Paper Menu >>
Journal Menu >>
 Vol.1, No.1, 17-23 (2010)s doi:10.4236/as.2010.11003 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ Agricultural Science Effects of different priming treatments and priming durations on germination percentage of parsley (Petroselinum crispum L.) seeds Atilla Dursun*, Melek Ekinci Atatürk University, Agriculture Faculty, Department of Horticulture, Erzurum, Turkey; *Corresponding Author: atilladursun@hotmail.com Received 16 March 2010; revised 15 April 2010; accepted 20 April 2010. ABSTRACT The effects of different priming treatments and priming durations on germination percenta ge at different temperatures in parsley seeds were studied. The seeds were treated for 2, 4, 6 and 8 days with the PEG 6000 (–0.5 MPa, –1.0 MPa and –1.5 MPa), KNO3 (0.30 mol/L and 0.35 mol/L), Mannitol (0.50 mol/L and 0.60 mol/L) and hy- dropriming (12h, 24h, 36h and 48h) and un- primed (control). Germination studies were made at 5, 10, 15, 20 and 25°C. Percentage of germination at different temperatures was sig- nificantly affected by priming treatments. Hy- dropriming (12h, 24h and 36 h) and mannitol 0.60 mol/L at 2 day generally had the highest germination percentages. In general, the high- est germination percentage with priming was determined at 10°C. It may be said that seed priming treatments increased seed germination percentage at both low and high temperatures. The highest germination percentages were ob- served in both hydropriming and mannitol treatments as compared with PEG and KNO3 treatments. The PEG and KNO3 (2 and 4 days) treatments were better than unprimed treatment in all of the temperatures. Keywords: Parsley; Germination Percentage; Seed Priming; PEG; KNO3; Hydropriming 1. INTRODUCTION Vegetables are important for human nutrition and its importance is getting increase in all around of the world. Good and high crop establishment is one of the major challenges to crop production in the world and its im- portance is recognized by farmers as well as researchers [1,2]. Constraints to good crop establishment include poor seedbed preparation, low quality seed, untimely seed sowing, and adverse weather conditions after sow- ing [2,3]. To improve vegetable stand establishment, priming techniques including osmopriming, solid matrix priming and hydropriming can be used. Seed priming is a pre-sowing physiological seed enhancement treatment involving the controlled hydration of seeds. Hydration is sufficient to allow pregerminative metabolic activation to take place, but insufficient to allow radicle protrusion through the seed coat [4]. This technique has been used in some vegetable seeds to increase the seed vigor, the germination speed, total germination rate and seedling uniformity, mainly under unfavorable environmental conditions. The technique can lead to better crop stands and higher crop yields [5,6]. Seed germination and emergence of parsley growing period especially in early spring, summer and late au- tumn are critical. Parsley is one of the most important vegetable that seed germination has taken long time and it is difficult to be germinated it especially under unfa- vorable environmental conditions [7]. The germination percentage of parsley seed is lower than 55-75% in these kinds of conditions [8]. Thus, slow emergence and low emergence rate lead to smaller seedlings [9]. However, uniformity and rapidity of seed germination and emer- gence are essential to increase yield, quality and profits in crops [9,10]. For this reason, seed priming techniques have recently been used for many plant species to in- crease crop stands and higher yields [11]. Also, it is re- ported that seed priming is a suitable method of shorten- ing the time to early crops and obtain flexibility in proc- essing plant schedules [12]. The aim of this study was to determine the effects of different priming treatments and durations on germina- tion percentage of parsley seeds. 2. MATERIALS AND METHODS The experiment was carried out at the Horticultural Re- search Laboratory at the Agricultural Faculty of Ataturk University. D’Giant İtaliana parsley seeds were treated at 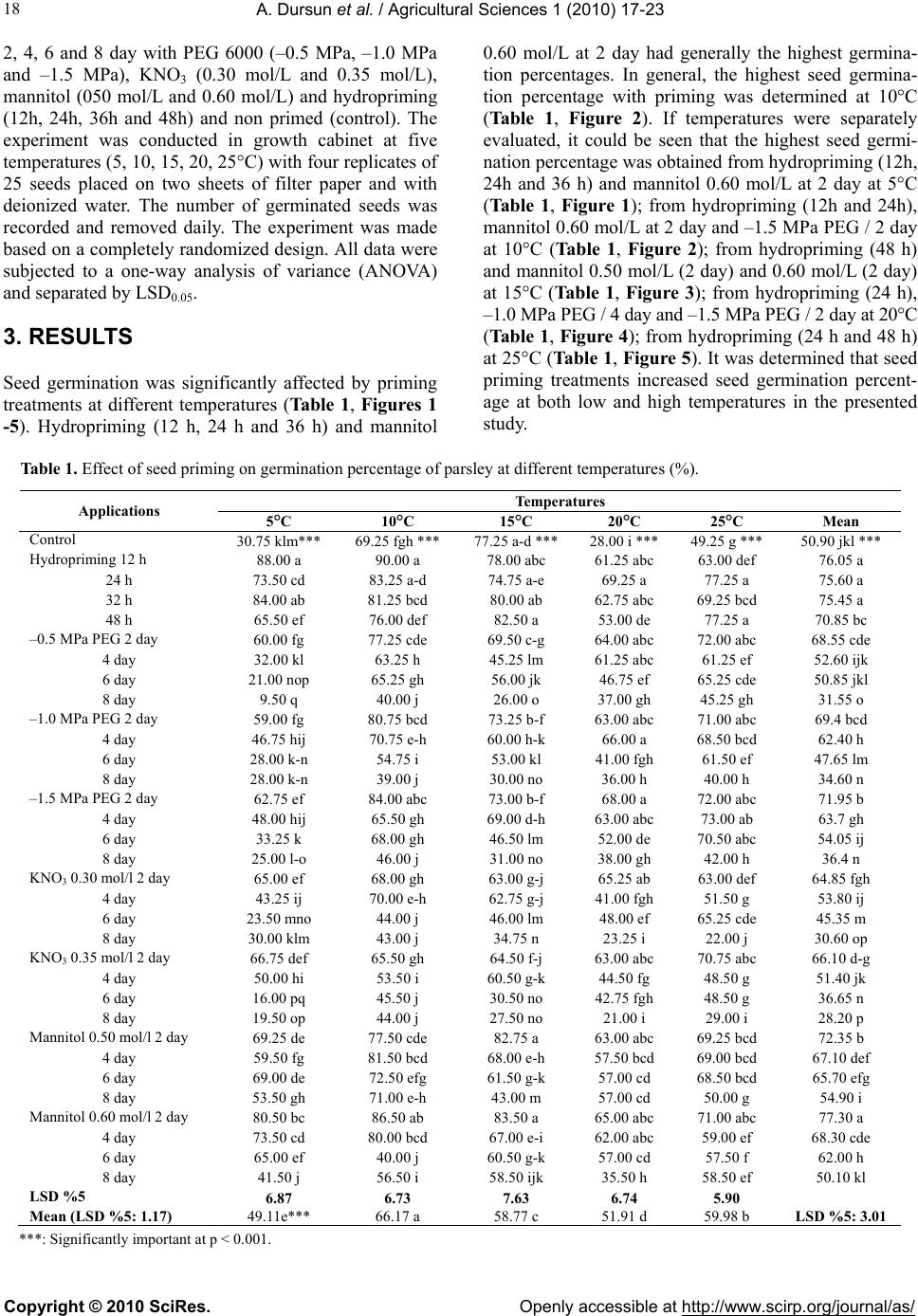 A. Dursun et al. / Agricultural Sciences 1 (2010) 17-23 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ 18 2, 4, 6 and 8 day with PEG 6000 (–0.5 MPa, –1.0 MPa and –1.5 MPa), KNO3 (0.30 mol/L and 0.35 mol/L), mannitol (050 mol/L and 0.60 mol/L) and hydropriming (12h, 24h, 36h and 48h) and non primed (control). The experiment was conducted in growth cabinet at five temperatures (5, 10, 15, 20, 25°C) with four replicates of 25 seeds placed on two sheets of filter paper and with deionized water. The number of germinated seeds was recorded and removed daily. The experiment was made based on a completely randomized design. All data were subjected to a one-way analysis of variance (ANOVA) and separated by LSD0.05. 3. RESULTS Seed germination was significantly affected by priming treatments at different temperatures (Ta b le 1, Figures 1 -5). Hydropriming (12 h, 24 h and 36 h) and mannitol 0.60 mol/L at 2 day had generally the highest germina- tion percentages. In general, the highest seed germina- tion percentage with priming was determined at 10°C (Table 1, Figure 2). If temperatures were separately evaluated, it could be seen that the highest seed germi- nation percentage was obtained from hydropriming (12h, 24h and 36 h) and mannitol 0.60 mol/L at 2 day at 5°C (Ta b l e 1 , Figure 1); from hydropriming (12h and 24h), mannitol 0.60 mol/L at 2 day and –1.5 MPa PEG / 2 day at 10°C (Ta b l e 1, Figure 2); from hydropriming (48 h) and mannitol 0.50 mol/L (2 day) and 0.60 mol/L (2 day) at 15°C (Ta ble 1, Figure 3); from hydropriming (24 h), –1.0 MPa PEG / 4 day and –1.5 MPa PEG / 2 day at 20°C (Table 1, Figure 4); from hydropriming (24 h and 48 h) at 25°C (Table 1, Figure 5). It was determined that seed priming treatments increased seed germination percent- age at both low and high temperatures in the presented study. Table 1. Effect of seed priming on germination percentage of parsley at different temperatures (%). ***: Significantly important at p < 0.001. Temperatures Applications 5°C 10°C 15°C 20°C 25°C Mean Control 30.75 klm*** 69.25 fgh *** 77.25 a-d *** 28.00 i ***49.25 g *** 50.90 jkl *** Hydropriming 12 h 88.00 a 90.00 a 78.00 abc 61.25 abc 63.00 def 76.05 a 24 h 73.50 cd 83.25 a-d 74.75 a-e 69.25 a 77.25 a 75.60 a 32 h 84.00 ab 81.25 bcd 80.00 ab 62.75 abc 69.25 bcd 75.45 a 48 h 65.50 ef 76.00 def 82.50 a 53.00 de 77.25 a 70.85 bc –0.5 MPa PEG 2 day 60.00 fg 77.25 cde 69.50 c-g 64.00 abc 72.00 abc 68.55 cde 4 day 32.00 kl 63.25 h 45.25 lm 61.25 abc 61.25 ef 52.60 ijk 6 day 21.00 nop 65.25 gh 56.00 jk 46.75 ef 65.25 cde 50.85 jkl 8 day 9.50 q 40.00 j 26.00 o 37.00 gh 45.25 gh 31.55 o –1.0 MPa PEG 2 day 59.00 fg 80.75 bcd 73.25 b-f 63.00 abc 71.00 abc 69.4 bcd 4 day 46.75 hij 70.75 e-h 60.00 h-k 66.00 a 68.50 bcd 62.40 h 6 day 28.00 k-n 54.75 i 53.00 kl 41.00 fgh 61.50 ef 47.65 lm 8 day 28.00 k-n 39.00 j 30.00 no 36.00 h 40.00 h 34.60 n –1.5 MPa PEG 2 day 62.75 ef 84.00 abc 73.00 b-f 68.00 a 72.00 abc 71.95 b 4 day 48.00 hij 65.50 gh 69.00 d-h 63.00 abc 73.00 ab 63.7 gh 6 day 33.25 k 68.00 gh 46.50 lm 52.00 de 70.50 abc 54.05 ij 8 day 25.00 l-o 46.00 j 31.00 no 38.00 gh 42.00 h 36.4 n KNO3 0.30 mol/l 2 day 65.00 ef 68.00 gh 63.00 g-j 65.25 ab 63.00 def 64.85 fgh 4 day 43.25 ij 70.00 e-h 62.75 g-j 41.00 fgh 51.50 g 53.80 ij 6 day 23.50 mno 44.00 j 46.00 lm 48.00 ef 65.25 cde 45.35 m 8 day 30.00 klm 43.00 j 34.75 n 23.25 i 22.00 j 30.60 op KNO3 0.35 mol/l 2 day 66.75 def 65.50 gh 64.50 f-j 63.00 abc 70.75 abc 66.10 d-g 4 day 50.00 hi 53.50 i 60.50 g-k 44.50 fg 48.50 g 51.40 jk 6 day 16.00 pq 45.50 j 30.50 no 42.75 fgh 48.50 g 36.65 n 8 day 19.50 op 44.00 j 27.50 no 21.00 i 29.00 i 28.20 p Mannitol 0.50 mol/l 2 day 69.25 de 77.50 cde 82.75 a 63.00 abc 69.25 bcd 72.35 b 4 day 59.50 fg 81.50 bcd 68.00 e-h 57.50 bcd 69.00 bcd 67.10 def 6 day 69.00 de 72.50 efg 61.50 g-k 57.00 cd 68.50 bcd 65.70 efg 8 day 53.50 gh 71.00 e-h 43.00 m 57.00 cd 50.00 g 54.90 i Mannitol 0.60 mol/l 2 day 80.50 bc 86.50 ab 83.50 a 65.00 abc 71.00 abc 77.30 a 4 day 73.50 cd 80.00 bcd 67.00 e-i 62.00 abc 59.00 ef 68.30 cde 6 day 65.00 ef 40.00 j 60.50 g-k 57.00 cd 57.50 f 62.00 h 8 day 41.50 j 56.50 i 58.50 ijk 35.50 h 58.50 ef 50.10 kl LSD %5 6.87 6.73 7.63 6.74 5.90 Mean (LSD %5: 1.17) 49.11e*** 66.17 a 58.77 c 51.91 d 59.98 b LSD %5: 3.01  A. Dursun et al. / Agricultural Sciences 1 (2010) 17-23 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ 19 Figure 1. Effect of seed priming on germination percentage of parsley at 5°C. Figure 2. Effect of seed priming on germination percentage of parsley at 10°C. 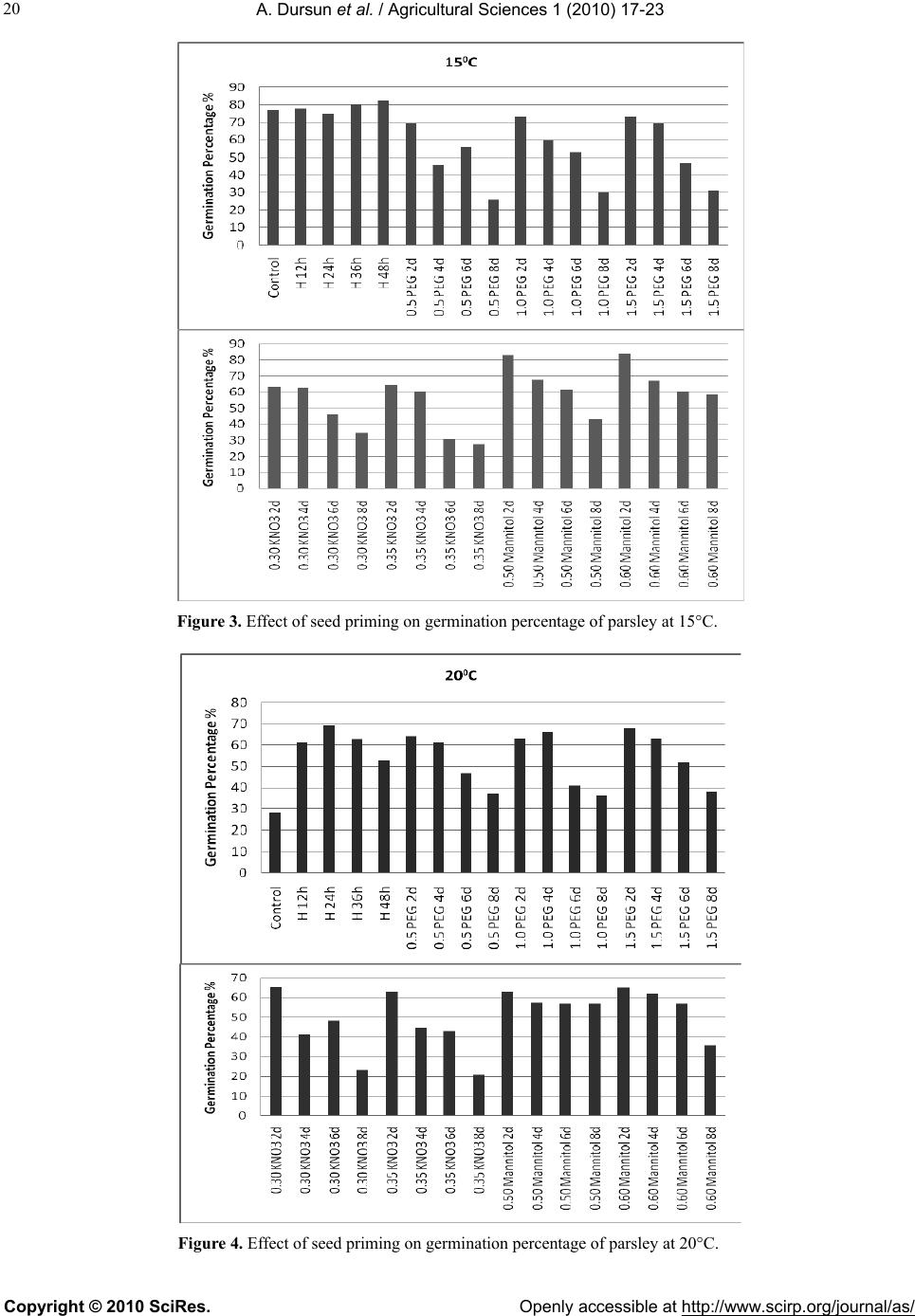 A. Dursun et al. / Agricultural Sciences 1 (2010) 17-23 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ 20 Figure 3. Effect of seed priming on germination percentage of parsley at 15°C. Figure 4. Effect of seed priming on germination percentage of parsley at 20°C. 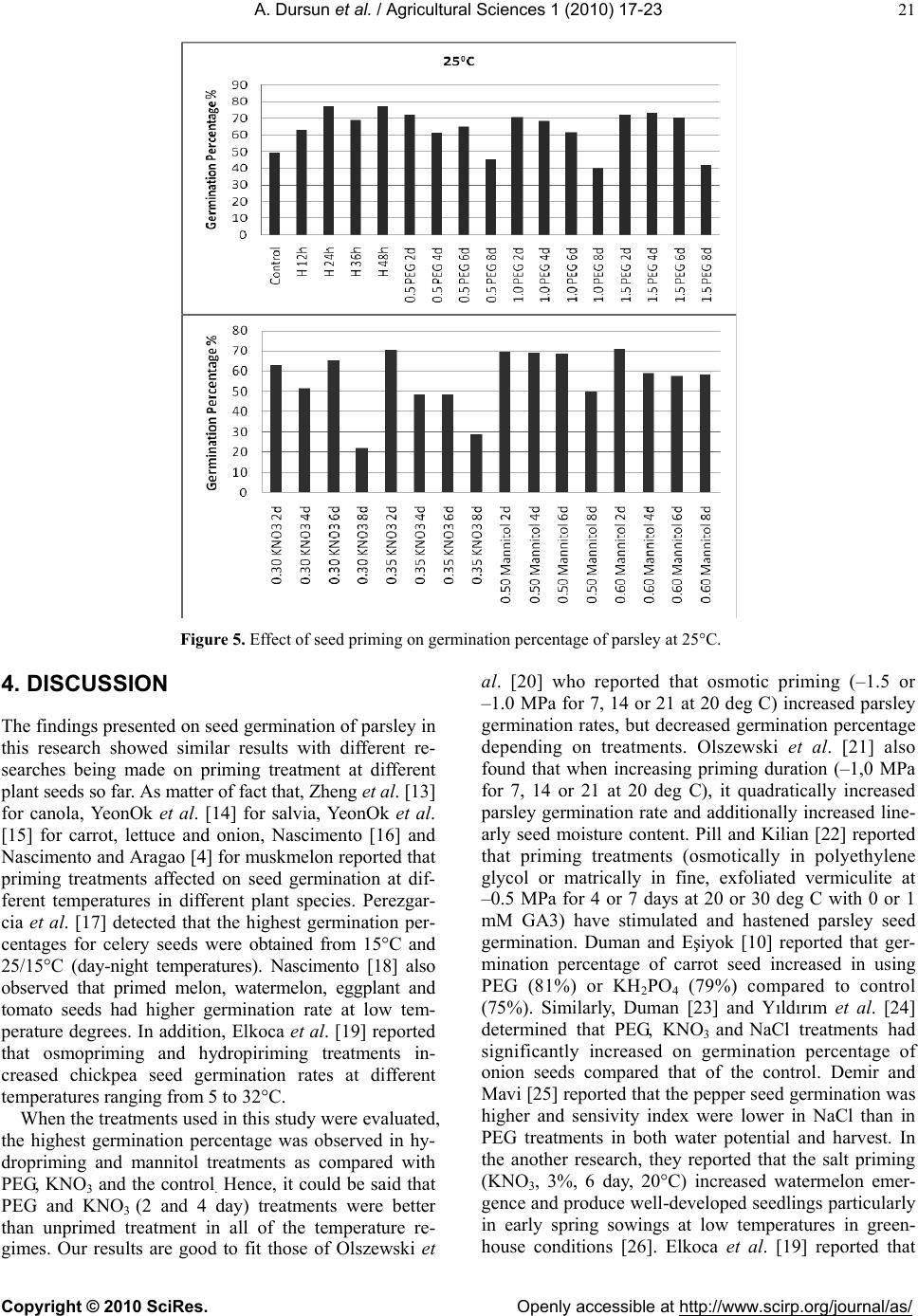 A. Dursun et al. / Agricultural Sciences 1 (2010) 17-23 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ 21 Figure 5. Effect of seed priming on germination percentage of parsley at 25°C. 4. DISCUSSION The findings presented on seed germination of parsley in this research showed similar results with different re- searches being made on priming treatment at different plant seeds so far. As matter of fact that, Zheng et al. [13] for canola, YeonOk et al. [14] for salvia, YeonOk et al. [15] for carrot, lettuce and onion, Nascimento [16] and Nascimento and Aragao [4] for muskmelon reported that priming treatments affected on seed germination at dif- ferent temperatures in different plant species. Perezgar- cia et al. [17] detected that the highest germination per- centages for celery seeds were obtained from 15°C and 25/15°C (day-night temperatures). Nascimento [18] also observed that primed melon, watermelon, eggplant and tomato seeds had higher germination rate at low tem- perature degrees. In addition, Elkoca et al. [19] reported that osmopriming and hydropiriming treatments in- creased chickpea seed germination rates at different temperatures ranging from 5 to 32°C. When the treatments used in this study were evaluated, the highest germination percentage was observed in hy- dropriming and mannitol treatments as compared with PEG, KNO3 and the control. Hence, it could be said that PEG and KNO3 (2 and 4 day) treatments were better than unprimed treatment in all of the temperature re- gimes. Our results are good to fit those of Olszewski et al. [20] who reported that osmotic priming (–1.5 or –1.0 MPa for 7, 14 or 21 at 20 deg C) increased parsley germination rates, but decreased germination percentage depending on treatments. Olszewski et al. [21] also found that when increasing priming duration (–1,0 MPa for 7, 14 or 21 at 20 deg C), it quadratically increased parsley germination rate and additionally increased line- arly seed moisture content. Pill and Kilian [22] reported that priming treatments (osmotically in polyethylene glycol or matrically in fine, exfoliated vermiculite at –0.5 MPa for 4 or 7 days at 20 or 30 deg C with 0 or 1 mM GA3) have stimulated and hastened parsley seed germination. Duman and Eşiyok [10] reported that ger- mination percentage of carrot seed increased in using PEG (81%) or KH2PO4 (79%) compared to control (75%). Similarly, Duman [23] and Yıldırım et al. [24] determined that PEG, KNO3 and NaCl treatments had significantly increased on germination percentage of onion seeds compared that of the control. Demir and Mavi [25] reported that the pepper seed germination was higher and sensivity index were lower in NaCl than in PEG treatments in both water potential and harvest. In the another research, they reported that the salt priming (KNO3, 3%, 6 day, 20°C) increased watermelon emer- gence and produce well-developed seedlings particularly in early spring sowings at low temperatures in green- house conditions [26]. Elkoca et al. [19] reported that  A. Dursun et al. / Agricultural Sciences 1 (2010) 17-23 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ 22 hydropriming for 12 h or osmopriming with –0.5 MPa PEG for 24h resulted in the highest seed germination of chickpea. Arin and Kiyak [27] stated that priming of tomato seeds (especially KNO3) improved emergence and seedling growth under stress (low temperature, drought and salinity) conditions. Osmopriming (PEG-6000) treatments had the best conditions for leek seeds in 7-10 days at 15°C and –15 bars [28]. Similarly, PEG solution (–1.1 and –1.8 MPa) improved germination soybean seed compared to control [29]. And also Pazdera and Hosnedl [30] reported that the osmopriming was suitable method for lettuce seed germination. Sivritepe and Eriş [31] detected that priming treatments (–0.25, –0.50 and –0.75 MPa with PEG-8000, 10-3 M and 10-4 M with ABA and 2, 4 and 6 days with distilled water (H2O) ) at 16°C increased the germination percentage and de- creased the mean germination time in pea seeds. Sadeghian and Yavari [32] reported that the highest concentration of mannitol decreased seedling growth and germination rates on sugar beet seed. Nascimento [16] suggested that salt solutions (0.30 mol/L or 0.35 mol/L potassium nitrate KNO3; 0.30 or 0.40 mol/L potassium dihydrogen phosphate (KH2PO4); 0.15 or 0.20 mol/L potassium nitrate +0.15 or 0.20 mol/L potassium dihy- drogen phosphate; 0.50 or 0.60 mol/L mannitol; and 0.04 or 0.05 mol/L polyethylene glycol/liter) had better ger- mination rate on seeds of muskmelon. It was also re- ported that the best treatment to seed of muskmelon for germination and emergence at low temperature was 2.5% KNO3 for 16 h in dark [33]. Demir et al. [34] re- ported that GA3 and KNO3 treatments influenced on eggplant seed germination and rate of germination re- spect comparing that of the control. In addition, Brock- lehurst et al. [35] stated that PEG gave suitable results for different vegetable species such as onion, leek, and celery and KH2PO4 had effect of reducing in percentage germination and emergence in leek and celery. Accord- ing to another research, 0.1 M KNO3 and 0.1 M KH2PO4 pre-storage treatments were the most effective for de- laying viability loss due to ageing in onion seed [36]. Sivritepe et al. [37-39] indicated that priming of melon seeds with NaCl resulted in increased salt tolerance in seedling, total germination and mean germination time. Results of presented research were similar and confirma- tive to the mentioned studies above. In conclusion, the effects of seed priming depend on the crop species. Seed priming is safe, effective and easily adopted by farmers. It also has the potential to benefit such farmers in many ways and hence, its importance is recognized by farmers as well as researchers. Priming in- creased the percentage of germination of parsley in this study. Therefore, parsley seed should be primed in order to perform better under unfavorable environmental con- ditions. REFERENCES [1] Chivasa, W., Harris, D., Chiduza, C., Nyamudeza, P. and Mashingaidze, A.B. (1998) Agronomic practices, major crops and farmer’s perceptions of the importance of good stand establishment in Musikavanhu Communal Area, Zimbabwe. Journal of Applied Science in Southern Af- rica, 4(2), 108-125. [2] Murungu, E.S., Chıduza, C., Nyamugafata, P., Clark, L.J. and Whalley, W.R. (2004) Effect of on-farm seed prim- ing on emergence, growth and yield of cotton and Maize in a semi-arid area of Zimbabwe. Experimental Agricul- ture, 40(1), 23-36. [3] Harris, D. (1996) The effects of manure, genotype, seed priming, depth and date of sowing on the emergence and early growth of Sorghum bicolor (L.) Moench in semi-arid Botswana. Soil and Tillage Research, 40(1-2), 73-88. [4] Nascimento, W.M. and Aragao, F.A.S. (2004) Musk- melon seed priming in relation to seed vigor. Scientia Agricola, 61(1), 114 -117. [5] Black, M. and Bawley, J.D. (2000) Seed technology and its biological basis. Sheffield Academic Press, Sheffield. [6] Duman, İ. (2005) Olumsuz çevre koşullarında tohum- ların çimlenme ve çıkış performansı nasıl artırılabilir? Hasad Dergisi, 21(246), 72-78. [7] Vural, H., Eşiyok, D. and Duman, İ. (2000) Kültür seb- zeleri (sebze yetiştirme). Ege Üniversitesi Ziraat Fakül- tesi Bahçe Bitkileri Bölümü, Bornova-İzmir, 440. [8] Günay, A. (2005) Sebze yetiştiriciliği cilt II. İzmir, 531. [9] Cantliffe, D.J. (2003) Seed enhancement. ISHS Acta Horticulturae 607: IX International Symposium on Tim- ing of Field Production in Vegetable Crops, Sao Paulo, 13 May 2003, 34(1). [10] Duman, İ. and Eşiyok, D. (1998) Ekim öncesi PEG ve KH2PO4 uygulamalarının havuç tohumlarının çimlenme ve çıkış oranı ile verim üzerine etkileri. Turkish Journal of Agriculture and Forestry, 22, 445-449. [11] Lee, J.M. (2004) Advances in seed treatments for horti- cultural crops. Chronica Horticulturae, 44(2), 11-20. [12] Barlow, E.W.R. and Haigh, A.M. (1987) Effect of seed priming on the emergence, growth and yield of UC 82B tomatoes in the field. ISHS Acta Horticultrae 200: II. In- ternational Symposium on Processing Tomatoes, XXII IHC, Davis, 1 March 1987, 22(1). [13] Zheng, G.H., Wilen, R.W., Slinkard, A.E. and Gusta, L.V. (1994) Enhancement of canola seed-germination and seedling emergence at low temperature by priming. Crop Science, 34(6), 1589-1593. [14] YeonOk, J., SeongMo, K. and JeoungLai, C. (2000) Priming conditions to improve germination of salvia (Salvia splendens F.) seeds. Korean Journal of Horti- cultural Science and Technology, 18(2), 98-102. [15] YeonOk, J., JongCheol, K. and JeoungLai, C. (2000) Effect of priming duration and temperature on the ger- minability of carrot, lettuce, onion, and welsh onion seeds. Korean Journal of Horticultural Science and Technology, 18(3), 327-333. [16] Nascimento, W.M. (2003) Muskmelon seed germination and seedling development in response to seed priming. Scientia Agri cola, 60(1), 71-75.  A. Dursun et al. / Agricultural Sciences 1 (2010) 17-23 Copyright © 2010 SciRes. Openly accessible at http://www.scirp.org/journal/as/ 23 [17] Perezgarcia, F., Pita, J.M., Gonzalezbenito, M.E. and Irıondo, J.M. (1995) Effects of light, temperature and seed priming on germination of celery seeds (Apium graveolens L.). Seed Science and Technology, 23(2), 377-383. [18] Nascimento, W.M. (2005) Vegetable seed priming to improve germination at low temperature. Horticultura Brasileria, 23(2), 211-214. [19] Elkoca, E., Haliloğlu, K., Eşitken, A. and Ercişli, S. (2007) Hydro and osmopriming improve chickpea ger- mination. Acta Agriculturae Scandinavica Section B Soil and Plant Science, 57(3), 193-200. [20] Olszweski, M.W., Evans, T.A., Gregory, N.F. and Pill, W.G. (2005) Enhanced germination of primed mericarps of parsley (Petroselinum crispum Mill. Nyman ex A.W.Hill) limited by Alternaria alternate proliferation. Journal of Horticultural Science and Biotechnology, 80(4), 427-432. [21] Olszewski, M., Pill, W., Pizzolato, T.D. and Pesek, J. (2005) Priming duration influences anatomy and germi- nation responses of parsley mericarps. Journal of the American Society for Horticultural Science, 130(5), 754- 758. [22] Pill, W.G. and Kilian, E.A. (2000) Germination and emergence of parsley in response to osmotic or matric seed priming and treatment with gibberellin. Horticul- tural Science, 35(5), 907-909. [23] Duman, İ. (2002) Soğan (Allium cepa L.) tohumlarının çimlenmesini iyileştirici farklı osmotik uygulama yöntemlerinin karşılaştırılması. Ege Üniversitesi Ziraat Fakültesi Dergisi, 39(2), 1-8. [24] Yıldırım, E., Dursun, A., Güvenç, İ. and Kumlay, A.M. (2002) Effects of different salt, biostumulant and tem- perature levels on seed germination of some vegetable species. Ac ta Agrobotanica, 55(2), 75-80. [25] Demir, İ. and Mavi, K. (2008) Effect of salt and osmotic stresses on the germination of pepper seeds of different maturation stages. Brazilian Archives of Biology and Technology, 51(5), 897-902. [26] Demir, İ. and Mavi, K. (2004) The effect of priming on seedling emergence of differentially matured watermelon (Citrullus lanatus (Thunb.) Matsum and Nakai) seeds. Scientia Horticulturae, 102(4), 467-473. [27] Arin, L. and Kiyak, Y. (2003) The effects of pre-sowing treatments on emergence and seedling growth of tomato seed (Lycopersicon esculentum Mill.) under several stress conditions. Pakistan Journal of Biological Science s, 6(11), 990-994. [28] Corbineau, F., Picard, M.A. and Come, D. (1994) Ger- minability of leek seeds and its improvement by osmo- priming. ISHS Acta Horticulture 371: VII International Symposium on Timing Field Production of Vegetables, Skierniewice, 1 July 1994, 57(1). [29] Khalil, S.K., Mexal, J.G. and Murray, L.W. (2001) Ger- mination of soybean primed in aerated solution of poly- ethylene glycol (8000). Online Journal of Biological Sciences, 1(3), 105-107. [30] Pazdera, J. and Hosnedl, V. (2002) Effect of hydration treatments on seed parameters of different lettuce (Lac- tuca sativa L.) seed lots. Horticultural Science, 29(1), 12-16. [31] Sivritepe, H.Ö. and Eris, A. (2000) The effects of post- storage priming treatments on viability and repair of ge- netic damage in pea seeds. ISHS: XXV International Horticultural Congress, Part 7: Quality of Horticultural Products, 517, 143-149. [32] Sadeghian, S.Y. and Yavari, N. (2004) Effect of water- deficit stress on germination and early seedling growth in sugar beet. Journal of Agronomy and Crop Science, 190(2), 138-144. [33] Dhillon, N.P.S. (1995) Seed priming of male sterile muskmelon (Cucumis melo L.) for low temperature germination. Seed Science and Technology, 23(3), 881- 884. [34] Demir, İ., Ellialtıoğlu, S. and Tipirdamaz, R. (1993) The effect of different priming treatments on reparability of aged eggplant seeds. ISHS: International Symposium on Agrotechnics and Storage of Vegetable and Ornamental Seeds, Bari, 14-16 June 1993, 362, 205-212. [35] Brocklehurst, P.A., Dearman, J. and Drew, R.L.K. (1987) Recent developments in osmotic treatment of vegetable seeds. ISHS Acta Horticulturae 215: Seed Research in Horticulture, 30(1). [36] İlbi, H. and Eser, B. (2004) The effects of seed treatments on ageing in onion seed. Ege Üniversitesi Ziraat Fakültesi Dergisi, 41(1), 39-48. [37] Sivritepe, H.Ö., Eris, A. and Sivritepe, N. (1999) The effect of NaCl priming on salt tolerance in melon seed- ling. ISTA: I International Symposium on Cucurb its, Adana, 1 May 1999, 492, 77-84. [38] Sivritepe, H.Ö., Eris, A. and Sivritepe, N. (1999) The effect of priming treatments on salt tolerance in melon seeds. ISTA: I International Symposium on Cucurbits, Adana, 1 May 1999, 492, 287-295. [39] Sivritepe, H.Ö., Sivritepe, N., Eriş, A. and Turhan, E. (2005) The effects of NaCl pre-treatments on salt toler- ance of melon grown under long-term salinity. Science Horticulturae, 106(4), 568-581. |

