 Open Journal of Radiology, 2012, 2, 14-21 http://dx.doi.org/10.4236/ojrad.2012.21003 Published Online March 2012 (http://www.SciRP.org/journal/ojrad) Diagnostic Accuracy of MRI in Primary Cervical Cancer Giuliano Rigon1, Cristina Vallone1, Andrea Starita1, Marco Flavio Michele Vismara2, Pasquale Ialongo3, Lorenza Putignani4*, Fabrizio Signore1* 1Department of Obstetrics and Gynaecology, S. Camillo-Forlanini Hospital, Rome, Italy 2Department of Molecular Medicine, Faculty of Medicine and Pharmacy, “Sapienza” University of Rome, Rome, Italy 3Department of Radiology, S. Camillo-Forlanini Hospital, Rome, Italy 4Microbiology Unit, Children’s Hospital and Research Institute “Bambino Gesù”, Rome, Italy Email: *fsignore@scamilloforlanini.rm.it, lorenza.putignani@opbg.net Received January 14, 2012; revised February 12, 2012; accepted February 22, 2012 ABSTRACT Introduction: Magnetic resonance imaging (MRI) studies obtained during the initial staging of patients affected by uterine cervical cancer were compared to the final histological report after surgery. Methods: Data were retrieved from published papers. Results: MRI detection of lymph node metastases shows a sensitivity of 49.3% (1209 patients) and a specificity of 87.7% (1182 patients). Parametrial involvement detection has 66.2% sensitivity (1288 patients) and 83.6% specificity (1282 patients). MRI tumor size evaluation shows significant error. Even detection of over 1 cm di- ameter primary tumor can fail. MRI appears promising in the detection of myometrial and endometrial involvement. Conclusions: Primary uterine cervical cancer evaluation with routine MRI has a limited accuracy especially in the de- tection of lymph node involvement and parametrial invasion. It is not sensitive enough to replace histology of dissected nodes and parametria. Tumor size estimation is imprecise. Detection of myometrial and endometrial invasion using MRI might be possible. Awareness of MRI limitations is crucial in primary cervical cancer staging. Keywords: Cervical Cancer; MRI; Parametrial; Lymph Node Involvement 1. Introduction Uterine cervical cancer is the third most common gynae- cologic malignancy reported in the United States [1]. In 2007 11,150 new cases were diagnosed and 3670 deaths were expected in the United States [1]. Currently, the International Federation of Gynecology and Obstetrics (FIGO) staging for cervical carcinoma is primarily based on physical examination. Staging errors are strongly as- sociated with poor prognosis [1]. Exclusive FIGO clini- cal staging has been reported as accurate in only 29% of cases [2]. FIGO staging does not include lymph node status and tends to underestimate in 20% - 30% of low stage cervi- cal cancer patients when compared to surgical staging. FIGO understaging has been reported in up to 23% of stage IIb disease, and 40% to 64% in stage IIIb. About 40% of the patients receive an inaccurate preoperative staging [3]. Together with parametrial extension and positive sur- gical margins, one of the most important prognostic fac- tors in cervical cancer is the presence of lymph node me- tastasis. Imaging technologies, as Computed Assisted Tomo- graphy (CAT), Magnetic Resonance Imaging (MRI) and Positron Emission Tomography (PET) might have an im- pact in determining the proper treatment for patients with cervical cancer. International papers screened using PubMed were re- viewed. Sensitivity and specificity of routine MRI for 1) primary tumour detection; 2) internal of involvement; 3) myometrial invasion; 4) lymph node involvement; 5) parametrial invasion; 6) bladder invasion; 7) rectal inva- sion and 8) vaginal invasion were noted. MRI tumor size evaluation was compared to histologic tumor size. Ra- diological findings were compared to the postoperative histological report. 2. Methods 2.1. Literature Search and Study Selection This study was approved by San Camillo-Forlanini In- stitutional Review Board. A literature search of international studies was carried out to identify articles on MRI preoperative diagnostic performance in patients with cervical carcinoma. MED- LINE database (2000 to 2011) was searched for the fol- lowing terms: “cervical cancer, cervical neoplasm, MRI, parametrial and lymph nodes” as medical subject head- *Corresponding author. C opyright © 2012 SciRes. OJRad 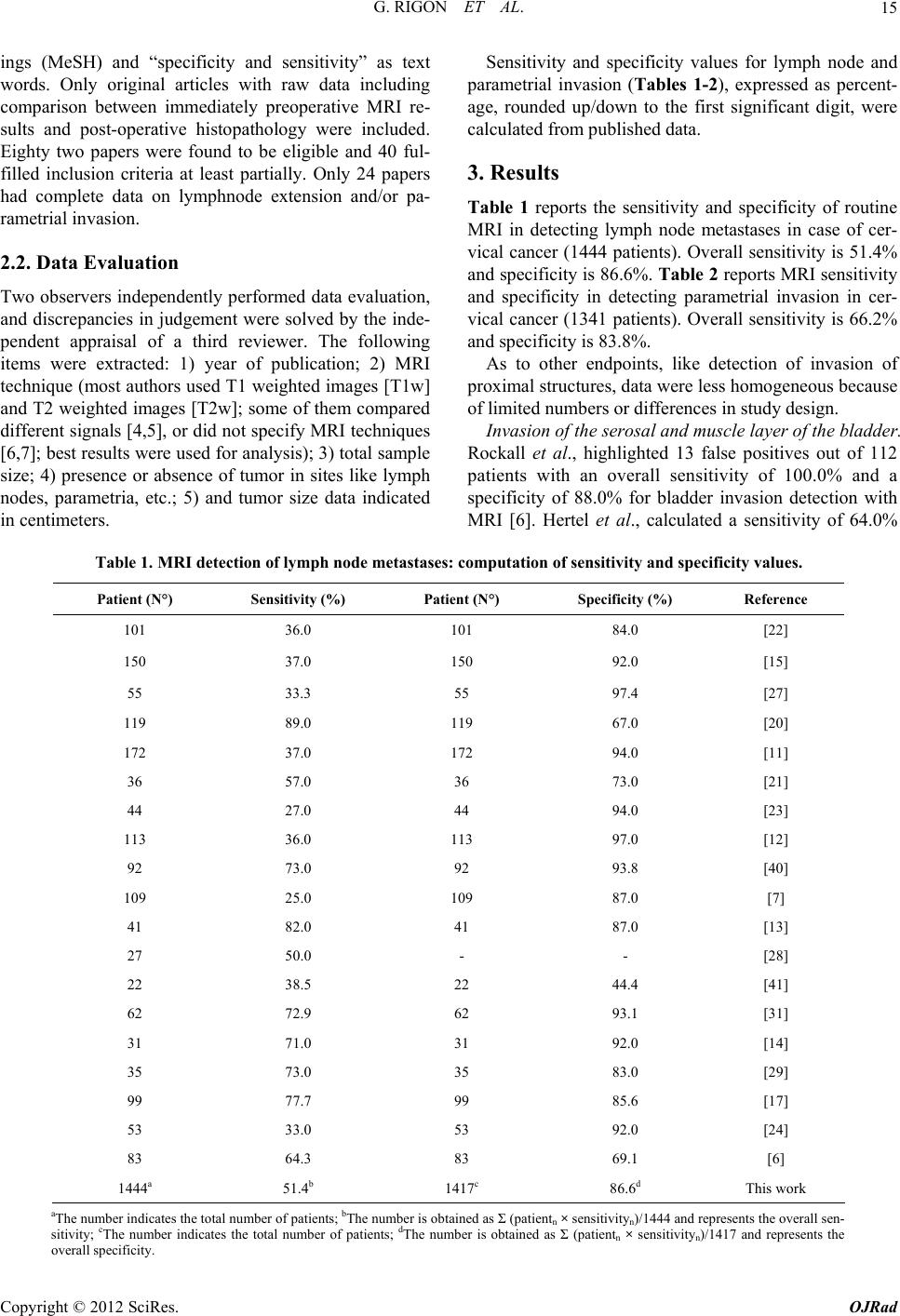 G. RIGON ET AL. 15 ings (MeSH) and “specificity and sensitivity” as text words. Only original articles with raw data including comparison between immediately preoperative MRI re- sults and post-operative histopathology were included. Eighty two papers were found to be eligible and 40 ful- filled inclusion criteria at least partially. Only 24 papers had complete data on lymphnode extension and/or pa- rametrial invasion. 2.2. Data Evaluation Two observers independently performed data evaluation, and discrepancies in judgement were solved by the inde- pendent appraisal of a third reviewer. The following items were extracted: 1) year of publication; 2) MRI technique (most authors used T1 weighted images [T1w] and T2 weighted images [T2w]; some of them compared different signals [4,5], or did not specify MRI techniques [6,7]; best results were used for analysis); 3) total sample size; 4) presence or absence of tumor in sites like lymph nodes, parametria, etc.; 5) and tumor size data indicated in centimeters. Sensitivity and specificity values for lymph node and parametrial invasion (Tables 1-2), expressed as percent- age, rounded up/down to the first significant digit, were calculated from published data. 3. Results Table 1 reports the sensitivity and specificity of routine MRI in detecting lymph node metastases in case of cer- vical cancer (1444 patients). Overall sensitivity is 51.4% and specificity is 86.6%. Table 2 reports MRI sensitivity and specificity in detecting parametrial invasion in cer- vical cancer (1341 patients). Overall sensitivity is 66.2% and specificity is 83.8%. As to other endpoints, like detection of invasion of proximal structures, data were less homogeneous because of limited numbers or differences in study design. Invasion of the serosa l and muscle la yer of th e bladd er. Rockall et al., highlighted 13 false positives out of 112 patients with an overall sensitivity of 100.0% and a specificity of 88.0% for bladder invasion detection with MRI [6]. Hertel et al., calculated a sensitivity of 64.0% Table 1. MRI detection of lymph node metastases: computation of sensitivity and specificity values. Patient (N°) Sensitivity (%) Patient (N°) Specificity (%) Reference 101 36.0 101 84.0 [22] 150 37.0 150 92.0 [15] 55 33.3 55 97.4 [27] 119 89.0 119 67.0 [20] 172 37.0 172 94.0 [11] 36 57.0 36 73.0 [21] 44 27.0 44 94.0 [23] 113 36.0 113 97.0 [12] 92 73.0 92 93.8 [40] 109 25.0 109 87.0 [7] 41 82.0 41 87.0 [13] 27 50.0 - - [28] 22 38.5 22 44.4 [41] 62 72.9 62 93.1 [31] 31 71.0 31 92.0 [14] 35 73.0 35 83.0 [29] 99 77.7 99 85.6 [17] 53 33.0 53 92.0 [24] 83 64.3 83 69.1 [6] 1444a 51.4b 1417c 86.6d This work aThe number indicates the total number of patients; bThe number is obtained as Σ (patientn × sensitivityn)/1444 and represents the overall sen- sitivity; cThe number indicates the total number of patients; dThe number is obtained as Σ (patientn × sensitivityn)/1417 and represents the overall specificity. Copyright © 2012 SciRes. OJRad 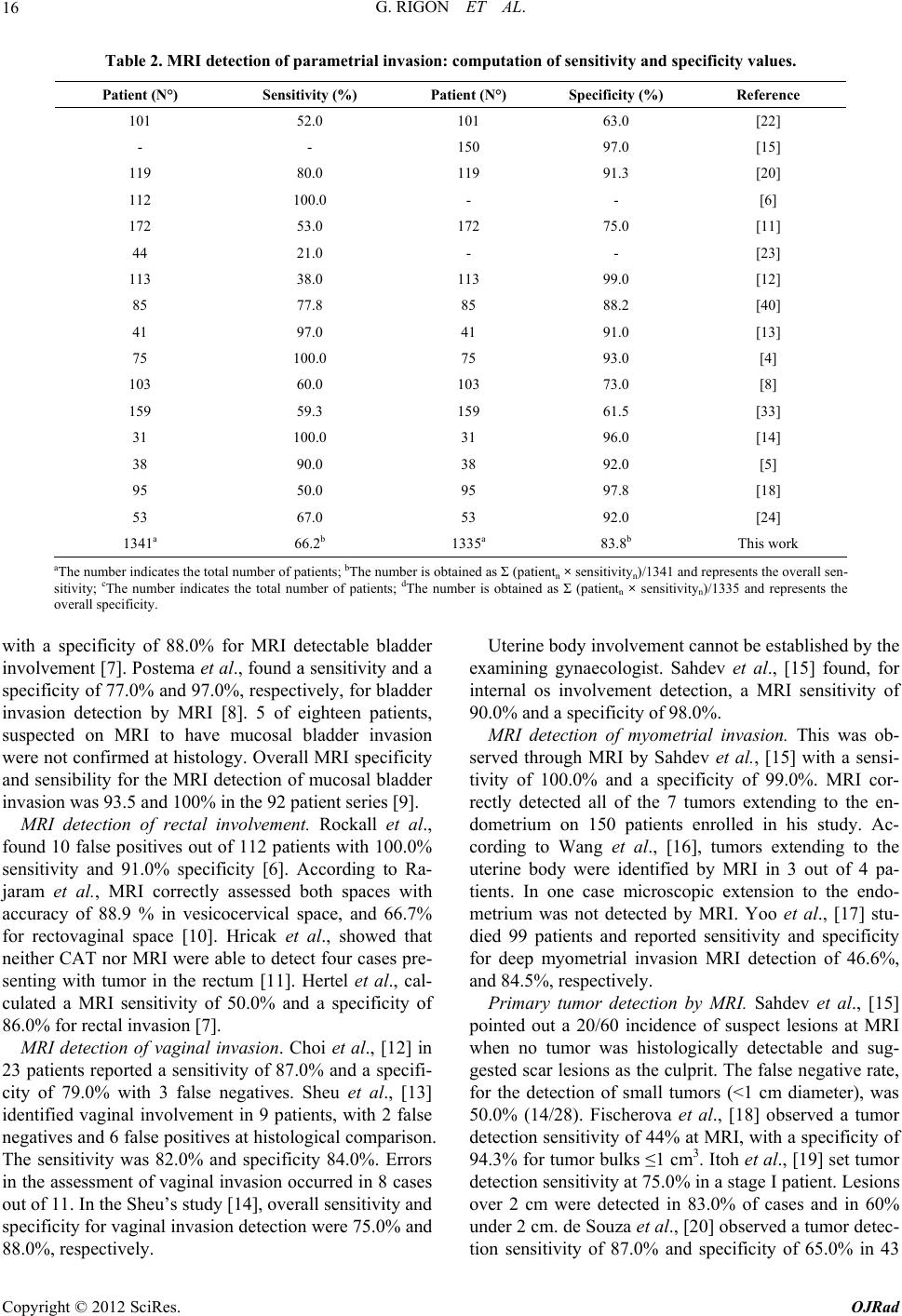 G. RIGON ET AL. 16 Table 2. MRI detection of parametrial invasion: computation of sensitivity and specificity value s. Patient (N°) Sensitivity (%) Patient (N°) Specificity (%) Reference 101 52.0 101 63.0 [22] - - 150 97.0 [15] 119 80.0 119 91.3 [20] 112 100.0 - - [6] 172 53.0 172 75.0 [11] 44 21.0 - - [23] 113 38.0 113 99.0 [12] 85 77.8 85 88.2 [40] 41 97.0 41 91.0 [13] 75 100.0 75 93.0 [4] 103 60.0 103 73.0 [8] 159 59.3 159 61.5 [33] 31 100.0 31 96.0 [14] 38 90.0 38 92.0 [5] 95 50.0 95 97.8 [18] 53 67.0 53 92.0 [24] 1341a 66.2b 1335a 83.8b This work aThe number indicates the total number of patients; bThe number is obtained as Σ (patientn × sensitivityn)/1341 and represents the overall sen- sitivity; cThe number indicates the total number of patients; dThe number is obtained as Σ (patientn × sensitivityn)/1335 and represents the overall specificity. with a specificity of 88.0% for MRI detectable bladder involvement [7]. Postema et al., found a sensitivity and a specificity of 77.0% and 97.0%, respectively, for bladder invasion detection by MRI [8]. 5 of eighteen patients, suspected on MRI to have mucosal bladder invasion were not confirmed at histology. Overall MRI specificity and sensibility for the MRI detection of mucosal bladder invasion was 93.5 and 100% in the 92 patient series [9]. MRI detection of rectal involvement. Rockall et al., found 10 false positives out of 112 patients with 100.0% sensitivity and 91.0% specificity [6]. According to Ra- jaram et al., MRI correctly assessed both spaces with accuracy of 88.9 % in vesicocervical space, and 66.7% for rectovaginal space [10]. Hricak et al., showed that neither CAT nor MRI were able to detect four cases pre- senting with tumor in the rectum [11]. Hertel et al., cal- culated a MRI sensitivity of 50.0% and a specificity of 86.0% for rectal invasion [7]. MRI detection of vaginal invasion. Choi et al., [12] in 23 patients reported a sensitivity of 87.0% and a specifi- city of 79.0% with 3 false negatives. Sheu et al., [13] identified vaginal involvement in 9 patients, with 2 false negatives and 6 false positives at histological comparison. The sensitivity was 82.0% and specificity 84.0%. Errors in the assessment of vaginal invasion occurred in 8 cases out of 11. In the Sheu’s study [14], overall sensitivity and specificity for vaginal invasion detection were 75.0% and 88.0%, respectively. Uterine body involvement cannot be established by the examining gynaecologist. Sahdev et al., [15] found, for internal os involvement detection, a MRI sensitivity of 90.0% and a specificity of 98.0%. MRI detection of myometrial invasion. This was ob- served through MRI by Sahdev et al., [15] with a sensi- tivity of 100.0% and a specificity of 99.0%. MRI cor- rectly detected all of the 7 tumors extending to the en- dometrium on 150 patients enrolled in his study. Ac- cording to Wang et al., [16], tumors extending to the uterine body were identified by MRI in 3 out of 4 pa- tients. In one case microscopic extension to the endo- metrium was not detected by MRI. Yoo et al., [17] stu- died 99 patients and reported sensitivity and specificity for deep myometrial invasion MRI detection of 46.6%, and 84.5%, respectively. Primary tumor detection by MRI. Sahdev et al., [15] pointed out a 20/60 incidence of suspect lesions at MRI when no tumor was histologically detectable and sug- gested scar lesions as the culprit. The false negative rate, for the detection of small tumors (<1 cm diameter), was 50.0% (14/28). Fischerova et al., [18] observed a tumor detection sensitivity of 44% at MRI, with a specificity of 94.3% for tumor bulks ≤1 cm3. Itoh et al., [19] set tumor detection sensitivity at 75.0% in a stage I patient. Lesions over 2 cm were detected in 83.0% of cases and in 60% under 2 cm. de Souza et al., [20] observed a tumor detec- tion sensitivity of 87.0% and specificity of 65.0% in 43 Copyright © 2012 SciRes. OJRad  G. RIGON ET AL. 17 patients with tumor volumes of ≤1 cm³. Sensitivity and specificity in patients with conization were 95.1% and 57.9%, respectively, while the same values in patients without conization were 98.2%, and 66.7%, respectively. According to Sahdev et al., [15], whose study included microscopic stage Ia tumors, detection sensitivity and specificity of the primary tumor was 65.0% and 77.0%, respectively. The total false-positive MRI rate was 30.6% (19/62) possibly due to tissue post-biopsy oedema or chronic inflammation. Fischerova et al., [18], had a more favourable experience and found MRI sensitivity for tu- mor detection of 82.9%, with a specificity of 84.2%. de Souza et al., [20] found false positives in 9 over 119 cases with an overall sensitivity of 96.9% and a specifi- city of 59.1%. Total results are influenced by low MRI performance in case of small primary tumors. Tumor size estimation. According to Sahdev et al., [15], there is an average tumor size estimation error of 9 mm, with 95% limits of agreement from 12.6 mm to 13.0 mm when compared with histology. Error drops to 3 mm if the tumor exceeds 10 mm. Park et al., [21] showed a volume estimation error of 71.9% if the lesion was con- fined to the cervix and up to 18.8% if extending to the vagina. Sheu et al., [13], for tumors over 1 cm, observed the overestimation of tumor size in 3 patients: this was due to inability to differentiate tumor from tissue oedema resulting from previous biopsy or previous cone biopsy. In two cases tumor size was underestimated and errors in measurement were due to indistinct lesion margin. Ac- cording to Sheu et al., [14], tumor size detected by MRI was 3.23 ± 1.75 cm (mean ± standard deviation) com- pared with 2.79 ± 1.76 cm at pathology evaluation. Over-estimation of tumor size was consistent in tumors larger than 10 mm. 4. Discussions Routine MRI equipment has been updated periodically, but no trend towards a better diagnostic accuracy has been found [22], and, in all the papers we reviewed, the authors used standard 1.5 Tesla equipment. Advanced MRI techniques, like ultra-small particles of iron oxide (USPIO) [23], endorectal/phased-array coils [16], con- trast media enhancement [14] and others (endovaginal opacification [15], dynamic MRI imaging, short tau in- version recovery [STIR] sequence [5]) have been used in a limited number of studies but are not addressed by our review. All these techniques might improve diagnostic accuracy of MRI studies. MRI results vary according to the radiologist’s experience [8,22]. Only the results with routine MRI in a clinical setting have been considered in this paper. The overall patient sample set is biased [23] as patients with inoperable tumors were excluded. However, it should be noted that bulky tumors were well represented in all reviewed papers. Tissue preparation for histology could possibly lead to dehydration and shrinkage of the tumor mass. MRI overestimation and underestimation of tumor volume were equally represented in the available data. Tumor detection and size estimation. MRI evaluation does not precisely correlate with histologic tumor size unless the tumor is bulky (at least 1 cm diameter) [24]. Tumor size estimation by MRI [25] seems accurate in Ib to IIb stages, with a correlation of 0.93%. The problems observed with MRI evaluation of primary tumor size are: 1) no detection; 2) impossibility of margin definition; 3) confusion with peritumoral reaction or scars. The issue is possibly relevant only if the tumor is confined to the cer- vix (Figures 1(A)-(B)). The incidence of false positive diagnoses with MRI in Ia1 tumors was 33.0% (20/60), while false negatives reached 50.0% [12]. Correct diag- noses were mostly obtained in cases with a previous cone biopsy suggesting scar and not tumor detection. Some recent data reported by Fischerova et al., are encouraging [18] showing an overall sensitivity of 82.9% and a speci- ficity of 84.2% in primary tumor detection. For volumes under 1 cm3, the values became 44.0% and 94.3%, re- spectively. Internal os involvement and myometrial invasion. Pre- liminary data suggest good results with MRI in the defi- nition of cervical internal os involvement. Myometrial and endometrial invasion detection is possible by MRI (Figure 1(D)) [9]. This issue is not unrelated to tumor bulk. Some lesions misdiagnosed as bulky first stages (Figure 1(C)), with unfavourable outcome might include relatively more advanced cases with uterine body inva- sion demonstrable identified by MRI. Bipat et a l., [26] in 21 patients shows that MRI has a good chance of visual- izing the internal os. No false-negatives for involvement of the uterine corpus and 3 false positives with suspect uterine body invasion were observed by the authors. Lymph node involvement. Lymph node invasion is dif- ficult to determine with certainty by MRI and no definite cut-off has been established as regards volume or maxi- mum diameter of nodes. Criteria for nodal involvement are not standardized. Sahdev et al., [15] suggests that the prediction of nodal status was most accurate when a 9 mm cut-off was ap- plied to the short-axis diameter of the lymph node [27]. de Souza et al., [20], using a cut-off volume of 5.2 cm³, observed that lymph node metastasis could be predicted with a 78.6% sensitivity and a 72.5% specificity. A cut- off of 2.8 cm³ improves sensitivity to 89.0% but this gives a poor specificity of only 67.0%. Park et al., [21] reports that using 1 cm diameter cut-off sensitivity and specificity were 57.0% and 73.0%, respectively, while Narayan et al., [28] with the same cut-off observes a MRI sensitivity of 50.0%. MRI apparently might fail Copyright © 2012 SciRes. OJRad 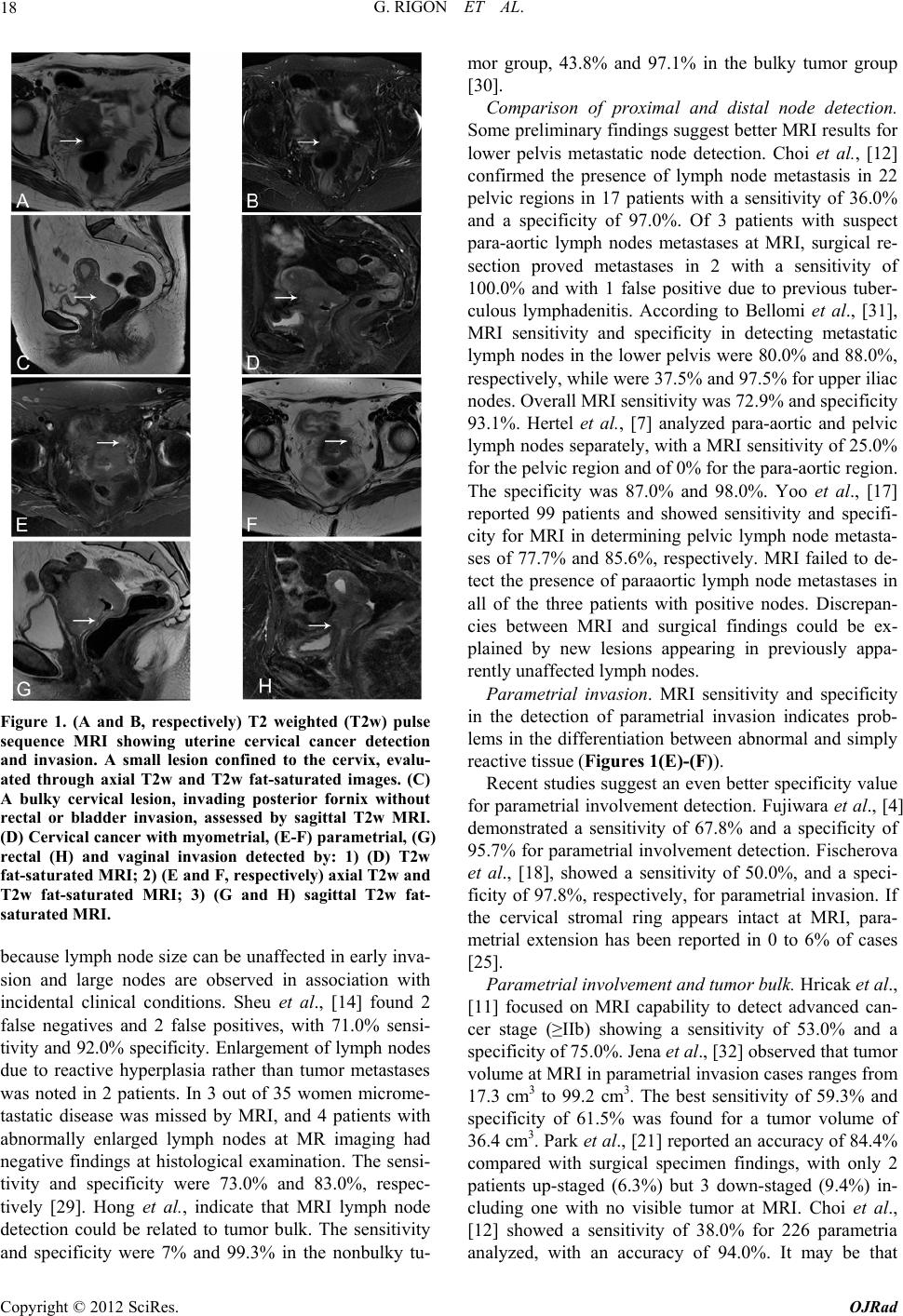 G. RIGON ET AL. 18 Figure 1. (A and B, respectively) T2 weighted (T2w) pulse sequence MRI showing uterine cervical cancer detection and invasion. A small lesion confined to the cervix, evalu- ated through axial T2w and T2w fat-saturated images. (C) A bulky cervical lesion, invading posterior fornix without rectal or bladder invasion, assessed by sagittal T2w MRI. (D) Cervical cancer with myometrial, (E-F) parametrial, (G) rectal (H) and vaginal invasion detected by: 1) (D) T2w fat-saturated MRI; 2) (E and F, respectively) axial T2w and T2w fat-saturated MRI; 3) (G and H) sagittal T2w fat- saturated MRI. because lymph node size can be unaffected in early inva- sion and large nodes are observed in association with incidental clinical conditions. Sheu et al., [14] found 2 false negatives and 2 false positives, with 71.0% sensi- tivity and 92.0% specificity. Enlargement of lymph nodes due to reactive hyperplasia rather than tumor metastases was noted in 2 patients. In 3 out of 35 women microme- tastatic disease was missed by MRI, and 4 patients with abnormally enlarged lymph nodes at MR imaging had negative findings at histological examination. The sensi- tivity and specificity were 73.0% and 83.0%, respec- tively [29]. Hong et al., indicate that MRI lymph node detection could be related to tumor bulk. The sensitivity and specificity were 7% and 99.3% in the nonbulky tu- mor group, 43.8% and 97.1% in the bulky tumor group [30]. Comparison of proximal and distal node detection. Some preliminary findings suggest better MRI results for lower pelvis metastatic node detection. Choi et al., [12] confirmed the presence of lymph node metastasis in 22 pelvic regions in 17 patients with a sensitivity of 36.0% and a specificity of 97.0%. Of 3 patients with suspect para-aortic lymph nodes metastases at MRI, surgical re- section proved metastases in 2 with a sensitivity of 100.0% and with 1 false positive due to previous tuber- culous lymphadenitis. According to Bellomi et al., [31], MRI sensitivity and specificity in detecting metastatic lymph nodes in the lower pelvis were 80.0% and 88.0%, respectively, while were 37.5% and 97.5% for upper iliac nodes. Overall MRI sensitivity was 72.9% and specificity 93.1%. Hertel et al., [7] analyzed para-aortic and pelvic lymph nodes separately, with a MRI sensitivity of 25.0% for the pelvic region and of 0% for the para-aortic region. The specificity was 87.0% and 98.0%. Yoo et al., [17] reported 99 patients and showed sensitivity and specifi- city for MRI in determining pelvic lymph node metasta- ses of 77.7% and 85.6%, respectively. MRI failed to de- tect the presence of paraaortic lymph node metastases in all of the three patients with positive nodes. Discrepan- cies between MRI and surgical findings could be ex- plained by new lesions appearing in previously appa- rently unaffected lymph nodes. Parametrial invasion. MRI sensitivity and specificity in the detection of parametrial invasion indicates prob- lems in the differentiation between abnormal and simply reactive tissue (Figur es 1(E)-(F)). Recent studies suggest an even better specificity value for parametrial involvement detection. Fujiwara et al., [4] demonstrated a sensitivity of 67.8% and a specificity of 95.7% for parametrial involvement detection. Fischerova et al., [18], showed a sensitivity of 50.0%, and a speci- ficity of 97.8%, respectively, for parametrial invasion. If the cervical stromal ring appears intact at MRI, para- metrial extension has been reported in 0 to 6% of cases [25]. Parametrial involvement and tumo r bulk. Hricak et al., [11] focused on MRI capability to detect advanced can- cer stage (≥IIb) showing a sensitivity of 53.0% and a specificity of 75.0%. Jena et al., [32] observed that tumor volume at MRI in parametrial invasion cases ranges from 17.3 cm3 to 99.2 cm3. The best sensitivity of 59.3% and specificity of 61.5% was found for a tumor volume of 36.4 cm3. Park et al., [21] reported an accuracy of 84.4% compared with surgical specimen findings, with only 2 patients up-staged (6.3%) but 3 down-staged (9.4%) in- cluding one with no visible tumor at MRI. Choi et al., [12] showed a sensitivity of 38.0% for 226 parametria analyzed, with an accuracy of 94.0%. It may be that Copyright © 2012 SciRes. OJRad  G. RIGON ET AL. 19 specificity is negatively affected by increasing tumor bulk. According to Testa et al., MRI provided low sensi- tivity (2/5, 40.0%) for the presence of parametrial infil- tration [33]. MRI interpretation might very according to different radiologists. Reviewing parametrial invasion, Postema et al. [8], showed that reader nr. 1 reported a sensitivity of 20.0% and a specificity of 97.0%, while reader nr. 2 ob- served a sensitivity and a specificity of 60.0% and 73.0%, respectively. Bladder, rectal and vaginal invasion. Data on rectal invasion detected by MRI in cervical cancer patients are scant and conflicting. Sensitivity varies from 50 to 100% in limited reports. MRI overdiagnoses bladder and rectal invasion. MRI might lead to detection of clinically un- suspected lesions and has determined restaging in one instance [14]. Absolute sensitivity and specificity for MRI detection of rectal invasion (Figure 1(G)) seems to be aligned to those observed in parametrial tumor detec- tion. Vaginal invasion might possibly be underdiagnosed by MRI (Figure 1(H)). 5. Conclusions MRI use is encouraged for cervical cancer staging. There seems to be good correlation between histological and MRI tumor bulk. There is a high probability of non ex- tension to parametria if cervical integrity is observed at MRI and good indication of extension to proximal struc- tures. Primary preoperative evaluation of uterine cancer by MRI does not accurately predict the nodal status and the degree of myometrial and parametrial invasion [17], but MRI shows encouraging progress in the detection of uterine myometrial and endometrial invasion, where CAT scans mostly fail. MRI has been proposed as a substitute for invasive cystoscopy and proctoscopy in the initial screening of cervical cancer and preliminary data seem encouraging. In general, the role of modern imaging techniques still remains undefined in the current management of uterine cervical cancer. MRI and CAT have been approved for use of initial staging in patients with cervical cancer by Medicare and Medicaid Services [33]. Due to its good tissue contrast, MRI is the preferred imaging technique for tumor detection and invasion evaluation in advanced stage disease [22]. In patients with negative results by MRI and CAT, the use of PET has been approved by health care providers such as Medicare [34]. A prelimi- nary report by Testa et al., [33] shows that Ultrasound (US) and MRI has similar sensitivity and specificity in preoperative staging. US has the advantages over MRI of low cost and widespread availability. Despite all limitations, introduction of MRI has im- proved staging in cervical cancer patients according to Chung et al., [3] and the American College of Radiology Imaging Network (ACRIN) study [11], from 30% - 40% to about 70% accuracy [17]. Selman et al., [35] reports a pooled negative likelihood ratio of 50% for lymph-node metastasis detection by MRI in a meta-analysis of the literature. Adding a senti- nel lymph node biopsy can raise the accuracy to 94%. Intraoperative histology could lead to a better primary staging of uterine cervical cancer. According to Rajaram, the incidence of positive nodes in stage IB patients, is approximately 15% [10]. Thus sentinel lymph node (SLN) evaluation, is a practical approach to reduce extensive lymph node dissection. SLN evaluation is an established technique for mela- noma and breast carcinoma staging and its use in vulva, lung and cervical carcinoma treatment is being actively discussed [36-39]. A combined imaging and histology approach might be warranted in the management of uter- ine cervical carcinoma. Awareness of MRI limitations is crucial in primary cervical cancer staging. 6. Acknowledgements Grant of “Fondazione Enrico ed Enrica Sovena” to C.V. REFERENCES [1] A. Jemal, R. Siegel, E. Ward, T. Murray, J. Xu and M. J. Thun, “Cancer Statistics,” CA: A Cancer Journal of Cli- nicians, Vol. 57, No. 1, 2007, pp. 43-66. doi:10.3322/canjclin.57.1.43 [2] H. J. Choi, W. Ju, S. K. Myung and Y. Kim, “Diagnostic Performance of Computed Tomography, Magnetic Reso- nance Imaging, and Positron Emission Tomography or Positron Emission Tomography/Computer Tomography for Detection of Metastatic Lymph Nodes in Patients with Cervical Cancer: Meta-Analysis,” Cancer Science, Vol. 10, No. 6, 2010, pp. 1471-1479. doi:10.1111/j.1349-7006.2010.01532.x [3] H. H. Chung, S. B. Kang, J. Y. Cho, J. W. Kim, N. H. Park, Y. S. Song, S. H. Kim and H. P. Lee, “Can Preop- erative MRI Accurately Evaluate Nodal and Parametrial Invasion in Early Stage Cervical Cancer?” Japanese Journal of Clinical Oncology, Vol. 37, No. 3, 2007, 370- 375. doi:10.1093/jjco/hym036 [4] K. Fujiwara, E. Yoden, T. Asakawa, M. Shimizu, M. Hirokawa, Y. Mikami, T. Oda, I. Joja, Y. Imajo and I. Kohno, “Negative MRI Findings with Invasive Cervical Biopsy May Indicate Stage IA Cervical Carcinoma,” Gy- necoloy Oncology, Vol. 79, No. 3, 2000, pp. 451-456. doi:10.1006/gyno.2000.5967 [5] W. W. M. Lam, N. M. C. So, W. T. Yang and C. Me- treweli, “Detection of Parametrial Invasion in Cervical Carcinoma: Role of Short Tau Inversion Recovery Se- quence,” Clinical Radiology, Vol. 55, No, 9, 2000, pp. 702-707. doi:10.1053/crad.2000.0506 [6] A. G. Rockall, S. Ghosh, F. Alexander-Sefre, S. Babar, M. T. Younis, S. Naz, I. J. Jacobs and R. H. Reznek, “Can Copyright © 2012 SciRes. OJRad  G. RIGON ET AL. 20 MRI Rule Out Bladder and Rectal Invasion in Cervical Cancer to Help Select Patients for Limited EUA?” Gyne- cology Oncology, Vol. 101, No. 2, 2006, pp. 244-249. doi:10.1016/j.ygyno.2005.10.012 [7] H. Hertel, C. Köhler, T. Elhawary, W. Michels, M. Poss- over and A. Schneider, “Laparoscopic Staging Compared with Imaging Techniques in the Staging of Advanced Cervical Cancer,” Gynecology Oncology, Vol. 87, No. 1, 2002, pp. 46-51. doi:10.1006/gyno.2002.6722 [8] S. Postema, P. T. M. Pattynama, A. Van den Berg-Huy- smans, P. W. Lex, G. Kenter and J. B. Trimbos, “Effect of MRI on Therapeutic Decisions in Invasive Cervical Carcinoma. Direct Comparison with the Pelvic Examina- tion as a Preoperative Test,” Gynecology Oncology, Vol. 79, No. 3, 2000, pp. 485-489. doi:10.1006/gyno.2000.5986 [9] H. Nam, S. J. Huh, W. Park, D. S. Bae, B. G. Kim, J. H. Lee, C. K. Kim and B. K. Park, “Prognostic Significance of MRI-Detected Bladder Muscle and/or Serosal Invasion in Patients with Cervical Cancer Treated with Radiother- apy,” British Journal of Radiology, Vol. 83, No. 994, 2010, pp. 868-873. doi:10.1259/bjr/6646798 [10] S. Rajaram, H. Sharma, S. K. Bhargava, R. P. Tripathi, N. Goel and S. Mehta, “Mapping the Extent of Disease by Multislice Computed Tomography, Magnetic Resonance Imaging and Sentinel Node Evaluation in Stage I and II Cervical Carcinoma,” Journal of Cancer Research and Therapeutics, Vol. 6, No. 3, 2010, pp. 267-271. doi:10.4103/0973-1482.73342 [11] H. Hricak, C. Gatsonis, D. S. Chi, M. A. Amendola, K. Brandt, L. H. Schwartz, S. Koelliker, E. S. Siegelman, J. J. Brown, R. B. McGhee Jr., R. Iyer, K. M. Vitellas, B. Snyder, H. J. Long III, J. V. Fiorica and D. G. Mitchell, “Role of Imaging in Pretreatment Evaluation of Early In- vasive Cervical Cancer: Results of the Intergroup Study American College of Radiology Imaging Network 6651- Gynecology Oncology Group 183,” Journal of Clinical Oncology, Vol. 23, No. 36, 2005, pp. 9329-9337. doi:10.1200/JCO.2005.02.0354 [12] S. H. Choi, S. H. Kim, H. J. Choi, B. K. Park and H. J. Lee, “Preoperative Magnetic Resonance Imaging Staging of Uterine Cervical Carcinoma,” Journal of Computer Assisted Tomography, Vol. 28, No. 5, 2004, pp. 620-627. [13] M. H. Sheu, C. Y, Chang, J. H. Wang and M. S. Yen, “Preoperative Staging of Cervical Carcinoma with MR Imaging: A Reappraisal of Diagnostic Accuracy and Pit- falls,” European Radiology, Vol. 11, No. 9, 2001, pp. 1828-1833. doi:10.1007/s003300000774 [14] M. H. Sheu, C. Y. Chang, J. H. Wang and M. S. Yen, “MR Staging of Clinical Stage I and IIa Cervical Carci- noma: A Reappraisal of Efficacy and Pitfalls,” European Journal of Radiology, Vol. 38, No. 3, 2001, pp. 225-231. doi:10.1016/S0720-048X(00)00278-3 [15] A. Sahdev, S. A. Sohaib, A. E. T. Wenaden, J. H. Shep- herd and R. H. Reznek, “The Performance of Magnetic Resonance Imaging in Early Cervical Carcinoma: A Long Term Experience,” International Journal of Gynecologi- cal Cancer, Vol. 17, No. 3, 2007, pp. 629-636. doi:10.1111/j.1525-1438.2007.00829.x [16] L. J. Wang, Y. C. Wong, C. J. Chen, K. G. Huang and S. Hsueh, “Cervical Carcinoma: MR Imaging with Inte- grated Endorectal/Phased-Array Coils: A Pilot Study,” European Radiology, Vol. 11, No. 9, 2001, pp.1822-1827. doi:10.1007/s003300000794 [17] S. C. Yoo, W. Y. Kim, J. H. Yoon, H. Y. Kim, E. J. Lee, S. J. Chang, K. H. Chang and H. S. Ryu, “Accuracy of Preoperative Magnetic Resonance Imaging in Assessing Lymph Node Metastasis and Myometrial Invasion in Pa- tients with Uterine Cancer,” European Journal of Gy- naecological Oncology, Vol. 30, No. 2, 2009, pp. 167- 170. [18] D. Fischerova, D. Cibula, H. Stenhova, H. Vondrichova, P. Calda, M. Zikan, P. Freitag, J. Slama, P. Dundr and J. Belacek, “Transrectal Ultrasound and Magnetic Reso- nance Imaging in Staging of Early Cervical Cancer,” In- ternational Journal of Gynecological Cancer, Vol. 18, No. 4, 2008, pp. 766-772. doi:10.1111/j.1525-1438.2007.01072.x [19] K. Itoh, T. Shiozawa, S. Ohira, S. Shiohara and I. Konishi, “Correlation between MRI and Histopathologic Findings in Stage I Cervical Carcinomas: Influence of Stromal Desmoplastic Reaction,” International Journal of Gyne- cological Cancer, Vol. 16, No. 2, 2006, pp. 610-614. doi:10.1111/j.1525-1438.2006.00383.x [20] N. M. de Souza, R. Dina, G. A. McIndoe and W. P. Sout- ter, “Cervical Cancer: Value of an Endovaginal Coil Magnetic Resonance Imaging Technique in Detecting Small Volume Disease and Assessing Parametrial Exten- sion,” Gynecology Oncology, Vol. 102, No. 1, 2006, pp. 80-85. doi:10.1016/j.ygyno.2005.11.038 [21] W. Park, Y. J. Park, S. J. Huh, B. G. Kim, D. S. Bae, J. Lee, B. H. Kim, J. Y. Choi, Y. C. Ahn and D. H. Lim, “The Usefulness of MRI and PET Imaging for the Detec- tion of Parametrial Involvement and Lymph Node Metas- tasis in Patients with Cervical Cancer,” Japanese Journal of Clinical Oncology, Vol. 35, No. 5, 2005, pp. 260-264. doi:10.1093/jjco/hyi079 [22] K. Hancke, V. Heilmann, P. Straka, R. Kreienberg and C. Kurzeder, “Pre-Treatment Staging of Cervical Cancer: Is Imaging Better Than Palpation? Role of CT and MRI in Preoperative Staging of Cervical Cancer: Single Institu- tion Results of 255 Patients,” Annals of Surgical Oncol- ogy, Vol. 15, No. 10, 2008, pp. 2856-2861. doi:10.1245/s10434-008-0088-7 [23] A. G. Rockall, S. A. Sohaib, M. G. Harisinghani, S. A. Babar, N. Singh, A. R. Jeyarajah, D. H. Oram, I. J. Jacobs, J. H. Shepherd and R. H. Reznek, “Diagnostic Perform- ance of Nanoparticle-Enhanced Magnetic Resonance Im- aging in the Diagnosis of Lymph Node Metastases in Pa- tients with Endometrial and Cervical Cancer,” Journal of Clinical Oncology, Vol. 23, No. 12, 2005, pp. 2813-2821. doi:10.1200/JCO.2005.07.166 [24] R. Manfredi, B. Gui, A. Giovanzana, S. Marini, M. Di Stefano, G. Zannoni, G. Scambia and L. Bonomo, “Lo- calized Cervical Cancer (Stage < IIB): Accuracy of MR Imaging in Planning Less Extensive Surgery,” La Ra- diologia Medica, Vol. 114, No. 6, 2009, pp. 960-975. doi:10.1007/s11547-009-0397-3 [25] E. Sala, S. Wakely, E. Senior and D. Lomas, “MRI of Copyright © 2012 SciRes. OJRad  G. RIGON ET AL. Copyright © 2012 SciRes. OJRad 21 Malignant Neoplasms of the Uterine Corpus and Cervix,” American Journal of Roentgenology, Vol. 188, No. 6, 2007, pp. 1577-1587. doi:10.2214/AJR.06.1196 [26] S. Bipat, R. A. van den Berg, J. van der Velden, J. Stoker and A. M. Spijkerboer, “The Role of Magnetic Resonance Imaging in Determining the Proximal Extension of Early Stage Cervical Cancer to the Internal OS,” European Journal of Radiology, Vol. 78, No. 1, 2009, pp. 60-64. doi:10.1016/j.ejrad.2009.06.006 [27] H. J. Choi, S. H. Kim, S. S. Seo, S. Kang, S. Lee, J. Y. Kim, Y. H. Kim, J. S. Lee, H. H. Chung, J. H. Lee and S. Y. Park, “MRI for Pretreatment Lymph Node Staging in Uterine Cervical Cancer,” American Journal of Roent- genology, Vol. 187, No. 5, 2006, pp. 538-543. doi:10.2214/AJR.05.0263 [28] K. Narayan, R. J. Hicks, T. Jobling, D. Bernshaw and A. F. Mckenzie, “A Comparison of MRI and PET Scanning in Surgically Staged Loco-Regionally Advanced Cervical Cancer: Potential Impact on Treatment,” International Journal of Gynecological Cancer, Vol. 11, No. 4, 2001, pp. 263-271. doi:10.1046/j.1525-1438.2001.011004263.x [29] M. J. Reinhardt, C. Ehritt-Braun, D. Vogelgesang, C. Ihling, S. Högerle, M. Mix, E. Moser and T. M. Krause, “Metastatic Lymph Nodes in Patients with Cervical Can- cer: Detection with MR Imaging and FDG PET,” Radi- ology, Vol. 218, No. 3, 2001, pp. 776-782. [30] K. S. Hong, W. Ju, H. J. Choi, J. K. Kim, M. H. Kim and K. S. Cho, “Differential Diagnostic Performance of Mag- netic Resonance Imaging in the Detection of Lymph Node Metastases According to the Tumor Size in Early- Stage Cervical Cancer Patients,” International Journal of Gynecological Cancer, Vol. 20, No. 5, 2010, pp. 841-846. doi:10.1111/IGC.0b013e3181db5140 [31] M. Bellomi, G. Bonomo, F. Landoni, G. Villa, M. E. Leon, L. Bocciolone, A. Maggioni and G. Viale, “Accuracy of Computed Tomography and Magnetic Resonance Imag- ing in the Detection of Lymph Node Involvement in Cervix Carcinoma,” European Radiology, Vol. 15, No. 12, 2005, pp. 2469-2474. doi:10.1007/s00330-005-2847-1 [32] A. Jena, R. Oberoi, S. Rawal, S. K. Das and K. K. Pandey, “Parametrial Invasion in Carcinoma of Cervix: Role of MRI Measured Tumour Volume,” British Journal of Ra- diology, Vol. 78, No. 936, 2005, pp. 1075-1077. doi:10.1259/bjr/36116150 [33] A. C. Testa, M. Ludovisi, R. Manfredi, G. Zannoni, B. Gui, D. Basso, A. Di Legge, A. Licameli, R. Di Bidino, G. Scambia and G. Ferrandina, “Transvaginal Ultrasonogra- phy and Magnetic Resonance Imaging for Assessment of preseNce, Size and Extent of Invasive Cervical Cancer,” Ultrasound in Obstetrics and Gynecology, Vol. 34, No. 3, 2009, pp. 335-344. doi:10.1002/uog.7325 [34] Conference Report, “The Contribution of New Imaging Techniques in Staging Cervical Cancer,” Gynecology Oncology, Vol. 107, Suppl. 1, 2007, pp. S10-S12. [35] T. J. Selman, C. Mann, J. Zamora, T. L. Appleyard and K. Khan, “Diagnostic Accuracy of Test for Lymph-Node Status in Primary Cervical Cancer: A Systematic Review and Meta-Analysis,” Canadian Medical Association Journal, Vol. 25, No. 7, 2008, pp. 855-862. doi:10.1503/cmaj.071124 [36] D. L. Morton, J. F. Thompson, R. Essner, R. Elashoff, S. L. Stern, O. E. Nieweg, D. F. Roses, C. P. Karakousis, N. Mozzillo, D. Reintgen, H. J. Wang, E. C. Glass and A. J. Cochran, “Validation of the Accuracy of Intraoperative Lymphatic Mapping and Sentinel Lymphadenectomy for Early-Stage Melanoma: A Multicenter Trial. Multicenter Selective Lymphadenectomy Trial Group,” Annals of Surgery, Vol. 230, No. 4, 1999, pp. 453-463. doi:10.1097/00000658-199910000-00001 [37] K. M. McMasters, S. L. Wong, C. Chao, C. Woo, T. M. Tuttle, R. D. Noyes, D. J. Carlson, A. L. Laidley, T. Q. McGlothin, P. B. Ley, C. M. Brown, R. L. Glaser, R. E. Pennington, P. S. Turk, D. Simpson, M. J. Edwards and University of Louisville Breast Cancer Study Group, “Defining the Optimal Surgeon Experience for Breast Cancer Sentinel Lymph Node Biopsy: A Model for Im- plementation of New Surgical Techniques,” Annals of Surgery, Vol. 234, No. 3, 2001, pp. 292-299. doi:10.1097/00000658-200109000-00003 [38] C. Levenback, T. W. Burke, D. M. Gershenson, M. Mor- ris, A. Malpica and M. I. Ross, “Intraoperative Lymphatic Mapping for Vulvar Cancer,” Obstetrics and Gynecology, Vol. 84, No. 2, 1994, pp. 163-167. [39] M. J. Liptay, G. A. Masters, D. J. Winchester, B. L. Edelman, B. J. Garrido, T. R. Hirschtritt, R. M. Perlman and W. A. Fry, “Intraoperative Radioisotope Sentinel Lymph Node Mapping in Non-Small Cell Lung Cancer,” The Annals of Thoracic Surgery, Vol. 70, No. 2, 2000, pp. 384-389. doi:10.1016/S0003-4975(00)01643-X [40] W. P. Soutter, J. Hanoch, T. D’Arcy, R. Dina, G. A. Mc- Indoe and N. M. DeSouza, “Pretreatment Tumour Vol- ume Measurement on High-Resolution Magnetic Reso- nance Imaging as a Predictor of Survival in Cervical Cancer,” British Journal of Obstetrics and Gynaecology, Vol. 111, No. 7, 2004, pp. 741-747. doi:10.1111/j.1471-0528.2004.00172.x [41] H. J. Choi, J. W. Roh, S. S. Seo, S. Lee, J. Y. Kim, S. K. Kim, K. W. Kang, J. S. Lee, J. Y. Jeong and S. Y. Park, “Comparison of the Accuracy of Magnetic Resonance Imaging and Positron Emission Tomography/Computed Tomography in the Presurgical Detection of Lymph Node Metastases in Patients with Uterine Cervical Carcinoma,” Cancer, Vol. 106, 4, 2006, pp. 914-922. doi:10.1002/cncr.21641
|