 Vol.1, No.1, 24-38 (2010)s doi:10.4236/as.2010.11004 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ Agricultural Science Symbiosis of nodule bacteria with perennial xerophyte leguminous plants of Central Asia Zair S. Shakirov*, Sardor A. Khakimov Institute of Microbiology of Uzbekistan Academy of Sciences, Tashkent, Republic of Uzbekistan; *Corresponding Author: zair@dostlink.net Received 27 April 2010; revised 3 May 2010; accepted 5 May 2010. ABSTRACT From nodules of perennial xerophyte desert leguminous plants – Ammodendron conollyi, Astragalus villossimus, Astragalus unifoliolatus – 151 bacterial isolates have been isolated. The study of nodulation showed that AC8-1, AC11, AC21, AC1-1, AC12-1 isolates (from Ammoden- dron conollyi) , AV1 , AV8 - 1, AV 9, AV2 6 -1 , AV 3 6- 1 isolates (from Astragalus villossimus) and AU17-1, AU30-1, AU30-2, AU20-1, AU23 isolates (from Astragalus unifoliolatus) formed an effective nitrogen-fixing symbiosis with the host plants. As a result of 16S rRNA gene study of the salt-resistant nodule bacteria it has been de- termined that bacteria were related to Rhizo- bium, Burkholderia and Achromobacter genera. The study of isolates growth has revealed that there were fast-growing and moderately-grow- ing isolates that possessed with doubling-time varying from 20 to 45 min. Their examination for antibiotic-resistance showed that the number of bacterial colonies of selected strains decreased to some extent in the presence of chloram- phenicol, but in all strains the resistance to an- tibiotics was detected. The further investiga- tions of resistance of the formed symbiosis to stresses (drought, salinity) showed that at 6.41% of moisture the maximal height and biomass of inoculated plants of Ammodendron conollyi we re 21 cm and 2320 mg, but at 3.8% moisture the height reduced by 4 times (up to 4.5 c m) and th e biomass – by 11 times (203 mg). The analogous effect was observed in Astragalus villossimus and Astragalus unifoliolatus symbiosises. The salinity equal to 100-200 mM NaCl did not affect practically on normal growth and development of desert leguminous plants symbiosis, while for Astragalus villossimus such affecting con- centration comprised up to 100 mM NaCl. The light microscopy and electron microscopy of Astragalus villossimus nodule sections showed that V1 nodule bacteria strain efficiently colo- nized the internal space within nodules, where they were transformed into bacteroids. At 100 mM NaCl salinity concentration the colonization of nodule bacteria within nodule plant cells re- duced in comparison with control nodules of plant s grown in non-salted conditions. Keywords: Ammodendron con ollyi; Astragalus villossimus; Astragalus unifoliolatus; Nodulation; Nitrogen Fixation; Salinity; Bacteroid; Rhizobium; Burkholderia; Achromobacter 1. INTRODUCTION Deserts occupy one of third of dry land and they are un- claimed potential for conducting human’s economical activity. In many regions the desertification process with its spreading to arable lands is going on, so the problem of combating desertification is actual for many countries. Desert legumes are represented with annual plants and perennial trees and shrubs, which serve as a frame basis for desert ecosystem. There are literature data about symbiosis of relatively hygrophilous acacia trees with their nodule bacteria [1,2], but there is a few data about development of nitrogen-fixing symbiosis of xerophyte desert legumes, their nodule bacteria and nodulation. Nodulation in hygrophilous Acacia auriculiformis and Acacia ampliceps reduced during the change of moistur e content from 0.008 МРа to 0.08 МРа twice and totally disappeared at 0.8 МРа [1]. Under inoculation of salt- resistant Acacia ampliceps species by salt-tolerant strain of nodule bacterium the nitrogen-fixing activity of formed symbiosis considerable increased in the presence of salt in comparison with nitrogen-fixing activity of symbiosis caused with inoculation by salt-intolerable strain [1]. In 20 Acacia species nodules formed after 5 months of growth, but in Leucaena leucocephala the formation of nodules was stimulated by low doses of nitrogen fertilizers (30-50 kg/Ha) and suppressed with  Z. S. Shakirov et al. / Agricultural Sciences 1 (2010) 24-38 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ 25 high doses (100 kg/Ha and more) [1]. More humid sands promoted the increase of nodulation frequency in Pro- sopis glangulosa (honey mesquite), desert perennial le- guminous shrub/tree. Rhizobia, isolated from Acacia, Prosopis and Leucaena were resistant to 500-850 mM NaCl [2]. For the most of rhizobia the optimal tempera- ture for growth in culture varies within range 28-31°C, however, some strains of rhizobia isolated from woody legumes of Acacia and Prosopis species grew well at 40-44°C [2]. The growth of plants, their nodulation and nitrogen fixation (nitrogen content) in Acacia redolens and Acacia cyclops, inoculated with salt-resistant nodule bacteria, grown in vegetation experiments within sand, were observed up to salinity value 80 mM NaCl, but in Acacia ampliceps plants during growing within sand — up to 200 mM NaCl [2]. Bacterial cells of rhizobia fro m Acacia nilotica displayed the stable growth up to 850 mM NaCl and formed effective nitrogen-fixing nodules in Acacia trees at 150 mM NaCl [2]. The objects of the present research were microbiologi- cal and symbiotic properties of nodule bacteria isolated from Ammodendron conollyi (“sandy acacia”, tree), As- tragalus villossimus (shrub) and Astragalus unifoliolatus (sandy semi-shrub), xerophyte perennial leguminous plants of Kyzylkum desert (Uzbekistan) that grow at av- erage annual rainfall norm 60 mm and temperature 45-50°C [3], and also the host specificity of their nitrogen-fixing symbiosis towards to host plant as well as resistance of their symbiosi s to st resses (salinity, drought). 2. MATERIALS AND METHODS 2.1. Description of Ammodendron conollyi, Astragalus villossimus, Astragalus unifoliolatus plants Ammodendron connollyi Bge. (“sandy Acacia”) belongs to Leguminosae family and Ammodendron genus. It looks like a shrub (in juvenile state), later (after 3-4 years) it turns into tree that in mature state can reach of height 2-3 m and maximal height – up to 8 m [3]. Astragalus villossimus Bge. belongs to Leguminosae family and Astragalus genus, Cercidothrix subgenus, Ammodendron Bge section. It is a branched shrub with height up to 70 cm and surface with wh ite hairs. Woody branches are long and thick; they are covered with light splintered bark. Annual stems (branches) are either very short or long (6-23 cm length), they twine round and, they are fluffy and white [3]. Astragalus unifolio latus Bge. b elong s to Legumino sae family, Astragalus genus, Cercidothrix subgenus, Am- modendron Bge. section. It is a shrub with woody stem (trunk). Annual branches are long, branchy, 13-22 cm length, cylindrical, closely twisted, white [3]. 2.2. Nodule Sampling and Isolation of Nodule Bacteria Nodules were sampled during intense blossoming and repeatedly rinsed with sterile distilled water, then they were sterilized in 96% ethanol with followed th eir burn- ing in open flame. Bacterial isolates of nodule bacteria were isolated from nodules and purified in correspon- dence with common methods [4,5], then they were re-sowed to medium of the following co mposition (g/L): glucose – 5, sucrose – 5, К2НРО4 – 0.5, КН2РО4 – 0.5, MgSO4•7H 2O – 0.5, CaSO4 – 0.2, pea – 50, agar – 20, water distilled – up to 1 L, pH – 6.8-7.0 (pea was boiled during 1 hour and th e medium w as pr epared on th e basis of pea’s broth). 2.3. Treatment and Germination of Seeds Seeds of plants were treated with 96% H2SO4 during 40 min for Ammodendron conollyi and 20 min for both Astragalus villossimus and Astragalus unifoliolatus, whereupon the seeds were repeatedly rinsed by sterile distilled water and transferred to 1 % water agar with moisture enough for swelling of seeds and their germi- nation in Petri dishes. Next 1-2 da ys after treatment with acid the seeds swelled and scarification of seed’s coat was conducted, then opened appeared roots of seeds were submerged into sterile agar for fast development of seeds` roots in Petri dishes that were introduced into thermostat at 30°C. After 2-3 days from the starting of seed germination the seedlings with developed roots were transplanted in potting substrate for their further development and growth. 2.4. Preparation of Potting Substrate for Microvegetation/Vegetation Experiments The ability of desert leguminous plants seedlings to grow in ascending flow of nutritive solution in tubes (microvegetation experiments) was tested. Tubes were filled for one third volume by sterile nutritive solution and filter paper strips were introduced into the tubes, the upper end of which was fixed on the height 1-2 cm from the surface of nutritive solution in tubes, but the lower end of strips was submerged into the nutritive solution for formation of ascend ing flow of th e nutritive solution . Composition of the nutritive solution for plant growing was following: MgSO4•4 H 2O – 5 mM, K2SO4 - 10 mM, CaCl2•2H2O – 1 mM, phosphate buffer (NaH2PO4 + Na2HPO4, pH 6.5) – 15 mM, microelements –0.05 ml/L of medium, Fe source –5 mM; microelements composi- tion (g/L) – H3BO3 – 17.16, MnSO4 – 7.2, ZnSO4 – 1.32, CuSO4 – 1.65, Na2MoO4 – 0.12 [4]. The seedlings were put on the upper end of strips and inoculated by nodule bacteria. As vessels for potting substrate 2-Litre volume black perforated bags (vegetation experiments) were used.  Z. S. Shakirov et al. / Agricultural Sciences 1 (2010) 24-38 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ 26 They were filled by combinations of substrates (ver- miculite, peat, sand, soil) that were sterilized at 1 at- mosphere during 30 min. 2.5. Nodulation Test Ten strains of every nodule bacteria isolated from Am- modendron conollyi (AC1-1, AC2, AC4-1, AC8-1, AC 11, AC12-1, AC13-1, AC15, AC18-1, AC21), Astragalus villossimus (AV1, AV2, AV3, AV6-1, AV8-1, AV9, AV9-1, AV26-1, AV30, AV36-1) and Astragalus unifoli- olatus (AU2-1, AU3-1, AU4, AU7, AU17-1, AU20-1, AU23, AU28, AU30-1, AU30-2) were selected for in- oculation. Strains of Azorhizobium caulinodans ORS571 (University of Nottingham, Centre for crop nitrogen fixation) and CXM1 (the Russian industrial strain for alfalfa inoculation) were also used. The bacteria were grown in 2 % hard (agar-containing) medium with pea’s broth during 3 days at 28°C. The plant seedlings were sowed into bags on the depth of substrate 2-3 cm and further there was done an inoculation of planted seed- lings with 5 ml of culture nodule bacterial suspension (109 cells/ml) per each variant and simultaneous irriga- tion with nutritive solution. 2.6. Determination of Nitrogen-Fixing Activity Nitrogen-f ixing activity was estimated by the acety- lene-reductase activity (ARA) assay described by Hardy [5]. The plant samples (with root nodules) were washed with sterile water and transferred into 60 ml capacity tube fitted with airtight rubber stoppers. Acetylene (10 volume %) was injected and the tube s were incubated at 30°C for 24 hours. The data was the mean of three replicates. The samples without acetylene were used as control. The quantitative estimation of ethylene gas produced in the samples was measured on a gas chromatograph (Hewlett Packard-5890) using a “Porapak-N” column and a H2-flame ionization detector (FID). The acetylene- reductase activity of the plants was expressed as ppm C2H4 tube/hour. 2.7. Soil Moisture Soil moisture was measured with help of both TDR-method (electrical conductivity of soil) and gra- vimetric method (weighing of soil samples). The soil was taken from 0-30 cm horizons and was dried twice at 105°C. 2.8. Drying of Plant Biomass Drying of plant biomass for determination of biomass was conducted at 70°C during day. 2.9. PCR Amplification of the 16S rRNA Gene The 16S rRNA gene from nodule bacteria of Ammoden- dron conollyi (АС1-1, АС8-1, AC11, AC15, АС21), As- tragalus villossimus (AV1, AV3, AV6-1, AV8-1, AV9) and Astragalus unifoliolatus (AU3-1, AU7, AU17-1, AU30-1, AU30-2) was amplified using universal primers 1070f (59-ACGGGCGGTGTG- TAC-39) and 1392r (59-CGCCCGCCGCGCCCCG- CGCCCGGCCCGCCG- CCCCCGCCCC-ACGGGCGGTGTGTAC-39) [6]. Each PCR mixture contained the following: 10 pmol each primer, 200 M dNTPs, 1U Tag DNA polymerase, 100-200 ng genomic DNA and Taq polymerase buffer in a final reaction volume of 50 l. The DNA thermal cy- cler used for PCR amplification was programmed as follows: an initial extensive denaturation step at 94°C for 5 min; 30 cycles of 94°C for 1 min, 53°C for 1 min and 72°C for 1.5 min; and a final extension step at 72°C for 10 mi n. 2.10. Phylogenetic Analysis The complete 302-343-bp 16S rRNA gene sequences were compared with the sequences available in the GenBank database using the standard Basic Local Alignment Search Tool, BLASTn [6], at the National Center for Biotechnology Information (NCBI) (http://blast. ncbi.nlm.nih.gov/Blast.cgi). From the aligned sequences, neighbor-joining dendrograms [8] were constructed with the software MEGA version 4.0.2 [9]. The robustness of the inferred trees was evaluated by 1000 bootstrap re- samplings. 2.11. Molecular-Genetic Investigations Symbiotic NIF- and NOD-genes were determined in nodule bacteria with help of correspondent probes - Nif-probe (from Klebsiella pneumoniaea) and Nod-ABC probe (from Sinorhizobium fredii) – by means of dot-(Southern)-hybridization in nylon membranes [10]. Plasmids were visualized by the in-gel lysis method of Eckhardt [11] as modified by Priefer [12]. 2.12. Salinity Experiments Plants were irrigated by the nutritive medium containing 100 mM, 200 mM, 300 mM and 500 mM NaCl, with/without addition of 1 mM NH4NO3, up to satura- tion of potting su bstrate with the medium and its leaking from the bottom of perforated bags every 5th day. 2.13. Doubling Time of Bacterial Cells of Nodule Bacteria Nodule bacteria were grown in liquid ТУ medium at 30о C in orbital shaker at 140 rpm/min and the increase of bacterial biomass was measured on optical density (photometrically). Obtained results were treated with special computer program supplied by Sevilla University. Composition of ТУ medium was as followed (g/L): bactotryptone – 4 ; yeast extract – 2; CaCl2•6H2O – 1.3 or CaCl2•2H2O – 0.87; phosphate buffer (Na2HPO4 – 2.4;  Z. S. Shakirov et al. / Agricultural Sciences 1 (2010) 24-38 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ 27 KH2PO4 – 1.0), H2O – up to 1 L, pH – 6.8-7.0 [13]. 2.14. Light and Electron Microscopy Nodules of Ammodendron conollyi, Astragalus villos- simus, Astragalus unifoliolatus were fixed in 2.5% glu- taric aldehyde and 0.025 M potassium phosphate pH 7.2 during 4 hours at room temperature with further post-fixation in buffered 1% osmium tetraoxide. Then nodules were dehydrated by acetone and in a series of alcohols with increased alcohol concentration and after this they were embedded into araldite. Mid-sections of nodules and ultra-thin sections were done in LKB-ultra- microtome (Sweden), they were contrasted with 1% uranyl-acetate and 0.8% led citrate prepared according Reynhold and we re v iew ed in electron microscope “JEM (Jeol)-10” (Japan) at accelerating voltage 60-80 kilo- volts. For study of sections in the light microscope the sec- tions were fixed in 2% osmium tetraoxide. After this they were coloured by mixture of equal parts of fuchsin and methylene blue in 1% acetic acid. It should colour preparations during 3-5 seconds, otherwise they will be re-coloured and colour palette will change. In prepara- tions nodule plant tissues are coloured in blue colour and bacteria – in red colour. Bacterial preparations were grown in TY hard medium (as mentioned above) during 18-20 hours. After growing up the bacterial cells 3-4 times were rinsed by 0.025 M potassium phosphate buffer (pH 7.2) and centrifuged at 6,000 rpm during 20 min, and then they were fixed by 3% formalin solution (preliminarily neutralized by K2CO3 beforehand 24 hours). Before their transfer to special metallic grids the bacterial cells were fixed (contrasted) by 2% phospho- rous-tungstic acid (pH 7.2) during 15 min and with help of sprinkler the cells were dusted to metallic grids. All electron microscopic samples were viewed in electron microscope “JEM (Jeol)-10” (Japan) in Central Asian Pediatric Institute (Tashkent, Uzbekistan). 3. RESULTS 3.1. Isolation and Screening of Microbiological and Symbiotic Properties of Bacterial Isolates of Nodule Bacteria 151 bacterial isolates of nodule bacteria were isolated from perennial leguminous plants of Kyzylkum Desert. During growth on hard pea’s medium there were col- ourless, whitish, greyish, faintly-grey and yellowish- reddish, transparent, semi-transparent and muddy with different degree of slime, convex, conical and spherical colonies, but rough colonies occurred also. They differed on growth rate – fast-growing bacteria prevailed and slowly-growing isolates occurred also. The time of col- ony appearance varied in average from 2 to 4 days (in slowly-growing ones — from 4-5 days and more). Bac- terial isolates, isolated from Astragalus villossimus, were mainly represented by fast-growing bacteria, while bac- terial isolates, isolated from from Ammodendron conollyi and Astragalus unifoliolatus, were represented by equal halves of both fast-grow ing bacteria and slowly-growing bacteria. During analysis of colonies and their slime pro- duction ability when their growing on the pea’s agar it was noted that there was some correlation between ap- pearance of colonies and their size; fast-growing bacte- rial isolates formed bigger colonies with middle slime production ability. The most of colonies, that had typical (characteristic features for nodule bacteria), they were greyish-white, semi-transparent, with abundant slime production. Bacterial colonies were mainly conical and they had convex profiles with even colony edges, that probably allows to suppose that they are S-forms of nodule bacteria. But, at the same time, there were also rough colonies, semi-transparent with uneven edges that perhaps can be considered like as R-формы [14]. After study of morphological-physiological properties and selection for growth rate (appearance of colonies in Petri dishes with medium for cultivation of bacteria) about 50 perspective isolates were selected. Analysis of cells growth of selected strains showed that fast-growing and moderately-growing strains occurred among them that had doubling time from 20 to 45 min. The presence of salt in TY medium increased doubling time of strains - by в 1.4-1.9 times in the presence of 0.75 M NaCl in comparison with control and by 1.6-4.0 times in the presence of 1 M NaCl (Ta b le 1 ). At higher salt concen- trations in medium composition the differences in salt-resistance became even more expressed: AV9-1 and AV30 strains lost an ability to grow, doubling time of AV3 and AV8-1 were by more than 26 times higher than control, while AV9 strain was less sensitive to salt and it had doubling time that exceeded control one only by 2 times. At salt content 1 .75 M NaCl AU7 and AV 3 strains lost a growth ability and in the rest of strains the increase of doubling time was observed. Finally, only AC11, AC15 and AV9 strains grew at 2 M NaCl, where AV9 strain showed doubling time that exceeded control only by 3 times, but growth of C21 strain was extremely slow. All strains were able to grow in the range of tempera- tures 12-40°C, and AV9 strain – at 45°C. Except for AV9 strain, all the rest strains grew in agar at 8°C (Ta ble 2). During study of growth ability at different values of pH (from 4.0 to 11.0), many strains were able to grow ex- cept AV8-1 and AV9-1 strains that lost growth ability at pH 4.0. AС11 strain grew only starting from pH 6.0, and AU30-2 strain was able to grow on ly at pH values lower than 10.0. Testing of strains for resistance to antibiotics showed  Z. S. Shakirov et al. / Agricultural Sciences 1 (2010) 24-38 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ 28 Tabl e 1. Effect of salt on growth. Bacteria were cultivated in TY medium containing 0.04 (control), 0.75, 1.0, 1.5, 1.75 or 2.0 M NaCl. Cultures were maintained in an orbital shaker at 30oC. Isolate Control td (h)(a) 0.75 M td (h) 1.0 M td (h) 1.5 M td (h) 1.75 M td (h) 2.0 M td (h) AC8-1(1) AC11 AC15 AC21 AU7(2) AU17-1 AU30-1 AU30-2 AV1(3) AV3 AV6-1 AV8-1 AV9 AV9-1 AV30 0.82 1.31 0.72 0.68 0.84 0.84 0.77 1.08 0.81 0.76 0.93 1.27 0.75 0.84 0.81 1.29 (1.6)(b) 2.51 (1.9) 1.06 (1.5) 1.00 (1.5) 1.29 (1.5) 1.46 (1.7) 1.08 (1.4) 1.66 (1.5) 1.31 (1.6) 1.44 (1.9) 1.64 (1.8) 1.98 (1.6) 1.13 (1.5) 1.44 (1.7) 1.29 (1.6) 1.72 (2.1) 3.17 (2.4) 1.72 (2.4) 1.51 (2.2) 2.01 (2.4) 2.08 (2.5) 2.52 (3.3) 2.6 (2.4) 1.75 (2.2) 2.94 (3.9) 2.32 (2.5) 5.04 (4.0) 1.20 (1.6) 3.16 (3.8) 2.12 (2.6) 4.27 (5.2) 10.33 (7.9) 5.45 (7.6) 5.12 (7.5) 15.57 (18.5) 8.85 (10.5) 11.92 (15.5) 12.04 (11.1) 7.46 (9.2) 20.19 (26.6) 7.04 (7.6) 37.62 (29.6) 1.50 (2.0) NG NG 11.58 (14.1) 13.68 (10.4) 19.11 (26.5) 10.38 (15.3) NG 33.44 (39.8) 42.13 (54.7) 45.60 (42.2) 24.27 (30.0) NG 11.04 (11.9) 150.48 (118.5) NG NG NG(c ) 19.8 (15.1) 66.88 (92.9) NG NG NG NG NG NG NG NG NG 2.26 (3.0) NG NG (a) Doubling time; (b) td: td of control; (c) No detectable growth. AС(1) – nodule bacteria isolated from Ammodendron connollyi nodules; AU(2) – nodule bacteria isolated from Astragalus unifo liolatus nodules; AV(3) – nodule bacteria isolated from Astragalus villossimus nodules. Table 2. Relevant characteristics of the isolates described in previous. Isolate td(a) (h) NaCl limit(b) (M) Plasmid profile (kbp) Antibiotic Resistance Growth temp., oC Growth pH Melanin pro- duction Colony Type AC8-1 AC11 AC15 AC21 AU7 AU17-1 AU30-1 AU30-2 AV1 AV3 AV6-1 AV8-1 AV9 AV9-1 AV30 0.82 1.31 0.72 0.68 0.84 0.84 0.77 1.08 0.81 0.76 0.93 1.27 0.75 0.84 0.81 1.75 2.00 2.00 1.75 1.50 1.75 1.75 1.75 1.75 1.50 1.75 1.75 2.00 1.00 1.00 370, 515 370, 515 118, 370, 515 370, 515 370, 515 370, 515 370, 515 370, 515 118, 370, 515 118, 370, 515 370, 515 118, 370, 515 118, 370, 515 118, 370, 515 370, 515 Amp Cm Amp Amp Amp, Km, Str, Tc Amp, Tc Amp, Km, Str, Tc Amp, Tc Amp, Tc Amp, Tc Amp, Cm Amp, Cm, Km, Str 8-40 8-40 8-40 8-40 8-40 8-40 8-40 8-40 8-40 8-40 8-40 8-40 12-40 8-40 8-40 4-11 6-10 4-11 4-11 4-11 4-11 4-11 4-10 4-11 4-11 4-11 5-11 4-11 5-11 4-11 – weak – – + + +++ + + – + – – – ++ I II I I I I III IV I II I I V IV VI (a) Doubling time in TY liquid medium at 30°C. (b) Highest concentration of NaCl in TY liquid medium at which growth was observed (see Table 1). that amount of colonies decreased somewhat in the presence of chloramphenicol (and in some cases in the presence of other antib iotics), but in all strains the resis- tance to antibiotics was observed. The ability to grow in dishes with ampicillin was the most widespread in all strains, it was followed further with ability to grow in the presence of tetracycline. AU7 and AU30-1 strains grew in the presence of four of the antib iotics (ampicillin , kanamycin, streptomycin, tetracycline, Table 2). During identification of Nod- and Nif-genes in se- lected bacterial isolates with help of dot-hybridization and specific probes the positive hybridization reflexes were obtained. In this study of selected bacterial isolates for salt-resistance about 30 perspective strains of nodule bacteria were revealed which were able to grow in the presence of 1 M NaCl and more (up to 2 M NaCl). Dur- ing study of plasmid profile of strains from 2 (in nodule bacteria strains of Ammodendron conollyi and Astraga-  Z. S. Shakirov et al. / Agricultural Sciences 1 (2010) 24-38 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ 29 lus unifoliolatus) to 3 megaplasmids (in Astragalus vil- lossimus nodule bacteria strains) with molecular masses 118, 370 and 515 kB correspondingly (Table 2). Melanin formation is a phenotypic feature which can also help in identification of closely-related rhizobia. Screening of isolates on this feature was conducted in a way of detection of diffusing dark-brown pigment that is produced with cells grown in Petri dishes containing TY medium with added tyrosine and copper [15]. The fast and abundant formation of melanin was observed in U30-1 strain and almost the same like it took place in V30 strain; lesser formation of melanin was observed in AU7, AU17-1, AU30-2, AV1 and AV6-1 strains. The rest of strains did not produce diffusing dark pigment and they were considered lik e as Mel–strains (Table 2). All strains grew in Luria-Bertani medium [12]. Test- ing of their growth in TY medium showed at least 6 dif- ferent ty p es of colonies: Type 1: Middle size, round, convex, light and white. Type 2: Young colonies like as in type 1, they b ecome yellow later. Type 3: Young colonies like as in type 1, they b ecome yellow-brown later. Type 4: As type 1 , but smaller in sizes. Type 5: Middle size, round, dim and curly. Type 6: Young colonies like as in type 1, later they become darker than type 3. All above-mentioned investigations have confirmed that the selected isolates are nitrogen-fixing nodule bac- teria which are able in the wide ranges of pH and tem- peratures. 3.2. Germination of Plant Seeds, Nodulation Test (Direct Inoculation and Cross-Inoculation) After study of microbiological properties of bacterial isolates of nodule bacteria next stage of investigations was a study of their symbiotic properties. It was neces- sary for this to conduct a search of optimal conditions for seed germination of wild desert leguminous plants (Ammodendron conollyi tree, Astragalus unifoliolatus and Astragalus villossimus shrubs), their growth and development as well as nodulation process of these plants roots upon inoculation with selected isolates (strains) of nodule bacter ia. No prolonged treatment of plant seeds with concen- trated sulphuric acid (Ammomodendron conollyi during 3 hours) nor their moistening in water during several days did not lead to increase of seed germination output of desert xerophyte plants higher than 10-15% (in natu- ral conditions 5-10% output of seed germination is ob- served) [16]. In literature there are data about efficiency of scarification of only hygrophilous foot-hill species of acacia [17]. As a result of search the combination of treatment with sulphuric acid and scarification of root part of seeds was found when almost total (about 90-95%) seed ger- mination of xerophyte desert wild leguminous plants in sterile conditions was obtained. The tasks of investiga- tions included growing up of seedlings up to mature state and study of root nodul ation of t he grown up plant s. First of all it was necessary to obtain normal vegeta- tion of plants (that would be favourable for their nodula- tion). During study of nodulation it was important to clear up two matters: 1) to determine the belonging (biological test) of iso- lated nodule bacteria to their maternal host-plants and, thus, to confirm their originality (“direct inoculation”); 2) to examine a host specificity of nodule bacteria to- wards other (non-maternal) host plants (“cross-inocu- lation”). During search of optimal conditions for plants grow- ing up the following types of potting substrates were used: 1) Microvegetation experiments: Strips of filter paper in agronomic tubes (60 ml) with ascending flow of nutritive solution for plants in sterile conditions. Veg etation experiments: 2) Sterile vermiculite impregnated with nutritive solu- tion for plants in pots (0.7 L) in greenhouse. 3) “Light structure” in bags (sterile, 2 L) – 1 part peat: 1 part vermiculite: 1 part soil: 2 parts sand. 4) “Structure close to natural conditions” (in bags) – 3 parts sand: 1 part vermiculite. 5) Natural conditions (fine-grained sand). 6) Field conditions (soil). In microvegetation experiments during growing and inoculation of plants seedlings with nodule bacteria on filter paper strips after 1 month both suppression and stoppage of their growth and development were ob- served. In vegetation experiments at growing on the 2nd type of substrate the height of plants was good, but after 45 days from the starting of experiment the stoppage of growth and development was observed also. In both pre- vious experiments there was no nodulation. Probably, plants being xerophytes and in natural conditions having only low moisture in the upper soil horizon (0-30 cm), they did not “manage” with moisture excess in two pre- vious variants, because the presence of available mois- ture exceeded the transpiration of water by desert plants. In this connection it was necessary to create the condi- tions close to natural co nditions. To achieve this aim the 3rd type with sand prevalence was taken together with “light” potting structures (vermiculite wou ld retain more moisture) which would accelerate drainage and removal of water from potting substrate, during this the moisture was supported within range 15-20 %. After 2.5 months (from moment of seedling planting up to the mature state) of growing in the 3rd type the height and development of plants corresponded to 1-2 yearly plants (15-30 cm) that were observed in nature. But, there was not nodulation  Z. S. Shakirov et al. / Agricultural Sciences 1 (2010) 24-38 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ 30 (there were only single nodules). During use of the 4th type of substrate, where sand was dominating part of substrate and conditions were close to natural ones, the intense growth and development of plants was observed. After 2 months of growing the nodulation was found in all plants, and thus, the search of optimal conditions for total nodulation finally has crowned with success. But, nodulation after inoculation with Azorhizobium cauli- nodans ORS571 and CXM1 was not observed. Further, all experiments on study of plant nodulation were con- ducted in sand, the 5th type of potting substrate. As it is seen from Figure 1(a) and Ta b le 3 the nod- ules of Ammodendron cono llyi, formed after inoculation, were pink and large (up to 7 mm in diameter). In vari- ants of Ammodendron conollyi plant inoculation the av- erage nodule number per plant was 1-5 and their bio- mass comprised 4.5-27.5 mg. At the same time the ni- trogen fixation intensity did not influence essentially on the height of shoot part and length of roots. High nitro- gen-fixing activity was recorded in direct inoculation with own nodule bacteria, isolated from Ammodendron conollyi (AC1-1, AC2, AC12-1, AC18-1, AC21). The cross-inoculation of Ammodendron conollyi plants by nodule bacteria isolated from Astragalus villossimus, has led to visibly low nitrogen-fixing activity in comparison with results of direct inoculation of the same plants with own nodule bacteria. It should be noted that cross- inoculation of Astragalus villossimus plants by Am- modendron conollyi nodule bacteria (AC8-1, AC11, AC15) gave comparatively higher values of nitrogen fixation than their direct inoculation with own Astraga- lus villossimus nodule bacteria, during this there was an increase of nodules amount in Astragalus villossimus plants. As it is seen from Table 3, AС1-1 strain was more specific towards maternal Ammodendron conollyi plants during direct inoculation than Astragalus villos- simus plants during their cross-inoculation. It is interest- ing to note that, on the whole, nitrogen-fixing activities of Ammodendron conollyi plants inoculated with direct inoculation and cross-inoculation were in several times higher than nitrogen-fixing activities of inoculated As- tragalus villossimus plants and Astragalus unifoliolatus plants. Highly-efficient symbiosis (under term “symbio- sis efficiency” is meant the increase of biomass of in- oculated plants in comparison with biomass of non-in- oculated plants) in Ammodendron conollyi plants was observed during inoculation with such nodule bacteria as AC1-1, AC8-1, AC11, AC12-1, AC18-1, AV1, AV8-1, AV9-1, AV30, when efficiency (biomass) of shoot part increased from 143.2 to 168.6 % in comparison with control. At observation of Astragalus villossimus plants (Table 3) during direct inoculation with AV1, AV2, AV3, AV9, AV26-1 and cross-inoculation by AC11, AC15, AC21 and AU30-1 numerous nodules were formed (av- erage nodule number 10-29 and 1-2.5 mm size, their colour varied from weakly-reddish to white and greenish) (Figure 1(b)). High nitrogen-fixing activ ity of symbiosis of inoculated Astragalus villossimus plants was observed during direct inoculation with their own nodule bacteria AV2, AV3, AV9, AV30 and AV36-1. At the same time during cross-inoculation of Astragalus v illossi mus plan ts with Astragalus unifoliolatus nodule bacteria the com- paratively low nitrogen-fix ing activity was obtained th an during direct inoculation. Efficiency from 121.5 to 128.4% was obtained during inoculation with AV1, AV3, AV8-1, AV9 and AC8-1 strains. During inoculation of Astragalus unifoliolatus plants the highest nodule number – up to 41 – was recorded, nodules were numerous and small (0.5-1.5 mm) (Figure 1(c)). Astragalus unifoliolatus plants, inoculated with their own nodule bacteria (direct inoculation) had con- siderably low nitrogen fixation than under cross-inocu- lation by nodule bacteria of Astragalus villossimus. Hi gh efficiency of Astragalus unifoliolatus symbiosis was observed at direct inoculation with AU23, AU28, AU30-1 and cross-inoculation by AV1, AV2, AV6-1, AV8-1 and AV9-1 strains. 3.3. Phylogenetic Analysis of the 16S rRNA Gene of Nodule Bacteria Strains For taxonomic identification and creation of phyloge- netic tree 15 salt-tolerant bacteria were re-isolated again from nodules formed on the roots of desert leguminous plants which were grown in sterile vegetation experi- ment. Results of BLAST-analysis for sequence of 16S rRNA gene of bacteria showed that studied bacteria were related to Alphaproteobacteria and Betaproteobacteria classes. The nucleotide sequence of 16S rRNA gene of АС1-1, АС8-1, АС21, AV1, AV3, AU3-1, AV8-1, AV9 and AU30-1 strains matched by 96-97% together with analogous genes of Rhizobium sp. GGNM 66; the genes of AC15, AU 17-1 , A U30 -2 and AU 7 bacteria w er e id en- tical by 89-97% to nucleotide sequ ences of Burkholderia cepacia NBRAJG97 species and the genes of AC11 and AV6-1 strains were identical by 95% and 98% to genes of Achromobacter xylosoxidans species. On phyloge- netic tree the studied bacteria could be grouped into 4 clusters (Figure 2): AU7 strain (Astragalus unifoliolatus) formed the 1st cluster, the 2nd cluster included AC11 (Ammodendron conollyi) and AV6-1 (Astragalus villos- simus) strains, the 3rd group was created by AC15 (Am- modendron conollyi), AU17-1 and AU30-2 (Astragalus unifoliolatus) strains, and, at last, the 4th big group was formed by АС1-1, АС8-1, АС21 (Ammodendron co- nollyi); AV1, AV3, AV8-1, AV9 (Astragalus villossimus), Astragalus unifoliolatus (AU3-1; AU30 -1) n odule bacte- ria strains. It may conclude from obtained results that nucleotide sequences of 16S rRNA gene of studied bac- teria that were highly identical between themselves within group and also bacteria isolated from each cor-  Z. S. Shakirov et al. / Agricultural Sciences 1 (2010) 24-38 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ 31 (a) (b) (c) Figure 1. Nodulation of desert leguminous perennial plants: (a) Inocu- lation of Ammodendron сonollyi plant with AС11 strain; (b) Inoculation of Astragalus villossimus plant with AV8-1 strain; (c) Inoculation of Astragalus unifoliolatus plant with AU30-2 strain. Figure 2. Phylogenetic tree based on the 16S rRNA gene strains of nodule bacteria from desert leguminous perennial plants. The branching pattern was produced by the neighbour-joining method. The GenBank accession numbers for the sequences used are indicated in parentheses. Symbionts of desert legume plants are shown in bold type. 0.02  Z. S. Shakirov et al. / Agricultural Sciences 1 (2010) 24-38 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ 32 respondent leguminous host plant were related to both Alpha class and Bet aproteobacteria class. 3.4. Study of Influence of Drought and Salinity on Symbiosis of Desert Leguminous Plants Experiment on study of influence of drought on xero- phyte desert leguminous plants was carried out in greenhouse in soil (field conditions, the 6th type) during 2.5 months. Plants, inoculated by correspondent nodule bacteria (AC21-1, AV1, AU30-1), were planted in rows into the soil and the first irrigation was done to equalize of soil moisture under the plants and one week later the soil moisture was within range 15-20%. Afterwards the further irrigation of soil (the area which did not include the square which was not occupied by plants) was done from one side across the rows of planted plants. For whole period of plants gr owing there w ere 3 (three) such irrigations in order to obtain a gradient of soil moisture. The gradient of so il moisture was obtained due to that irrigation was conducted from the one side (side of the first plants from each row), but on the contrary side of the last plants (according to order of row) there were constantly dried dehydrated soils. As a result the gradi- ent of soil moisture in the range from 4 to 7% was ob- tained. During growing of plants the gradient of plants height – from the side of conducted irrigation (the be- ginning of row) to the contrary side (end of row) – was obtained. Thus, model system for study of plants drought resistance was created. From Figure 3 it is seen that from the moment of starting of experiment up to plants harvesting the gradient of soil moisture comprised from 3.8 to 6.41%. The height and biomass of inoculated plants also decreased during this. Maximal height and biomass of inoculated plants of Ammodendron conollyi were 21 cm and 2320 mg (at 6.41% soil moisture), but at 3.8% moisture the height decreased by 4 times (up to 4.5 cm) and biomass - by 11 times (203 mg). Analogous situation was observed in Astragalus unifoliolatus. For mation of nodules in this experiment was not observed. As our experiments showed, even under insignificant increase of soil moisture from 3.8 to 4.32% (by 0.52%), the biomass of all studied plants increased by 2-3 times, and under further increase from 3.8 to 6.41% (by 2.61%) the biomass of all plants increased by 10-15 times. At the same time it should be noted that under increase of moisture from 5.48 to 6.41% (by 0.93%) the biomass of all plants increased only by 1.5 times. From here one may conclude that 6.41% of soil moisture for these plants is emergent value which is close to stationary level of moisture that is optimal for growth and devel- opment of these plants (Figure 3). Analogous results were received for plants Astragalus villossimus as well. For conducting of exp eriment on salin ity 20-da ily sap- lings of these plants were taken, that further were sub- jected to salt stress under their irrigation with nutritive medium for plants that contained 100 mM, 200 mM, 300 mM and 500 mM NaCl for each irrigation. Experiment was conducted in two modifications: a) without added nitrogen, and b) on the background of added nitrogen (1 mM NH4NO3). Plant inoculation was done with the cor- respondent nodule bacteria during seedling plantings into the bags: Ammodendron conollyi – by AC8-1 strain, Astragalus villossimus – by AV9 strain and Astragalus unifoliolatus – by AU30-1 strain. As it is seen from Fig- ure 4, under salinity treatments with 100 and 200 mM NaCl both in the absence and in the presence of added nitrogen (1 mM NH4NO 3) as a sole nitrogen source, the survival of Ammodendron conollyi inoculated plants remained almost entirely – 75-100%, and average green biomass of 1 plant increased by 16.2% in comparison with control. Further increase of NaCl concentration up 3,8 4,32 4,86 5,48 6,41 0 200 400 600 800 1000 1200 Dry biomass, mg Moistu re, % Astraga lus un ifoliola tus Ammodendron conollyi Astraga lus villossimus Figure 3. The influence of drought on dry biomass of perennial desert leguminous plants. 6.41 5.48 4.86 4.32 3.8  Z. S. Shakirov et al. / Agricultural Sciences 1 (2010) 24-38 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ 33 to 500 mM negatively influenced on yieldness and sur- vival of Ammodendron conollyi plants under both pres- ence and in the absence of nitrogen source, the survival decreased up to 25 and 50% accordingly (Figure 4). Study of salinity influence on Astragalus species showed that at 100 mM NaCl the survival of Astragalus villossimus was 46.1% and for Astragalus unifoliolatus – 66.7%. It is interesting to note that in spite to decrease of survival of Astragalus villossimus in the presence 100 mM NaCl, the average green biomass (yieldness) of one plant increased by 40% in comparison with control, and at 300 mM NaCl the yieldness got equalized with con- trol. At salinity from 100 to 500 mM NaCl both in the absence and in the presence of nitrogen the survival of Astragalus villossimus and Astragalus unifoliolatus regularly decreased. Nodulation was observed in Am- modendron conollyi in amount 1-2 nodules /1 plant at 100 mM NaCl, while in Astragalus villossimus (11.0 - 0.25 nodules/ plant) – up to 200 mM NaCl and for Astragalus unifoliolatus (12.5 – 1 nodules/1 plant) – up to 300 mM NaCl. Individual plants of Ammodendron conollyi and As- tragalus unifoliolatus were resistant to 100-200 mM NaCl, these salinity values did not influence practically on normal growth and development, while in Astragalus villossimus such concentration of salt-resistance was up to 100 mM NaCl. This testified about individual adapta- tion of studied plants subjected to salt stress. Further study of influence of NaCl on growth and development of Ammodendron conollyi, Astragalus villossimus and Astragalus unifolilatus showed that salinity up to 500 mM considerably decreased yieldness and survival of plants, but it also indicated to the fact that th e last concentration of NaCl was not critical value for salt-resistance of stud- ied plants. 3.5. Light/Electron Microscopy of Nodule Bacteria and Transections of Nodules During electron-microscopic study of typical samples of 100 200 300 500 0 10 20 30 40 50 60 70 80 90 100 Survavility, % Salinity, mM NaCl Ammodendron con ollyi Astragalus unifoliolatus Astragalus villossimus Figure 4. The influence of salinity on survival of perennial desert legu- minous plants (in the presence of nitrogen source). (a) (b) (c) Figure 5. The desert nodule bacteria in free-living state: (a) – Astragalus villossimus’s nodule bacterium AV1 strain (magnification 5,000); (b) – Ammodendron conollyi’s nodule bacterium AC8-1 strain (magnification 5,000); (c) – Astragalus unifoliolatus’s nodule bacterium AU30-1 strain (magnification 6,000). 0.17 mk 0.17 mkm 0.2 mk  Z. S. Shakirov et al. / Agricultural Sciences 1 (2010) 24-38 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ 34 selected samples of nodule bacteria the unipolar mono- trichous mobile rod-shaped forms of bacterial cells were revealed (Figure 5). Under differential colouring of nodule transections (preparations of light microscopy) of desert leguminous plant – Ammodendron conollyi tree or “sandy acacia”, Astragalus villossimus and Astragalus unifoliolatus shrubs it was possible to observe the simi- larity in organization and deve l opm ent of speci fi c cel l ular structures, and also some difference between them. So, for example, under inoculation of Astragalus villossimus plants with AV1 strain it took place a wide and intensive colonization of internal plant cells of nodule (located to the nodule’s center) by bacterial cells of this strain. As it is shown in Figure 6, after branchy (coloured in pink colour) bacterial net in o uter covering cells of nodule th e zone of formation and development of centres of infec- tion threads followed further which finally (at least) led to formation of completely colonized (densely-colonized) deep internal plant cells of Astragalus villossimus nodule by bacterial cells of nodule bacteria. Under higher amplifications it was possible to see a formation of “stairs-like” structures that seemingly are intracellular infection threads from which nodule bacteria further released into plant nodule cells and are trans- formed into non-mobile symbiotic forms – “bacteroids” within plant cells. It is important to note that infection threads move through and develop along perimeter of plant cells, i.e. along membranes of internal cells of plant nodule cells [18,19]. The microscopy results concerning to influence of salinity effect on the formed symbiosis showed that number of nodule bacteria cells internally- colonizing plant nodule decreased already at 100 mM NaCl in comparison with control (Figure 6(g)), and un- der the further increase of NaCl salinity concentration u p to 200 mM the nodule bacteria colonization of nodule internal spaces reduced practically (Figure 6(h)). Figure 6. Light microscopy of nodule transection of Astragalus villossimus plant, inoculated with its own Astragalus villossimus AV1 nodule bacteria strain: (a) – General picture of longitudinal part of nodule (magnification 148); (b) – Part of general picture taken under higher magnification (magni- fication x 315); not only central plant cells of nodule densely-colonized by bacterial cells, but also outer covering plant cells of nodule are visible, the zone of formation and development of infection threads centres follows further; (c) – Higher magnification of the same part (magnification 530); the outer bacterial net on plant bark (cortical) part is visible; (d) and (e) – Higher magnification of the infection thread zone (magnification 974); “stairs-like” intracellular infection threads are visible (they are shown by arrows) and perhaps some elements of inter-cellular infection threads (spherical-like black-red structures) ; (f) – The highest magnification (mag- nification 2835); development and distribution of thick infection threads along plant membranes of nodule and through nodule cells with releasing bacterial cells of nodule bacteria; (g)-(h) – The light microscopy of Astra- galus villossimus nodule section of plant grown at salinity 100 mM NaCl (g) and 200 mM NaCl (h). (a) (b) (c) (d) (e) (f) (g) (h)  Z. S. Shakirov et al. / Agricultural Sciences 1 (2010) 24-38 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ 35 For study and observations of more fine structural prop- erties of legume-rhizobial symbiosis of desert legumi- nous plants and selected nodule bacteria the elec- tron-microscopic preparations were used. In the whole, bacteroids were represented by polymorphic structural forms – from globular (spherical) up to mace-like and many other random-shaped forms (Figures 7(a)-(c)). As to infection threads, the use of electron microscopy en- abled to observe them more in detail. Infection threads formed in both inter-cellular free spaces between plant nodule cells (Figure s 7(d) and (e)) and within plant nodule cells (Figures 7(f)-(i)). The form of infection threads also varied – from roundish to rectangular (Figures 7(d)-(i)). Thus, as a result of legume-rhizobial symbiosis the structural reconstructions of both nodule bacteria and legume host plant took place (occurred). Under this not only penetration of bacteria into plant nodule cells and their colonization by bacteria occurs, but also transfor ma- tion (transition) of free-living uniform mobile cells of nodule bacteria into symbiotic polymorphic non-motile forms (bacteroids). Penetration of nodule bacteria into correspondent host plant is realized through special struc- ture – infection thread – that moves forward and distribu- tion of which occurs through inter-cellular free spaces between plant nodule cells within thei r volum es [20,21]. (a) (b) (c) (d) (e) (f) (g) (h) (i) Figure 7. Electron microscopy of nodules sections for desert legume plant Ammodendron conollyi: (a) (AV1), (b) (AC8-1 ) and (c) (AU30-1) – poly- morphism of bacteroids, (d) (AV1) and (e) (AC8-1 ) – intercellular infec- tion threads, (f)-(i) – intracellular infection threads. Localization of infec- tion threads is indicated by arrows. 0.7 mkm 0.5 mkm 0.5 mkm 2 mkm 2 mkm 2.5 mkm 2 mkm 2 mkm 2.5 mkm  Z. S. Shakirov et al. / Agricultural Sciences 1 (2010) 24-38 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ 36 4. DISCUSSION From obtained results one can conclude that there was correlation between nitrogen-fixing activity and effi- ciency of symbiosis by nodule bacteria AС1-1, AC12-1, AC18-1, AC8-1, AV1, AV3, AV9, AU23 and AU30-1. On the basis of this data one can estimate the specificity of nodule bacteria towar ds legum inous host plant. Duri ng this it is necessary to consider both direct host nodula- tion specificity (belonging of nodule bacterium to defi- nite maternal host plant from which this bacterium originates from) and wide host/cross host nodulation specificity (ability of this bacterium to form nitro- gen-fixing symbiosis with other non-maternal host plants). So, under analysis of nitrogen-fixing activity and nodulation in Ta ble 3 one can note that nodule bacteria of Ammodendron conollyi have the highest host specific- ity towards both their own maternal host plant and also to other host plants (wide host specificity), and in this connection they are the most perspective inoculants for obtaining of efficient symbiosis in perennial desert plants. Nodule bacteria of Astragalus villossimus have the middle specificities towards to its matern al ho st plan t and other plants. Nodule bacteria of Astragalus unifoli- olatus have lower specificity towards own maternal host plant and other plants. Thus, one can suppose that there is some regularity between attaching of nodule bacteria to concrete habitat and their specificity to come into symbiosis with different host plants. Since the collection of nodules of Ammodendron conollyi (they were sam- pled from Kara-Kata, Kyzylkum Desert), Astragalus villossimus (they were taken from Kyzylkum Desert Biostation) and Astragalus unifoliolatus (they were gathered from Yamandjar, Kyzylkum Desert) was con- ducted in separate places which were distant from each other on the distance 80-100 km, probably there were separate populations of local nodule bacteria although during expeditions the common habitats of Ammoden- dron conollyi with Astragalus villossimus as well as the areas of common inhabiting of Ammodendron conollyi with Astragalus unifoliolatus occurred. But it was nec- essary to collect nodules in places distant from each other in order to have distinct groups of nodule bacteria for their study. If Ammodendron conollyi plant can grow in pure sands and semi-sand soils, then Astragalus vil- lossimus plant grows in poorer dried soils and semi-sand soils, while Astragalus unifoliolatus plant - mainly in pure sands. Therefore, Astragalus unifoliolatus plant has comparatively narrower area of habitat and maybe its low specificity to other plants is determined with this feature. According to observations of some authors, Astraga- lus genera form nodules by the 2nd year of their vegeta- tion in natural conditions. At the same time, from expe- ditional observations of both our botanists and us, the nodules and roots of Ammodendron conollyi were brown and black colors correspondently in natural conditions (in our microvegetation experiments after 2.5 months of growth the initially pink-colored fragile nodules and white roots became dark-brown color; the appearance and development of 2.5 monthly plants of Ammodendron conollyi and Astragalus looked like as 1-2 yearly natur al plants). Probably, due to increase of requirements in ni- trogen by the 2nd year in natural conditions the plants begin to form nodules that are necessary for intense growth and development of young plants as well as their adaptation to surroun ding environ mental conditions. Th e studied strains of desert rhizobia are fast-growing rhizo- bia, but they are slower than Azorhizobium caulinodans (its generation time is 40-45 min [22]. They have 2 similar megalpasmids and some of them have additional 3rd megalpasmid, they do not form nodules in alfalfa. Microbiological and molecular-genetic characteristics of rhizobial symbiont of Sesbania revealed the availability of 2 megaplasmids (300 and 450 MDa), generation (doubling-time) time 2 hours 15 min and capability for growth at 2% NaCl, sensitivity to antibiotics and capa- bility for nodule formation in Vigna ung uiculata [23]. In literature the rhizobia isolated from Astragalus are re- lated to Mezorhizobium [24-26], but at the same time the authors noted that the most of studied rhizobia differed on DNA-homological groups from all known species of rhizobia and one subgroup was a unique genetic line [27]. Other authors on the basis of fingerpriniting noted that rhizobia of Astragalus adsurgens were related to Agrobacterium, Mezorhizobium, Rhizobium and Si- norhizobium genera [28]. During study of rhizobia (Si- norhizobium, Mezorhizobium), which cause nodulation in leguminous tress of Africa and South America, it was noted that there were both similarity and differences in symbiotic determinants of rhizobia [29]. In our investi- gations it was determined that nucleotide sequence of 16S rDNA gene of АС1-1, АС8-1, АС21, AV1, AV3, AU3-1, AV8-1, AV9 and AU30-1 nodule bacteria strains matched by 96-97% together with analogous genes of Rhizobium sp. GGNM 66 species; the genes of AC15; AU17-1; AU30-2 and AU7 89-97% were identical to genes of Burkholderia cepacia NBRAJG97 species and genes of AC11 and AV6-1 strains were identical by 95% and 98% to the genes from Achromobacter xylosoxidans species. In whole, according to phylogenetic tree the evolutionary origin of studied bacteria is close each other and they belong to both Alpha and Betaproteobac- teria classes. Thus, as a result of conducted work, the conditions for study of growth and nodulation of desert leguminous perennial plants have been chosen and found, the inter- actions between nodule bacteria and perennial xerophyte tree and shrubs have been determined. On the basis of this it may conclude that model system of efficient ni- trogen-fixing symbiosis “nodule bacterium-perennial 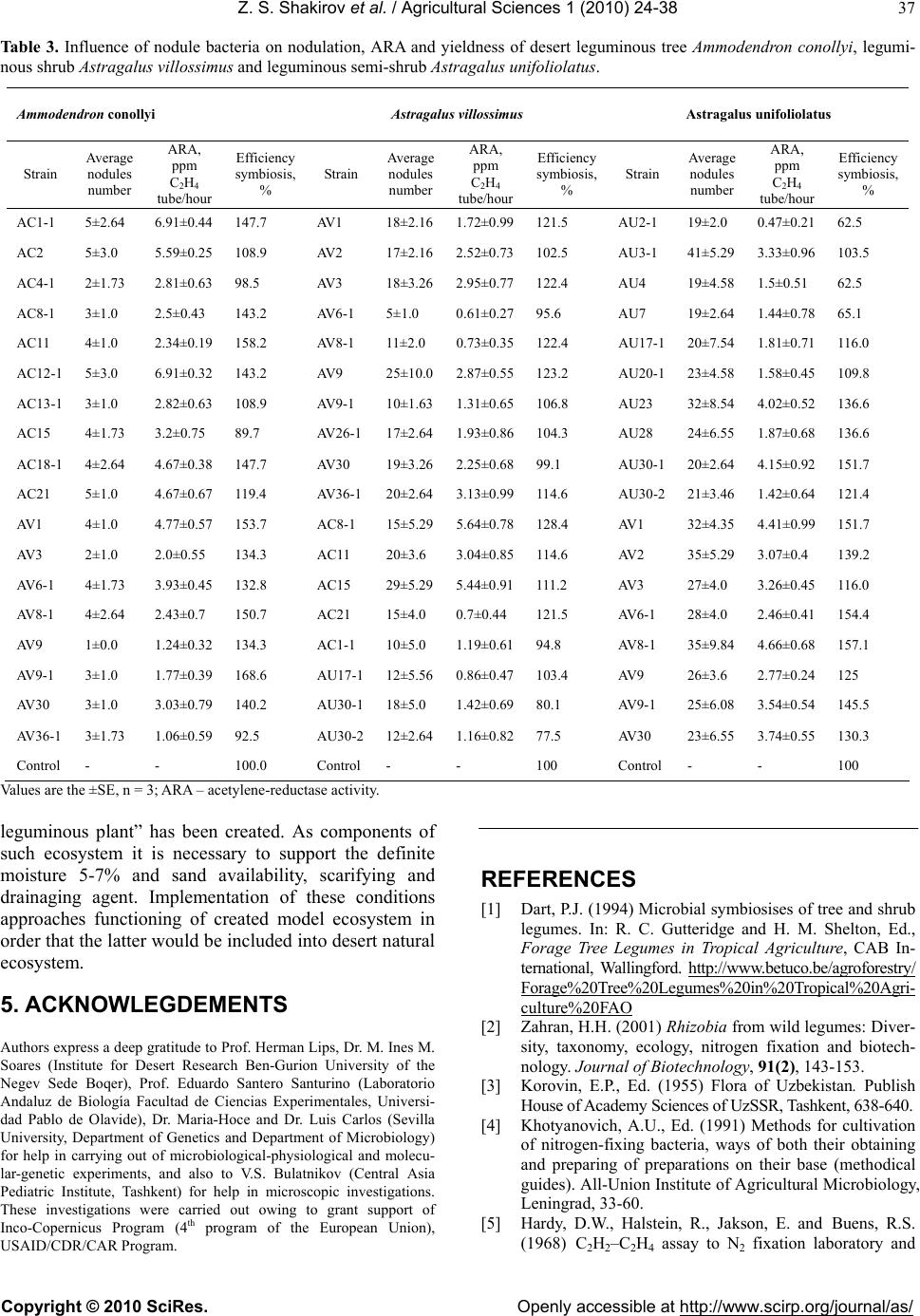 Z. S. Shakirov et al. / Agricultural Sciences 1 (2010) 24-38 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ 37 Table 3. Influence of nodule bacteria on nodulation, ARA and yieldness of desert leguminous tree Ammodendron conollyi, legumi- nous shrub Astragalus villossimus and leguminous semi-shrub Astragalus unifoliolatus. Ammodendron conollyi Astragalus villossimus Astragalus u nifoliola tus Strain Average nodules number ARA, ppm C2H4 tube/hour Efficiency symbiosis, % Strain Average nodules number ARA, ppm C2H4 tube/hour Efficiency symbiosis, % Strain Average nodules number ARA, ppm C2H4 tube/hour Efficiency symbiosis, % AC1-1 5±2.64 6.91±0.44 147.7 AV1 18±2.161.72±0.99121.5 AU2-1 19±2.0 0.47±0.21 62.5 AC2 5±3.0 5.59±0.25 108.9 AV2 17±2.162.52±0.73102.5 AU3-1 41±5.29 3.33±0.96 103.5 AC4-1 2±1.73 2.81±0.63 98.5 AV3 18±3.262.95±0.77122.4 AU4 19±4.58 1.5±0.51 62.5 AC8-1 3±1.0 2.5±0.43 143.2 AV6-1 5±1.0 0.61±0.2795.6 AU7 19±2.64 1.44±0.78 65.1 AC11 4±1.0 2.34±0.19 158.2 AV8-1 11±2.0 0.73±0.35122.4 AU17-120±7.54 1.81±0.71 116.0 AC12-1 5±3.0 6.91±0.32 143.2 AV9 25±10.02.87±0.55123.2 AU20-123±4.58 1.58±0.45 109.8 AC13-1 3±1.0 2.82±0.63 108.9 AV9-1 10±1.631.31±0.65106.8 AU23 32±8.54 4.02±0.52 136.6 AC15 4±1.73 3.2±0.75 89.7 AV26-117±2.641.93±0.86104.3 AU28 24±6.55 1.87±0.68 136.6 AC18-1 4±2.64 4.67±0.38 147.7 AV30 19±3.262.25±0.6899.1 AU30-120±2.64 4.15±0.92 151.7 AC21 5±1.0 4.67±0.67 119.4 AV36-120±2.643.13±0.99114.6 AU30-221±3.46 1.42±0.64 121.4 AV1 4±1.0 4.77±0.57 153.7 AC8-1 15±5.295.64±0.78128.4 AV1 32±4.35 4.41±0.99 151.7 AV3 2±1.0 2.0±0.55 134.3 AC11 20±3.6 3.04±0.85114.6 AV2 35±5.29 3.07±0.4 139.2 AV6-1 4±1.73 3.93±0.45 132.8 AC15 29±5.295.44±0.91111.2 AV3 27±4.0 3.26±0.45 116.0 AV8-1 4±2.64 2.43±0.7 150.7 AC21 15±4.0 0.7±0.44 121.5 AV6-1 28±4.0 2.46±0.41 154.4 AV9 1±0.0 1.24±0.32 134.3 AC1-1 10±5.0 1.19±0.6194.8 AV8-1 35±9.84 4.66±0.68 157.1 AV9-1 3±1.0 1.77±0.39 168.6 AU17-112±5.560.86±0.47103.4 AV9 26±3.6 2.77±0.24 125 AV30 3±1.0 3.03±0.79 140.2 AU30-118±5.0 1.42±0.6980.1 AV9-1 25±6.08 3.54±0.54 145.5 AV36-1 3±1.73 1.06±0.59 92.5 AU30-212±2.641.16±0.8277.5 AV30 23±6.55 3.74±0.55 130.3 Control - - 100.0 Control - - 100 Control - - 100 Values are the ±SE, n = 3; ARA – acetylene-reductase activity. leguminous plant” has been created. As components of such ecosystem it is necessary to support the definite moisture 5-7% and sand availability, scarifying and drainaging agent. Implementation of these conditions approaches functioning of created model ecosystem in order that the latter would be included into desert natural ecosystem. 5. ACKNOWLEGDEMENTS Authors express a deep gratitude to Prof. Herman Lips, Dr. M. Ines M. Soares (Institute for Desert Research Ben-Gurion University of the Negev Sede Boqer), Prof. Eduardo Santero Santurino (Laboratorio Andaluz de Biología Facultad de Ciencias Experimentales, Universi- dad Pablo de Olavide), Dr. Maria-Hoce and Dr. Luis Carlos (Sevilla University, Department of Genetics and Department of Microbiology) for help in carrying out of microbiological-physiological and molecu- lar-genetic experiments, and also to V.S. Bulatnikov (Central Asia Pediatric Institute, Tashkent) for help in microscopic investigations. These investigations were carried out owing to grant support of Inco-Copernicus Program (4th program of the European Union), USAID/CDR/CAR Program. REFERENCES [1] Dart, P.J. (1994) Microbial symbiosises of tree and shrub legumes. In: R. C. Gutteridge and H. M. Shelton, Ed., Forage Tree Legumes in Tropical Agriculture, CAB In- ternational, Wallingford. http://www. betuco.be/agroforest ry / Forage%20Tree%20Legumes%20in%20Tropical%20Agri- culture%20FAO [2] Zahran, H.H. (2001) Rhizobia from wild legumes: Diver- sity, taxonomy, ecology, nitrogen fixation and biotech- nology. Journal of Biotechnology, 91(2), 143-153. [3] Korovin, E.P., Ed. (1955) Flora of Uzbekistan. Publish House of Academy Sciences of UzSSR, Tashkent, 638-640. [4] Khotyanovich, A.U., Ed. (1991) Methods for cultivation of nitrogen-fixing bacteria, ways of both their obtaining and preparing of preparations on their base (methodical guides). All-Union Institute of Agricultural Microbiology, Leningrad, 33-60. [5] Hardy, D.W., Halstein, R., Jakson, E. and Buens, R.S. (1968) C2H2–C2H4 assay to N2 fixation laboratory and  Z. S. Shakirov et al. / Agricultural Sciences 1 (2010) 24-38 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ 38 field evaluation. Plant Physiology, 43, 9-13. [6] Ferris, M.J., Muyzer, G. and Ward, D.M. (1996) Dena- turing gradient gel electrophoresis profiles of 16S rRNA- defined populations inhabiting a hot spring microbial mat community. Applied Environmental Microbiology, 62(2), 340-346. [7] Altschul, S.F., Madden, T.L., Schaffer, A.A., Zhang, J., Zhang, Z., Miller, W. and Lipman, D.J. (1997) Gapped: BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Research, 25(17), 3389-3402. [8] Saitou, N. and Nei, M. (1987) The neighbour-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology Evolution, 4(4), 406-425. [9] Tamura, K., Dudley, J., Nei, M. and Kumar, S. (2007) MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24, 1596-1599. http://www.ncbi.nlm.nih.gov/sites/entrez? cmd=retrieve&db=pubmed&list_uids=17488738&dopt= AbstractPlus [10] Collavoli, A., Storti, S., Dell’Amico, C. and Iascone, M.R. (2000) Northern blot analysis with digoxigenin in PCR-labeled probes in research samples from myocardial biopsies. Biochemica, 3, 19-20. http://www.roche-applied- science.com/PROD_INF/BIOCHEMI/no3_2000/PDF/p19- 20.pdf [11] Eckhardt, T. (1978) A rapid method for the identification of plasmid deoxyribonucleic acid in bacteria. Plasmid, 1(4), 584-588. [12] Priefer, U.B. (1984) Characterization of plasmid DNA by agarose gel electrophoresis. In: Pühler, A. and Timmis, K.N., Ed., Ad vanced Molecu lar Genetics, Springer -Verlag, Berlin, 26-37. [13] Glazer, V.M., Ed. (1972) Big practical course on genetics of microorganisms. Publish House of Moscow State of University, Moscow, 1-25. [14] Nechaeva, N.T., Ed., (1985) Improvement of desert ranges in Soviet Central Asia. Harwood Academic Publishers, London, 88. [15] Cubo, M.T., Buendia-Claveria, A.M., Beringer, J.E. and Ruiz-Sainz, J.E. (1988) Melanin production by Rhizo- bium strains. Applied Environmental Microbiology, 54(7), 1812-1817. [16] Teketay, D. (1998) Germination of Acacia origena, Aca- cia pilispirina and Pterolobium stellatum in response to different pre-sowing seed treatments, temperature and light. Journal of Arid Environments, 38, 551-560. [17] Abulfatih, H.A. (1995) Seed germination in Acacia spe- cies and their relation to altitudinal gradient in south- western Saudi Arabia. Journal of Arid Environments, 31(2), 171-178. [18] Yakovleva, Z.M. and Mishustin, E.N., Ed. (1975) Bac- teroids of nodule bacteria. Publish House Nauka, Mos- cow, 171. [19] Gordienko, N.Y. and Yakovleva, Z.M. (1979) Topogra- phy of infection threads in root nodules of legume plants. Proceedings of the USSR Academy of Sciences, Series Biologic, 3, 466-471. [20] Yakovleva, Z.M. (1981) Microstructure of pea nodules upon infectioning by neomycin-resistant nodule bacteria mutant. Mikrobiologiya, 50(3), 528-534. [21] Newcomb, W., Syono, K. and Torrey, J.G. (1977) Devel- opment of an ineffective pea root nodule: Morphogenesis, fine structure and cytokinin biosynthesis. Canadi an Jour- nal of Botan y, 55(14), 1891-1907. [22] B. Dreyfus, J.L. Garcia, M. Gillis, “Characterization of Azorhizobium caulinodans gen.nov. sp.nov., a stem-nodulating nitrogen-fixing bacterium isolated from Sesbania rostrata”, Internation Journal of Systematic Bacteriology, vol. 38, 1988, pp. 89-98. [23] Rana, D. and Krishnan, H.B. (1995) A new root-nodu- lating symbiont of the tropical legume Sesbania, Rhizo- bium sp. SIN-1, is closely related to Rhizobium galegae, a speсies that nodulate temperate Legumes. FEMS Mi- crobiology Letters, 134(1), 19-25. [24] Wdowiak, S. and Malek, W. (2000) Numerical analysis of Astragalus cicer microsymbionts. Current Microbiol- ogy, 41(2), 142-148. [25] Chen, W.X., Li, G.S., Qi, Y.L., Wang, E.T., Yuan, H.L. and Li, J.L. (1991) Rhizobium huakuii sp. nov., isolated from the root nodules of Astragalus sinicus. International Journal of Systematic Bacteriology, 41(2), 275-280. [26] Zhang, X.X., Turner, S.L., Guo, X.W., Yang, H.J., De- belle, F., Yang, G.P., Denarie, J., Young, J.P. and Li, F.D. (2000) The common nodulation genes of Astragalus sinicus rhizobia are conserved despite chromosomal di- versity. Applied Environmental Microbiology, 66(7), 2988- 2995. [27] Wang, S. and Chen, W. (1997) A study of taxonomy of Rhizobia isolated from Astragalus sp. Acta Microbi- ologica Sinica, 37(5), 335-343. [28] Gao, J., Terefework, Z., Chen, W. and Lindstrom, K. (2001) Genetic diversity of rhizobia isolated from As- tragalus adsurgens growing in different geographical re- gions of China. Journal of Biotechnology, 91(2-3), 155- 168. [29] Haukka, K., Lindstrom, K. and Young, P.W. (1998) Three phylogenetic groups of nodA and nifH genes in Si- norhizobium and Mezorhizobium isolates from Lehumi- nous trees in Africa and Latin America. Applied Envi- ronmental Microbiology, 64, 419-426.
|