increase in stomatal density under elevated [CO2] [8] [24] [44] [50] [51] . Studies suggest that the density is not only relatively plastic and can potentially modify to environmental changes [52] -[54] but also genotypically differentiated [55] .
As expected, the interaction of [CO2], water levels and population differences further demonstrated effect on stomatal area, pore area and guard cell width. More importantly, supporting our hypothesis, elevated [CO2] with limited water level had reduced stomatal area, pore area and guard cell width. This observation is in agreement with the conclusion that elevated [CO2] and water stress reduce stomatal area in Arabidopsis [56] , Populus trichocarpa [57] , and Pistacia atlantica [58] , respectively. Consistent to paper birch, a study on Arabidopsis reported reduction in stomatal area (including pore area and guard cell width) under reduced water availability and explained that small stomata would support maximal stomatal conductance [56] . Thus, it has been suggested that smaller stomata and guard cells increase carbon dioxide diffusion per unit area of stomata and reduce water loss compared to larger stomata and guard cells [16] . Our result is not consistent with a study on paper birch populations from water deficit sites that had larger and fewer stomata per unit area [21] . Although the birch populations in this study increased stomatal area under low water levels, stomatal size per unit leaf area remained relatively same due to a decrease in stomatal density. The trade-off between stomatal size and density; that is, either larger stomata with low density or smaller stomata with high density, revealed by the strong correlations in our study, is consistent with other studies [8] [59] . Alternatively, previous studies suggested that a leaf with high stomatal density and smaller stomata can reduce stomatal conductance and increase water-use efficiency [60] which might be the case in this study.
Under environmental stress such as elevated [CO2] and/or water deficiency, plants modify leaf morphology and anatomy that either diminishes the water loss or increases water use efficiency [61] . Thus, small leaf area with less stomatal density would alter water use efficiency for a species. Supporting our hypothesis, the result showed significant correlations within and between leaf anatomical and morphological characteristics. All these features provided a structural basis in reducing water loss through leaves and increase water use efficiency. Therefore, the plasticity of leaf area and stomatal characteristics played a major role in the survival of paper birch under environmental stress.
In conclusion, the results of this study confirmed the significant effects of elevated [CO2] on paper birch treated at low water level. This finding helps to understand how the birch would change its leaf structures under future elevated [CO2] and altered precipitation patterns.
Acknowledgements
The research was founded by NSERC discovery grant to JW. We appreciate J. Lee for providing logistic support at the greenhouse. We acknowledge Profs. Leitch and Hutchion for laboratories support; and Dr. Danyagri, Bartels and anonymous reviewer for their comments.
References
- Intergovernmental Panel on Climate Change-IPCC (2007) Climate Change 2007: The Physical Science Basis. In: Solomon S., Qin D. and Manning M., Eds., Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge.
- Sitch, S., Huntingford, C., Gedney, N., Levy, P. E., Lomas, M., Piao, S.L., Betts, R., Ciais, P., Cox P., Friedlingstein, P., Jones, C.D., Prentice, I.C. and Woodward, F.I. (2008) Evaluation of the Terrestrial Carbon Cycle, Future Plant Geography and Climate-Carbon Cycle Feedbacks Using Five Dynamic Global Vegetation Models (DGVMs). Global Change Biology, 14, 2015-2039.
- Houghton, J.T., Ding, Y., Griggs, D.J., Noguer, M., van der Linden, P.J., Dai, X., Maskell, K. and Johnson, C.A. (2001) Climate Change 2001: The Scientific Basis. Cambridge University Press, Cambridge.
- Catovsky, S. and Bazzaz, F.A. (1999) Elevated CO2 Influences the Responses of Two Birch Species to Soil Moisture: Implications for Forest Community Structure. Global Change Biology, 5, 507-518. Doi/10.1046/J.1365-2486.1999.00247.X
- Volk, M., Niklaus, P.A. and Korner, C. (2000) Soil Moisture Effects Determine CO2 Responses of Grassland Species. Oecologia, 125, 380-388.
- Korner, C. (2003) Ecological Impacts of Atmospheric CO2 Enrichment on Terrestrial Ecosystems. Philosophical Transactions of the Royal Society, 361, 2023-2041. http://dx.doi.org/10.1098/rsta.2003.1241
- Ferris, R., Sabatti, M., Miglietta, F., Mills, R.F. and Taylor, G. (2001) Leaf Area is Stimulated in Populus by Free Air CO2 Enrichment (POPFACE), through Increased Cell Expansion and Production. Plant, Cell & Environment, 24, 305- 315. http://dx.doi.org/10.1046/j.1365-3040.2001.00684.x
- Hetherington, A.M. and Woodward, F.I. (2003) The Role of Stomata in Sensing and Driving Environmental Change. Nature, 424, 901-908. http://dx.doi.org/10.1038/nature01843
- Pritchard, S.G., Rogers, H.H., Prior, S.A. and Peterson, C.M. (1999) Elevated CO2 and Plant Structure: A Review. Global Change Biology, 5, 807-837. doi/10.1046/j.1365-2486.1999.00268.x
- McLellan, T. (2000) Geographic Variation and Plasticity of Leaf Shape and Size in Begonia dregei and B. homonyma (Begoniaceae). Botanical Journal of the Linnean Society, 132, 79-95. http://dx.doi.org/10.1111/j.1095-8339.2000.tb01855.x
- Heath, J. and Kerstiens, G. (1997) Effects of Elevated CO2 on Leaf Gas Exchange in Beech and Oak at Two Levels of Nutrient Supply: Consequences for Sensitivity to Drought in Beech. Plant, Cell & Environment, 20, 57-67.
- Kerstiens, G., Townend, J., Heath, J. and Mansfield, T.A. (1995) Effects of Water and Nutrient Availability on Physiological Responses of Woody Species to Elevated CO2. Forestry, 68, 303-315. http://dx.doi.org/10.1093/forestry/68.4.303
- Norby, R.J. and O’Neill, E.G. (1991) Leaf Area Compensation and Nutrient Interactions in CO2-Enriched Seedlings of Yellow-Poplar (Liriodendron tulipifera L.). New Phytologist, 117, 515-28.
- Meng, T.T., Ni, J. and Harrison, S.P. (2009) Plant Morphometric Traits and Climate Gradients in Northern China: A Meta-Analysis Using Quadrat and Flora Data. Annals of Botany, 104, 1217-1229. http://dx.doi.org/10.1093/aob/mcp230
- Mediavilla, S., Escudero, A. and Heilmeier, H. (2001) Internal Leaf Anatomy and Photosynthetic Resource-Use Efficiency: Interspecific and Intraspecific Comparisons. Tree Physiology, 21, 251-259. http://dx.doi.org/10.1093/treephys/21.4.251
- Abrams, M.D. (1999) Adaptations and Responses to Drought in Quercus Species of North America. Tree Physiology, 7, 227-238. http://dx.doi.org/10.1093/treephys/7.1-2-3-4.227
- Bruschi, P., Vendramin, G.G., Bussotti, F. and Grossoni, P. (2000) Morphological and Molecular Differentiation between Quercus petraea (Matt.) Liebl. and Quercus pubescens Wild. (Fagaceae) in Northern and Central Italy. Annals of Botany, 85, 325-333. http://www.ipmd.ir/Papers/707.pdf http://dx.doi.org/10.1006/anbo.1999.1046
- de Lillis, M. (1991) An Ecomorphological Study of the Evergreen Leaf. Braun, Blanquetia.
- Norby, R.J., Wullschleger, S.D., Gunderson, C.A. and Nietch, C.T. (1995) Increased Growth Efficiency of Quercus alba Trees in a CO2-Enriched Atmosphere. New Phytologist, 131, 91-97.
- Sims, D.A., Seemann, J.R. and Luo, Y. (1998) Elevated CO2 Concentration Has Independent Effects on Expansion Rates and Thickness of Soybean Leaves across Light and Nitrogen Gradients. Journal of Experimental Botany, 49, 583-591. http://dx.doi.org/10.1006/anbo.1999.1046
- Li, W.L., Berlyn, G.P. and Ashton, P.M.S. (1996) Polyploids and Their Structural and Physiological Characteristics Relative To Water Deficit in Betula papyrifera (Betulaceae). American Journal of Botany, 83, 15-20. http://dx.doi.org/10.2307/2445949
- Pettersson, R., McDonald, A.J.S. and Stadenberg, I. (1993) Response of Small Birch Plants (Betula pendula Roth.) to Elevated CO2 and Nitrogen Supply. Plant, Cell & Environment, 16, 1115-1121.
- Beerling, D.J., Heath, J., Woodward, F.I. and Mansfield, T.A. (1996) Drought-CO2 Interactions in Trees: Observations and Mechanisms. New Phytologist, 134, 235-242.
- Woodward, F.I. and Kelly, C.K. (1995) The Influence of CO2 Concentration on Stomatal Density. New Phytologist, 131, 311-327.
- Lin, J., Jach, M.E. and Ceulemans, R. (2001) Stomatal Density and Needle Anatomy of Scots Pine (Pinus sylvestris) Are Affected By Elevated CO2. New Phytologist, 150, 665-674.
- Xiao, C.W., Sun, O.J., Zhou, G.S., Zhao, J.Z. and Wu, G. (2005) Interactive Effects of Elevated CO2 and Drought Stress on Leaf Water Potential and Growth in Caragana intermedia. Trees, 19, 712-721. http://dx.doi.org/10.1007/s00468-005-0435-2
- Paoletti, E., Nourrisson, G., Garrec, J.P. and Raschi, A. (1998) Modifications of the Leaf Surface Structures of Quercus ilex L. in Open, Naturally CO2-Enriched Environments. Plant, Cell & Environment, 21, 1071-1075.
- Dancik, B.P. and Barnes, B.V. (1974) Leaf Diversity in Yellow Birch (Betula alleghaniensis). Canadian Journal of Botany, 52, 2407-2414.
- Pyakurel, A. and Wang, J.R. (2013) Leaf Morphological Variation among Paper Birch (Betula papyrifera Marsh.) Genotypes across Canada. Open Journal of Ecology, 3, 284-295. http://dx.doi.org/10.4236/oje.2013.34033
- Senn, J., Hanhimaki, S. and Haukioja, E. (1992) Among-Tree Variation in Leaf Phenology and Morphology and Its Correlation with Insect Performance in the Mountain Birch. Oikos, 63, 215-222. http://www.jstor.org/stable/3545381 http://dx.doi.org/10.2307/3545381
- Sharik, T.L. and Barnes, B.V. (1979) Natural Variation in Morphology among Diverse Populations of Yellow Birch (Betula alleghaniensis) and Sweet Birch (B. lenta). Canadian Journal of Botany, 57, 1932-1939.
- Safford, L., Bjorkbom, J.C. and Zasada, J.C. (1990) Betula papyrifera Marsh. Paper Birch. Forest Services, Washington DC.
- Tschaplinski, T.J. and Norby, R.J. (1991) Physiological Indicators of Nitrogen Response in a Short Rotation Sycamore Plantation.I.CO2 Assimilation, Photosynthetic Pigments and Soluble Carbohydrates. Physiologia Plantarum, 82, 117-126.
- Tschaplinski, T.J., Stewart, D.B., Hanson, P.J. and Norby, R.J. (1995) Interactions between Drought and Elevated CO2 on Growth and Gas Exchange of Seedlings of Three Deciduous Tree Species. New Phytologist, 129, 63-71. http://dx.doi.org/10.1111/j.1469-8137.1995.tb03010.x
- Bacelar, E.A., Correia, C.M., Moutinho-Pereira, J.M., Gonazalves, B.C., Lopes, J.I. and Torres-Pereira, J.M.G. (2004) Sclerophylly and Leaf Anatomical Traits of Five Field-Grown Olive Cultivars Growing under Drought Conditions. Tree Physiology, 24, 233-239. http://dx.doi.org/10.1093/treephys/24.2.233
- Xu, Z. and Zhou, G. (2008) Responses of Leaf Stomatal Density to Water Status and Its Relationship with Photosynthesis in a Grass. Journal of Experimental Botany, 59, 3317-3325. http://dx.doi.org/10.1093/jxb/ern185
- Batos, B., Vilotic, D., Orlovic, S. and Miljkovic, D. (2010) Inter and Intra-Population Variation of Leaf Stomatal Traits of Quercus robur L. in Northern Serbia. Archives of Biological Sciences, Belgrade, 62, 1125-1136. http://dx.doi.org/10.2298/ABS1004125B
- Sagaram, M., Lombardini, L. and Grauke, L.J. (2007) Variation in Leaf Anatomy of Pecan Cultivars from Three Ecogeographic Locations. Journal of American Society of Horticultural Science, 132, 592-596.
- Teklehaimanot, Z., Lanek, J. and Tomlinson, H.F. (1998) Provenance Variation in Morphology and Leaflet Anatomy of Parkia biglobosa and Its Relation to Drought Tolerance. Trees, 13, 96-102. http://dx.doi.org/10.1007/PL00009742
- Mousseau, M. and Enoch, H.Z. (1989) Carbon Dioxide Enrichment Reduces Shoot Growth in Sweet Chestnut Seedlings (Castanea sativa Mill.). Plant, Cell & Environment, 12, 927-934.
- Radoglou, K.M. and Jarvis, P.G. (1992) The Effects of CO2 Enrichment and Nutrient Supply on Growth Morphology and Anatomy of Phaseolus vulgaris L. Seedlings. Annals of Botany, 70, 245-256.
- Radoglou, K.M. and Jarvis, P.G. (1990) Effects of CO2 Enrichment on Four Poplar Clones. I. Growth and Leaf Anatomy. Annals of Botany, 65, 617-626. aob.oxfordjournals.org/content/65/6/617
- Gielen, B., Calfapietra, C., Sabatti, M. and Ceulemans, R. (2001) Leaf Area Dynamics in a Closed Poplar Plantation under Free-Air Carbon Dioxide Enrichment. Tree Physiology, 21, 1245-1255. http://dx.doi.org/10.1093/treephys/21.17.1245
- Mansfield, T.A., Hetherington, A.M. and Atkinson, C.J. (1990) Some Current Aspects of Stomatal Physiology. Annual Review of Plant Physiology and Plant Molecular Biology, 41, 55-75. http://dx.doi.org/10.1146/annurev.pp.41.060190.000415
- Woodward, F.I. (1987) Stomatal Numbers Are Sensitive to Increases in CO2 from Pre-Industrial Levels. Nature, 327, 617-618. http://dx.doi.org/10.1038/327617a0
- Knapp, A.K., Cocke, M., Hamerlynck, E.P. and Owensby, C.E. (1994) Effect of Elevated CO2 on Stomatal Density and Distribution in a C4 Grass and a C3 Forb under Field Conditions. Annals of Botany, 74, 595-599. aob.oxfordjournals.org/content/74/6/595 http://dx.doi.org/10.1006/anbo.1994.1159
- Woodward, F.I., Lake, J.A. and Quick, W.P. (2002) Stomatal Development and CO2: Ecological Consequences. New Phytologist, 153, 477-484.
- Banon, S., Fernandez, J.A., Franco, J.A., Torrecillas, A., Alarcon, J.J. and Sanchez-Blanco, M.J. (2004) Effects of Water Stress and Night Temperature Preconditioning on Water Relations and Morphological and Anatomical Changes of Lotus creticus Plants. Scientia Horticulturae, 101, 333-342. http://dx.doi.org/10.1016/j.scienta.2003.11.007
- Pyakurel, A. and Wang, J. (Unpublished) Leaf Morphological and Stomatal Variations in Paper Birch Populations across Canada. Ph.D. Dissertation, Lakehead University, Thunder Bay.
- Malone, S.R., Mayeux, H.S., Johnson, H.B. and Polley, H.W. (1993) Stomatal Density and Aperture Length in Four Plant Species Grown across a Sub-Ambient CO2 Gradient. American Journal of Botany, 80, 1413-1418.
- Tricker, P.J., Trewin, H., Kull, O., Clarkson, G.J., Eensalu, E., Tallis, M.J., Colella, A., Doncaster, C.P., Sabatti, M. and Taylor, G. (2005) Stomatal Conductance and not Stomatal Density Determines the Long-Term Reduction in Leaf Transpiration of Poplar in Elevated CO2. Oecologia, 143, 652-660. http://dx.doi.org/10.1007/s00442-005-0025-4
- Lake, J.A. and Woodward, F.I. (2008) Response of Stomatal Numbers to CO2 and Humidity: Control by Transpiration Rate and Abscisic Acid. New Phytologist, 179, 397-404.
- Richardson, A.D., Ashton, P.M.S., Berlyn, G.P., McGroddy, M.E. and Cameron, I.R. (2001) Within-Crown Foliar Plasticity of Western Hemlock, Tsuga heterophylla, in Relation to Stand Age. Annals of Botany, 88, 1007-1015. http://dx.doi.org/10.1006/anbo.2001.1538
- Sekiya, N. and Yano, K. (2008) Stomatal Density of Cowpea Correlates with Carbon Isotope Discrimination in Different Phosphorus, Water and CO2 Environments. New Phytologist, 179, 799-807.
- Fraser, L.H., Greenall, A., Carlyle, C., Turkington, R. and Friedman, C.R. (2009) Adaptive Phenotypic Plasticity of Pseudoroegneria spicata: Response of Stomatal Density, Leaf Area and Biomass to Changes in Water Supply and Increased Temperature. Annals of Botany, 103, 769-775. http://dx.doi.org/10.1093/aob/mcn252
- Doheny-Adams, T., Hunt, L., Franks, P.J., Beerling, D.J. and Gray, J.E. (2012) Genetic Manipulation of Stomatal Density Influences Stomatal Size, Plant Growth and Tolerance to Restricted Water Supply across a Growth Carbon Dioxide Gradient. Philosophical Transactions of the Royal Society London B: Biological Sciences, 367, 547-555. http://dx.doi.org/10.1098/rstb.2011.0272
- Dunlap, J.M. and Stettler, R.F. (2001) Variation in Leaf Epidermal and Stomatal Traits of Populus trichocarpa from Two Transects across the Washington Cascades. Canadian Journal of Botany, 79, 528-536. http://dx.doi.org/10.1139/b01-029
- Belhadj, S., Derridj, A., Moriana, A., Gijon, M.D.C., Mevy, J.P. and Gauquelin, T. (2007) Comparative Morphology of Leaf Epidermis in Eight Populations of Atlas Pistachio (Pistacia atlantica Anacardiaceae), Microscopy Research and Technique, 70, 837-846. http://dx.doi.org/10.1002/jemt.20483
- Camargo, M.B. and Marenco, R.A. (2011) Density, Size and Distribution of Stomata in 35 Rainforest Tree Species in Central Amazonia. Acta Amazonica, 41, 205-212. http://dx.doi.org/10.1590/S0044-59672011000200004
- Poulos, H.M., Goodale, U.M. and Berlyn, G.P. (2007) Drought Response of Two Mexican Oak Species, Quercus laceyi and Q. sideroxyla (Fagaceae), in Relation to Elevational Position. American Journal of Botany, 94, 809-818. http://dx.doi.org/10.3732/ajb.94.5.809
- Dudley, S.A. (1996) Differing Selection on Plant Physiological Traits in Response to Environmental Water Availability: A Test of Adaptive Hypotheses. Evolution, 50, 92-102. jstor.org/2410783


