 Open Journal of Marine Science, 2012, 2, 51-57 http://dx.doi.org/10.4236/ojms.2012.22007 Published Online April 2012 (http://www.SciRP.org/journal/ojms) Taxonomic Study on the Feather Stars (Crinoidea: Echinodermata) from Egyptian Red Sea Coasts and Suez Canal, Egypt Ahmed M. Hellal Marine Biology and Fish Science Section, Zoology Department, Faculty of Science, Al-Azhar University, Cairo, Egypt Email: Funyahem@Yahoo.com Received November 27, 2011; revised January 16, 2012; accepted January 25, 2012 ABSTRACT A taxonomic study on the crinoids (feather stars) collected from 34 sites from the Red Sea coasts and islands as well as the Suez Canal was done during the period from 1992 to 2003. A total of 15 species are now known from the Red Sea belonging to eleven genera under six families. Among them four species are endemic to the Red Sea and the two spe- cies, Decametra chadwicki and Lamprometra klunzingeri, are recorded from the Suez Canal for the first time. Also, the two species, Oligometra serripinna and Dorometra aegyptica, are new record from Gulf of Suez, and Decametra mollis from Gulf of Aqaba and Northern Red Sea. This study represents the first proper documentation of crinoid species in the study area. Summaries are provided of the specific habitats and geographical distribution. Keywords: Crinoidea; Red Sea; Suez Canal; Taxonomy; Habitats; Geographical Distribution 1. Introduction Feather-stars constitute group of echinoderms belonging to class Crinoidea and order Comatulida, having five to hundreds of arms surrounding their cup-like bodies [1,2]. Just like their closest relatives, the sea lilies, feather stars are stalked only in the juvenile stage but detach their cup-like bodies in the adult stage to become freely mov- ing or motile crinoids [2]. Feather stars are regarded as primitive echinoderms and today’s living species all be- long to the subclass Articulata [3]. Order Comatulida is composed of 18 extant families, with family Comasteri- dae being the most common in tropical shallow-water in both the Indo-West Pacific and the Western Atlantic [4- 7]. Feather stars are among the least known echinoderms attributable to difficulty in their collection on account of their fragile nature, secretive habits, and distribution in deep waters. Also, their identification requires patience and painstaking attention to morphological details [8,9]. In the Red Sea, although it is believed that shallow wa- ters (<50 m deep) are inhabited by living species of feather stars [10,11], there are no documented studies to warrant this claim. In present study, identified key of all crinoid species known from the Red Sea was applied. Also summaries of the specific habitats and geographical distributio n are provided. 2. Material and Methods 2.1. Field Observation, Collection and Preservation Many field trips were made to the Egyptian Red Sea coasts and islands, Gulfs of Aqaba and Suez and Suez Canal lakes during the period from 1992 to 2003. A total of 34 sites were surveyed and intensive collections of feather stars were done. The survey included both tidal and subtidal habitats (e.g. coral reefs and rocky h abitats). At each site, characteristics of specific habitats, position and site name, community structure, substrate type and crinoid distribution were recorded (Table 1). To loosen the animal’s grip on the substrate, a small metal bar was inserted between the cirri and the substrate, a technique employed to avoid possible breakage to the fragile arms. The animals were then placed in containers filled with sea-water and transported back to land, the natural color was noted. The specimens were then carefully lifted and immersed into their respective sea-water-filled containers to which 95% ethanol (3 parts sea water: 1 part 95% ethanol) was added, oral side down with arms spread out. Using the fingers, pressure was gently applied for about 30 second to restrain and keep the specimen in place and hasten fixation. When the animal became totally immo- bile, the seawater-95% ethnol solution was replaced a C opyright © 2012 SciRes. OJMS 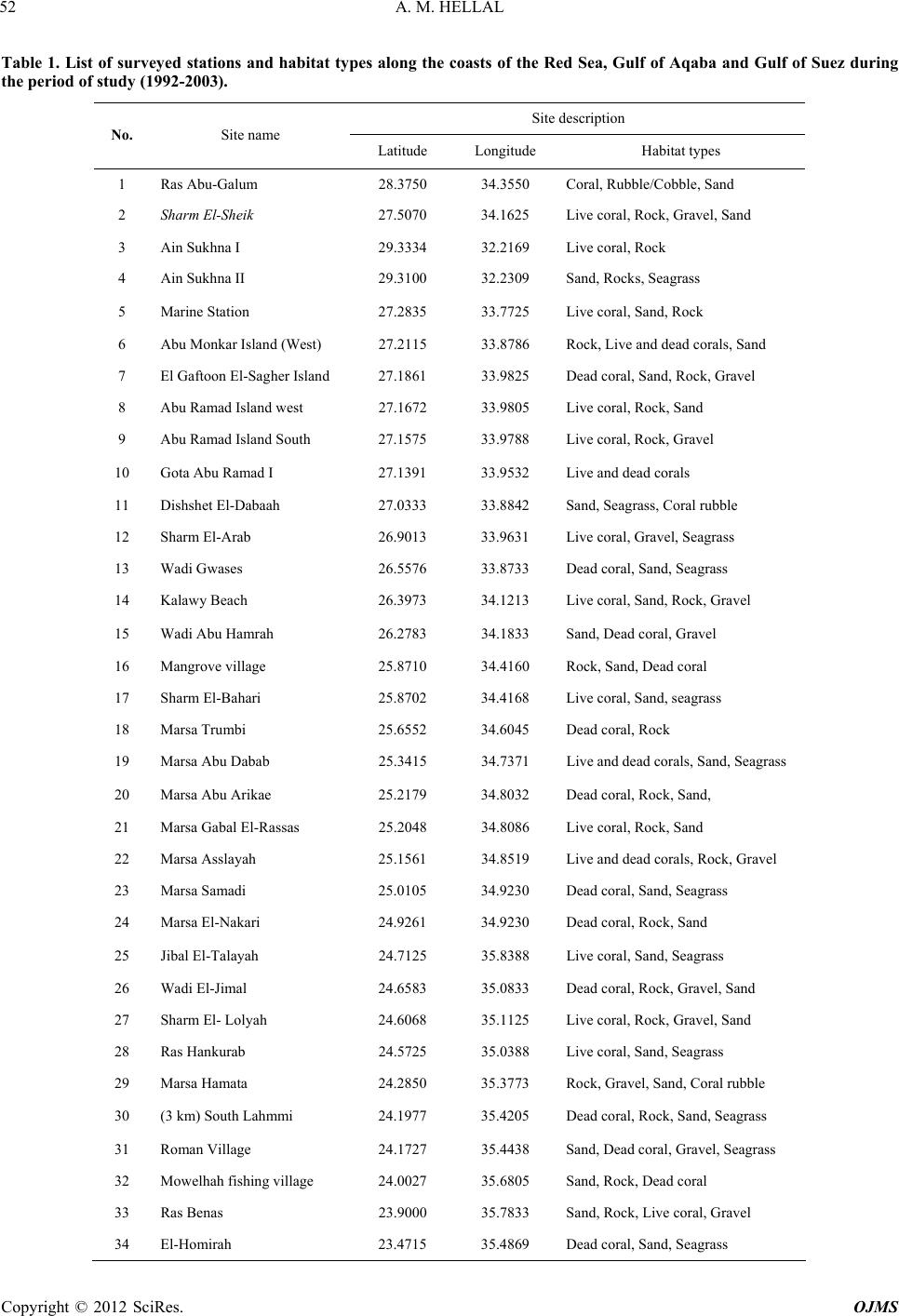 A. M. HELLAL 52 Table 1. List of surveyed stations and habitat types along the coasts of the Red Sea, Gulf of Aqaba and Gulf of Suez during the period of study (1992-2003). Site description No. Site name Latitude Longitude Habitat types 1 Ras Abu-Galum 28.3750 34.3550 Coral, Rubble/Cobble, Sand 2 Sharm El-Sheik 27.5070 34.1625 Live coral, Rock, Gravel, Sand 3 Ain Sukhna I 29.3334 32.2169 Live coral, Rock 4 Ain Sukhna II 29.3100 32.2309 Sand, Rocks, Seagrass 5 Marine Station 27.2835 33.7725 Live coral, Sand, Rock 6 Abu Monkar Island (West) 27.2115 33.8786 Rock, Live and dead corals, S and 7 El Gaftoon El-Sagher Island 27.1861 33.9825 Dead coral, Sand, Rock, Gravel 8 Abu Ramad Island west 27.1672 33.9805 Live coral, Rock, Sand 9 Abu Ramad Island South 27.1575 33.9788 Live coral, Rock, Gravel 10 Gota Abu Ramad I 27.1391 33.9532 Live and dead corals 11 Dishshet El-Dabaah 27.0333 33.8842 Sand, Seagrass, Coral rubble 12 Sharm El-Arab 26.9013 33.9631 Live coral, Gravel, Seagrass 13 Wadi Gwases 26.5576 33.8733 Dead coral, Sand, Seagrass 14 Kalawy Beach 26.3973 34.1213 Live coral, Sand, Rock, Gravel 15 Wadi Abu Hamrah 26.2783 34.1833 Sand, Dead coral, Gravel 16 Mangrove village 25.8710 34.4160 Rock, Sand, Dead coral 17 Sharm El-Bahari 25.8702 34.4168 Live coral, Sand, seagrass 18 Marsa Trumbi 25.6552 34.6045 Dead coral, Rock 19 Marsa Abu Dabab 25.3415 34.7371 Live and dead corals, Sand, Seagrass 20 Marsa Abu Arikae 25.2179 34.8032 Dead coral, Rock, Sand, 21 Marsa Gabal El-Rassas 25.2048 34.8086 Live coral, Rock, Sand 22 Marsa Asslayah 25.1561 34.8519 Live and dead corals, Rock, Gravel 23 Marsa Samadi 25.0105 34.9230 Dead coral, Sand, Seagrass 24 Marsa El-Nakari 24.9261 34.9230 Dead coral, Rock, Sand 25 Jibal El-Talayah 24.7125 35.8388 Live coral, Sand, Seagrass 26 Wadi El-Jimal 24.6583 35.0833 Dead coral, Rock, Gravel, Sand 27 Sharm El- Lolyah 24.6068 35.1125 Live coral, Rock, Gravel, Sand 28 Ras Hankurab 24.572 5 35.0388 Live coral, Sand, Seagrass 29 Marsa Hamata 24.2850 35.3773 Rock, Gravel, Sand, Coral rubble 30 (3 km) South Lahmmi 24.1977 35.4205 Dead coral, Rock, Sand, Seagrass 31 Roman Village 24.1727 35.4438 Sand, Dead coral, Gravel, Seagrass 32 Mowelhah fishing village 24.0027 35.6805 Sand, Rock, Dead coral 33 Ras Benas 23.9000 35.7833 Sand, Rock, Live coral, Gravel 34 El-Homirah 23.4715 35.4869 Dead coral, Sand, Seagrass Copyright © 2012 SciRes. OJMS  A. M. HELLAL 53 with the 70% etha nol, as the final fixative [12,13]. 2.2. Morphological Examination, Identification and Measurement Specimens were examined, noting important diagnostic features [14]. The body of crinoids is supported by cal- cium carbonate skeleton covered by a tissue layer (=skin). The central body (=theca) that houses the viscera is composed of a series of articulated ossicles forming the calyx. The theca and pinnule-bearing arms (brachials) of stalked juvenile feather stars make up the crown, while unstalked adult feather stars have cirri characterized as long hook-like structures for attachment. The calyx is composed of five basal ossicles (=basals) which may be absent or reduced, and five radial ossicles (radials) that support the central disk. Ambulacral grooves extend from the mouth to the arms and pinnules. The centrodorsal (discoidal, hemispherical, cylindrical, star- or cone-shap ed) is a large ossicle at the center of the aboral side of the body, where there are sockets for cirri attachment. Two arms, cirri, and the diameter of the central disk were measured in cm. The status of each species was deter- mined using the following categories. “Rare species” was applied when only 1 - 5 indi- viduals of a species were present. “Common species” was given for a species having 5 - 10 individuals. “Abundant species” for a species has more than 10 individuals. At the laboratory, crinoid specimens were sorted and identified using standard keys [10,11,15,16]. In addition, some important taxonomic works [17-21] were used in the identification of the specimen at the family, genus and species level. The species list for the crinoids of the Red Sea and adjacent waters such as Arabian Gulf, Southeast Arabia and East Africa was compiled using the present data and information from the works of available literatures. The geographical distribution patterns of crinoid species, re- corded in the present study and adjacent waters were compared. The following terms are used in the identification and description of crinoid species (Figure 1). Brachials: the calcareous ossicles of the arm (extend- ing the division series). Centrodorsal: the large plates occupying the center of the dorsal or aboral side, in shape discoidal, hemispheri- cal, or sometimes conical and usually bearing cirri except on its apex or do r sal pol e . Cirri: the jointed appendages arising from the centro- dorsal, for temporary attachment to the substrate. Radials: the five plates from which the division series (or arms) arise; superficially they are only narrowly visi- Figure 1. Introductory figure of crinoid, omitting to the arms, showing parts mentioned in terms (after Clark and Rowe, 1971). ble in adults of most species. Division series: the ossicles between the radials and the first brachials of the undivided arms absent only in five armed genera; the distalmost ossicle of the division series is an axillary. Pinnules: the slender jointed appendages arising from the brachials on alternate sides, the proximal one or more of which are modified as oral pinnules. The pinnules on the outer (inter-radial) side of the arm are designated P1, P2, etc. and the inner side Pa, Pb, etc. Zyzygy: a rigid breaking-joint occurring at intervals in division series and arms, often regularly placed; the ar- ticulation is by ligament rather th an muscles and the joint faces bear numerous fine radiating ridges so that exter- nally the suture may appe ar discontinuous or undulating. 3. Results Key to the Species, Genera and Families Recorded in the Present Study 1 Proximal pinnules very flexible and with some of the terminal segments modified to form a comb “Figure 2(a)”; mouth near the edge of the disc and anal tube approximately central. COMASTERIDAE 2 - No comb on the proximal pinnules, their terminal segments simply tapering or only very finely thorny. 3 2(1) Only 10 arms; up to 13 cirrus segments at arm length 30 - 40 mm. Comissia hartmeyeri A. H.Clark, 1912. - More than 10 arms; 14 or 15 cirrus segments at arm length 35 - 40 mm. Capillaster multiradiatus (Linnaeus, 1758). 3(1) Middle and distal cirrus segments with a pair of Copyright © 2012 SciRes. OJMS  A. M. HELLAL 54 dorsal spines or tubercles, one each side of the midline, rarely transverse ridge “Figure 2(b)”. COLOBOMETRIDAE. 4 - Distal cirrus segments with a median prominence (dorsal spine, tubercle or longitudinal crest) or quite smooth “Figures 2(c) and (d)”. 7 4(3) Cirri large with 36 - 48 segments, which have distinctly spinose distal edge. Colobometr a a rabi ca A. H. Clark, 1937. - Cirri smaller with <30 segments, their distal ends smooth. 5 5(4) Cirri wide and flat on the dorsal side, with curved transverse ridges or only small blunt paired tu- bercles; P2 stout and markedly prismatic with a saw-like profile (Figure 2(e)); Pa (the pinnule in the inner side of the fourth brachial) present on all or most of the arm. Oligometra serripinna (P. H. Carpenter, 1881). - Cirri laterally compressed, not markedly flat- tened dorsally, the paired tubercles usually coni- cal; P2 not consp icuously modified “Figure 2(f)”; Pa absent often more than not. DECAMETRA. 6 6(5) P2 over three times the length of P1; cirri numbering X V II -X XI I, usually c. XX. Decametra mollis (A. H. Clark, 1909). - P 2 rarely more than twice the length of P1; cirri numbering XIII-XIX, usually c. XVI. Decametra chadwicki (A. H. Clark, 1911). 7(3) The proximal pinnules at least with a slight, sometimes well marked, keel or series of proc- esses on the dorsal side basally “Figure 2(g)”, or else all the pinnules prismatic for their entire length; often more than 10 arms; the second bra- chial zyzygy usually farther out than brachials 9 and 10; the brachials distinctly wider than long for at least the proximal half of the arm, some- times discoidal in shape. 8 - No keel on the dorsal surface of any of the pin- nules; only 10 arms; the second brachial zyzygy almost invariably at 9 + 10; brachials after about the fourteenth usually as long as wide or longer and distinctly wedge-shape. ANTEDONIDAE 13 8(7) Only the proximal pinnules at all prismatic; the distal pinnules, if not all of them, flexible and not conspicuously stiffened. 9 - All the pinnules prismatic and conspicuously straight and stiff; 10 arms only and carinate dor- sally, at least in the proximal half; no dorsal or ventral processes on the cirrus segments “Figure 2(d)”. (TROPIOMETRIDAE) Tropiometra carina ta (Lamarck, 1816). 9(8) Ten or more arms; if more than 10 then the ex- ternal IIBr series at least usually of four ossicles “Figure 2(k)”. HIMEROMETRIDAE. 10 - Always more than 10 arms, the IIBr series and any other division series of 2 ossicles “Figure 2(j)”. MARIAMETRIDAE. 11 10(9) Dorsal spines on the distal cirrus segments sharp and usually long, some cirrus segments longer than broad; usually 20 arms; P2 and P3 similar in size “Figure 2(f)”. Heterometra savignii (Muller, 1841). - Spines or tubercles developed gradually over several cirrus segments, usually all short and blunt, cirri segments broader than long; 14 arms; P2 smaller than P3. Heterometra atra (A. H. Clark, 1911). 11(9) One or more of the enlarged proximal pinnules (P2, sometimes P3) very stiff and spike-like, of- ten recurved over the disc; division series well separated with rounded ventrolateral extensions “Figure 2(h)”. STEPHANOMETRA. 12 - Enlarged proximal pinnules (P2) tapering, slen- der and usually flexible; division series without ventrolateral extension, variable in form but the adjacent ones often straight-sided and closely approximating laterally “Figure 2(i)”. Lamprometra kl unzi ngeri (Hartlaub, 189 0). 12(11) P3 and the following pinnules smaller and more flexible than P2 which is the only spike-like pin- nule. Stephanometra indica (Smith, 1876). - P3 spike-like, resembling P2 but usually smaller. Stephano metra spicata (P. H. Carpenter, 1881). 13(7) P3 the largest pinnule; cirrus segments overlap- ping the succeeding segments. DOROMETRA. 14 - P 1 with only 8 - 11 segments and larger than P3. Antedon parvi fl or a (A. H. Clark, 1912). 14(13) Cirri relatively small, their length usually be- tween a fifth and sixth of the arm length. Dorometra parvicirra (P. H. Carpenter, 1888). - Cirri larger, about a third of the arm length. Dorometr a aegyptica (A. H. Clark, 1911). 4. Discussion Crinoids are prominent part of coral reef crypto fauna [22]. Although they are not as species rich (15 species) as ophiuroids 49 species [23], asteroids 36 species [24], holothuroids 28 species [25] or brachyuran crabs 361 species [26] and bivalves 180 species [27] many of such Copyright © 2012 SciRes. OJMS 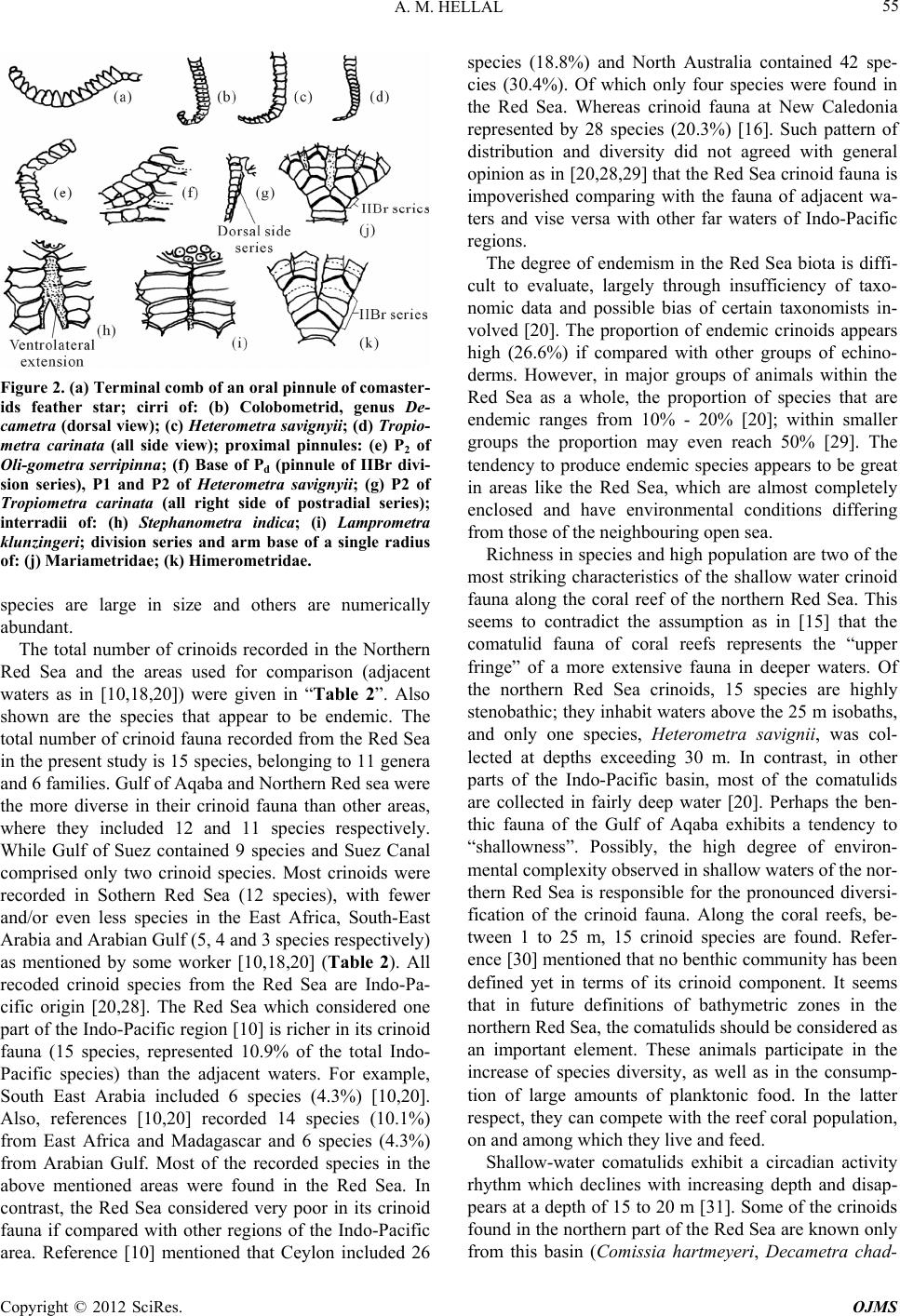 A. M. HELLAL 55 Figure 2. (a) Terminal comb of an oral pinnule of comaster- ids feather star; cirri of: (b) Colobometrid, genus De- cametra (dorsal view); (c) Heterometra savignyii; (d) Tropio- metra carinata (all side view); proximal pinnules: (e) P2 of Oli-gometra serripinna; (f) Base of Pd (pinnule of IIBr divi- sion series), P1 and P2 of Heterometra savignyii; (g) P2 of Tropiometra carinata (all right side of postradial series); interradii of: (h) Stephanometra indica; (i) Lamprometra klunzingeri; division series and arm base of a single radius of: (j) Mariametridae; (k) Himerometridae. species are large in size and others are numerically abundant. The total number of crinoids recorded in the Northern Red Sea and the areas used for comparison (adjacent waters as in [10,18,20]) were given in “Table 2”. Also shown are the species that appear to be endemic. The total number of crinoid fauna reco rded from the Red Sea in the present stud y is 15 species, belonging to 11 gen era and 6 families. Gulf of Aqaba and Northern Red sea were the more diverse in their crinoid fauna than other areas, where they included 12 and 11 species respectively. While Gulf of Suez contained 9 species and Suez Canal comprised only two crinoid species. Most crinoids were recorded in Sothern Red Sea (12 species), with fewer and/or even less species in the East Africa, South-East Arabia and Arabian Gulf (5, 4 and 3 species respectively) as mentioned by some worker [10,18,20] (Table 2). All recoded crinoid species from the Red Sea are Indo-Pa- cific origin [20,28]. The Red Sea which considered one part of the Indo-Pacific region [10] is richer in its crin oid fauna (15 species, represented 10.9% of the total Indo- Pacific species) than the adjacent waters. For example, South East Arabia included 6 species (4.3%) [10,20]. Also, references [10,20] recorded 14 species (10.1%) from East Africa and Madagascar and 6 species (4.3%) from Arabian Gulf. Most of the recorded species in the above mentioned areas were found in the Red Sea. In contrast, the Red Sea considered very poor in its crinoid fauna if compared with other regions of the Indo-Pacific area. Reference [10] mentioned that Ceylon included 26 species (18.8%) and North Australia contained 42 spe- cies (30.4%). Of which only four species were found in the Red Sea. Whereas crinoid fauna at New Caledonia represented by 28 species (20.3%) [16]. Such pattern of distribution and diversity did not agreed with general opinion as in [20,28,29] that the Red Sea crinoid fauna is impoverished comparing with the fauna of adjacent wa- ters and vise versa with other far waters of Indo-Pacific regions. The degree of endemism in the Red Sea biota is diffi- cult to evaluate, largely through insufficiency of taxo- nomic data and possible bias of certain taxonomists in- volved [20]. The proportion of endemic crinoids appears high (26.6%) if compared with other groups of echino- derms. However, in major groups of animals within the Red Sea as a whole, the proportion of species that are endemic ranges from 10% - 20% [20]; within smaller groups the proportion may even reach 50% [29]. The tendency to produce endemic species appears to be great in areas like the Red Sea, which are almost completely enclosed and have environmental conditions differing from those of t he ne i g hbouring open sea. Richness in species and high population are two of the most striking characteristics of the shallow water crinoid fauna along the coral reef of the northern Red Sea. This seems to contradict the assumption as in [15] that the comatulid fauna of coral reefs represents the “upper fringe” of a more extensive fauna in deeper waters. Of the northern Red Sea crinoids, 15 species are highly stenobathic; they inhabit waters above the 25 m isobaths, and only one species, Heterometra savignii, was col- lected at depths exceeding 30 m. In contrast, in other parts of the Indo-Pacific basin, most of the comatulids are collected in fairly deep water [20]. Perhaps the ben- thic fauna of the Gulf of Aqaba exhibits a tendency to “shallowness”. Possibly, the high degree of environ- mental complexity observ ed in shallow waters of the nor- thern Red Sea is responsible for the pronounced diversi- fication of the crinoid fauna. Along the coral reefs, be- tween 1 to 25 m, 15 crinoid species are found. Refer- ence [30] mentioned that no benthic community has been defined yet in terms of its crinoid component. It seems that in future definitions of bathymetric zones in the northern Red Sea, the comatulids should be considered as an important element. These animals participate in the increase of species diversity, as well as in the consump- tion of large amounts of planktonic food. In the latter respect, they can compete with the reef coral population, on and among which they live and feed. Shallow-water comatulids exhibit a circadian activity rhythm which declines with increasing depth and disap- pears at a depth of 15 to 20 m [31]. Some of the crinoids found in th e northern part of th e Red Sea are known on ly from this basin (Comissia hartmeyeri, Decametra chad- Copyright © 2012 SciRes. OJMS 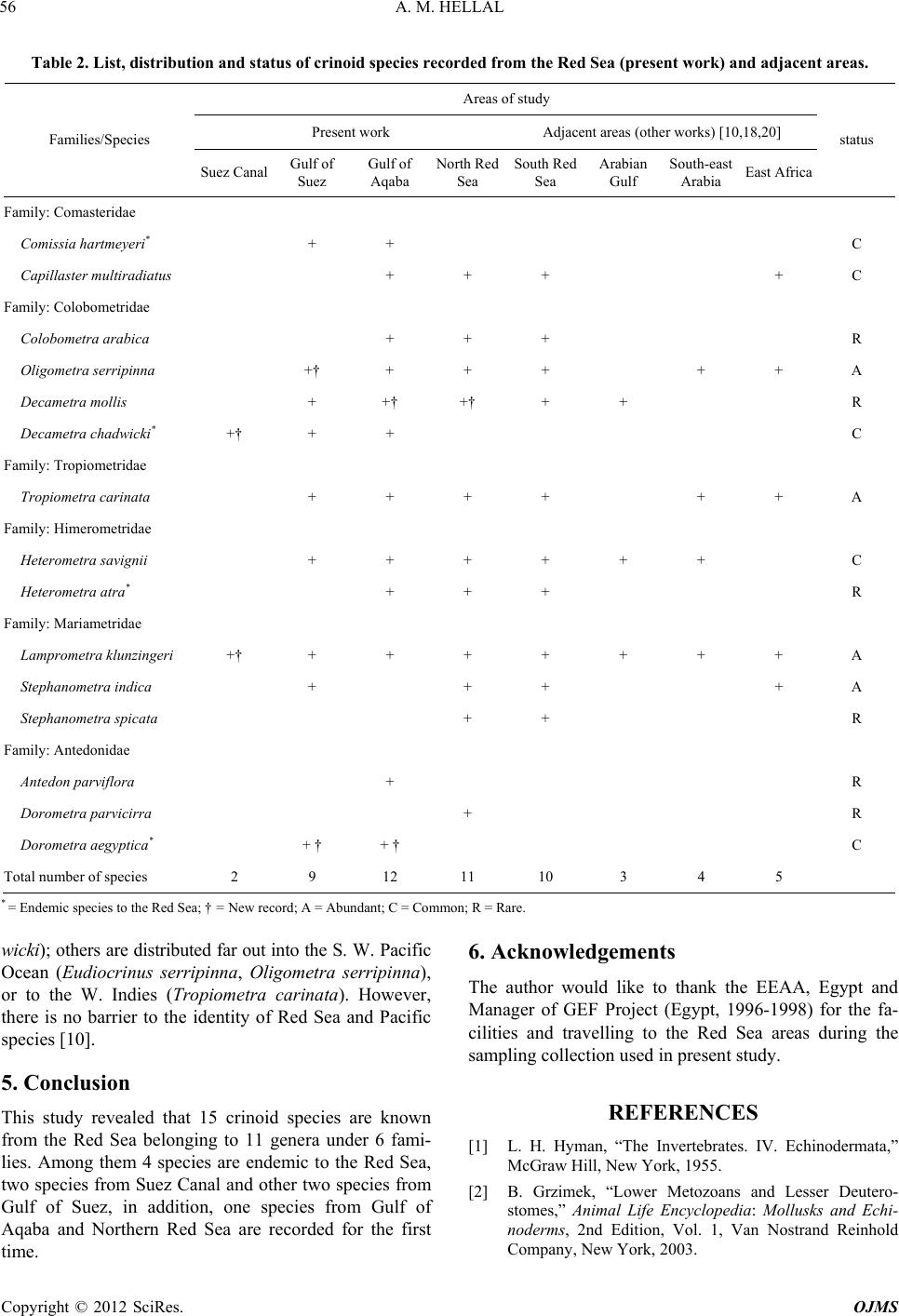 A. M. HELLAL Copyright © 2012 SciRes. OJMS 56 Table 2. List, distribution and status of crinoid species recorded from the Red Sea (present work) and adjacent areas. Areas of study Present work Adjacent areas (other works) [10,18,20] Families/Species Suez Canal Gulf of Suez Gulf of Aqaba North Red Sea South Red Sea Arabian Gulf South-east Arabia East Africa status Family: Comasteridae Comissia hartmeyeri* + + C Capillaster multiradiatus + + + + C Family: Colobometridae Colobometra arabica + + + R Oligometra serripinna +† + + + + + A Decametra mollis + +† +† + + R Decametra chadwicki* +† + + C Family: Tropiometridae Tropiometra carinata + + + + + + A Family: Himerometridae Heterometra savignii + + + + + + C Heterometra atra* + + + R Family: Mariametridae Lamprometra klunzingeri +† + + + + + + + A Stephanometra indica + + + + A Stephanometra spicata + + R Family: Antedonidae Antedon parviflora + R Dorometra parvicirra + R Dorometra aegyptica* + † + † C Total number of species 2 9 12 11 10 3 4 5 * = Endemic species to the Red Sea; † = New record; A = Abundant; C = Common; R = Rare. wicki); others are distributed far out into the S. W. Paci fic Ocean (Eudiocrinus serripinna, Oligometra serripinna), or to the W. Indies (Tropiometra carinata). However, there is no barrier to the identity of Red Sea and Pacific species [10]. 5. Conclusion This study revealed that 15 crinoid species are known from the Red Sea belonging to 11 genera under 6 fami- lies. Among them 4 species are endemic to the Red Sea, two species from Suez Canal and other two species from Gulf of Suez, in addition, one species from Gulf of Aqaba and Northern Red Sea are recorded for the first time. 6. Acknowledgements The author would like to thank the EEAA, Egypt and Manager of GEF Project (Egypt, 1996-1998) for the fa- cilities and travelling to the Red Sea areas during the sampling collection used in present study. REFERENCES [1] L. H. Hyman, “The Invertebrates. IV. Echinodermata,” McGraw Hill, New York, 1955. [2] B. Grzimek, “Lower Metozoans and Lesser Deutero- stomes,” Animal Life Encyclopedia: Mollusks and Echi- noderms, 2nd Edition, Vol. 1, Van Nostrand Reinhold Company, New York, 2003.  A. M. HELLAL 57 [3] W. I. Ausich and C. G. Messing, “Crinoidea. Sea Lilies and Feather Stars,” Version 21, The Tree of Life Web Project, 1998. http://tolweb.org/ [4] D. I. Meyer and D. B. Macurda Jr., “Ecology and Distri- bution of the Shallow-Water Crinoids (Echinodermata) of the Palau Islands and Guam (Western Pacific),” Microne- sica, Vol. 16, 1980, pp. 59-99. [5] C. G. Messing, “A Revision of the Recent Indo-West Pacific Comatulid Genus Comaster Agassiz. Part 1: The Type Species of Comaster and Phanogenia Lovén (Ech- inodermata: Crinoidea: Comasteridae),” Invertebrate Ta- xonomy, Vol, 12, No. 2, 1998, pp. 191-209. doi:10.1071/IT97004 [6] C. G. Messing, “Three New Species of Comasteridae (Echinodermata: Crinoidea) from the Tropical Western Pacific,” Zoosystm, Vol. 25, No. 1, 2003, pp. 149-162. [7] L. Kirkendale and C. G. Messing, “An Annotated Che- cklist and Key to the Crinoidea Guam and the Common- wealth of the Northern Mariana Islands,” Micronesica, Vol. 35-36, 2003, pp. 523-546. [8] K. B. Tomasz, “Crinoid Ecological Morphology,” Annual Review of Earth and Planetary Sciences, Vol. 36, No. 7, 2008, pp. 221-249. doi:10.1146/annurev.earth.36.031207.124116 [9] C. Carmen, R. M. Arguelles, D. Paz1 and G. C. Florencia, “Identification of Feather Stars (Echinodermata: Crinoidea: Comatulida) at Subic Bay, Zambales, Philippines,” Phil- ippine Journal of Science, Vol. 139, No. 1, 2010, pp. 51-60. [10] A. M. Clark and F. E. W. Rowe, “Monograph of Shallow Water Indo-West Pacific Echinoderms,” Trustees of the British Museum (Natural History), London, 1971. [11] A. R. G. Price, “Echinoderms of Saudai Arabia. Echi- noderms of the Arabian Gulf Coast of Saudi Arabia,” Fauna of Saudi Arabia, Vol. 5, 1983, pp. 28-108. [12] G. Hendler, “Collecting, Preserving and Archiving Echi- noderms,” Natural History of Los Angeles, County, Los Angeles, 2004. http://clade.ansp.org/malacology/people/rosenberg/archivi ng/taxa/echinoderms.html [13] C. G. Messing, “Charles Messi ng’s Crinoid Pages Florida: Nova Southeastern University Oceanographic Center,” 2006. http://www.nova.edu/ocean/ messing/crinoids/ [14] G. Rouse, C. G. Messing and L. Johnston, “Crinoidea: Featherstars and Sea Lilies Australia: Australian Biologi- cal Resources Study,” 2006. http://geolog008.geology.adeliade.edu.au/CrinoideaSite/ webcontent/pages/welcome.html [15] H. L. Clark, “A Monograph of the Existing Crinoids. Vo- lume 1. The Comatulids. Part 2,” Bulletin of the United States National Museum, Vol. 82, 1921, pp. 1-57. [16] A. Guille, A. Laboute and J. L. Moneu, “Guide des E to il es de Mer Oursins et Outers Echinodermes du Lagoon de Novelle Caledonie,” ORSTONO, 1986, p. 283. [17] A. H. Clark, “A Monograph of the Existing Crinoids. I (3): Superfamily Comasterida,” Bulletin of the American National Museum, Vol. 82, 1931, pp. 31-816. [18] D. B. James and J. S. Pearse, “Echinoderms from the Gulf of Suez and Northern Red Sea,” Journal of the Marine Biological Association of India, Vol. 1, No. 1-2, 1969, pp. 78-125. [19] A. R. G. Price, “Studies on the Echinoderm Fauna of the Western Arabian Gulf,” Journal of Natural History, Vol. 15, No. 1, 1981, pp. 1-15. doi:10.1080/00222938100770011 [20] A. R. G. Price, “Echinoderms of Saudi Arabia. Com- parison between Echinoderm Faunas of Arabian Gulf, SE-Arabia, Red Sea and Gulfs of Aqaba and Suez,” Fauna of Saudi Arabia, Vol. 4, 1982, pp. 3-21. [21] F. E. W. Rowe, A. K. Hoggett, R. A. Birtles and L. L. Vail, “Revision of Some Comasterid Genera from Aus- tralia (Echinodermata: Crinoidea), with Descriptions of the Two New Genera and Nine New Species,” Zoological Journal of the Linnean Society, Vol. 86, 1986, pp. 197- 277. doi:10.1111/j.1096-3642.1986.tb01812.x [22] N. A. Sloan, “Size and Structure of Echinoderm Popu- lations Associated with Different Coexisting Coral Spe- cies at Aldabra Atoll,” Marine Biology, Vol. 66, 1982, pp. 67-75. [23] A. M. Hellal, “Taxonomy and Zoogeography of the Red Sea Ophiuuoidea (Brittlestars),” Ph.D. Thesis, Al-Azhar University, Cairo, 1990. [24] M. M. Fauda and A. M. Hellal, “Fauna and Flora of Egypt. The Echinoderms of the Northwestern Red Sea. Asteroidea,” Natural History Museum of Egypt, Cairo, 1987, pp. 1-71. [25] A. M. Hellal, “Contribution to the Sea Cucumber Fauna (Echinodermata: Holothuroidea) at the Vicinity of Bab El-Mandab, Red Sea Yemen,” Al-Azhar Bulletin of S c i e nce, Vol. 21, No. 1, 2010, pp. 27-65. [26] P. Vine, “Red Sea Invertebrates,” Immel Publishing, Lon- don, 1986, pp. 1-224. [27] D. Sharabati, “Red Sea Shells,” Chapman and Hall, Routledge, 1985, pp. 1-128. [28] A. C. Campbell, “Echinoderms of the Red Sea,” In: A. J. Edwards and S. M. Head, Eds., Key Environments Red Sea, Pergamon Press, Oxford, 1987, pp. 215-232. [29] G. Bermet and R. Ormond, “Red Sea Coral Reefs,” Keegan Paul International Ltd., Lon do n, 1981. [30] H. B. Fell, “The Echinodermata,” In: T. J Parker and W. A Haswell, Eds., Text Book of Zoology, 7th Edition, Vol. 1, Macmillan, London, 1966. [31] J. L. Rutman and L. Fishelson, “Comparison of Repro- duction in the Red Sea Feather-Stars Lamprometra klunzingeri (Hartlaub), Heterometra savignii (J. Muller) and Capillaster multiradiatus (L.),” Echinoderms, 1984- 1985, pp. 195-220. Copyright © 2012 SciRes. OJMS
|