 Open Journal of Medicinal Chemistry, 2012, 2, 1-9 http://dx.doi.org/10.4236/ojmc.2012.21001 Published Online March 2012 (http://www.SciRP.org/journal/ojmc) The Reaction of Cyanoacetylhydrazine with Chloroacetone: Synthesis of 1,2,4-Triazine, 1,3,4-Oxadiazine and Their Fused Derivatives with Antitumor Activities Rafat M. Mohareb1,2*, Eman M. Samir3 1Department of Organic Chemistry, Faculty of Pharmacy, October University for Modern Sciences & Arts, Giza, Egypt 2Department of Chemistry, Faculty of Science, Cairo University, Giza, Egypt 3National Organization for Drug Control & Research, Cairo, Egypt Email: *raafat_mohareb@yahoo.com Received January 17, 2012; revised February 15, 2012; accepted February 25, 2012 ABSTRACT The reaction of cyanoacetylhydrazine with chloroacetone gave the N-(1-chloropropan-2-ylidene)-2-cyanoacetohy- drazide. This compound reacted with either hydrazine hydrate or phenylhydrazine to give the corresponding 1,2,4-tri- azine derivatives. On the other hand, its reaction with either benzenediazonium chloride or benzaldehyde gave in each case the 1,3,4-oxadiazine derivatives. Moreover, the reaction of the cyanoacetylhydrazine with 2-boromocyclohexa- none gave the corresponding hydrazine-hydrozon derivative. The antitumor evaluation of the newly synthesized prod- ucts against three cancer cell lines, namely breast adenocarcinoma (MCF-7), non-small cell lung cancer (NCI-H460) and CNS cancer (SF-268) was recorded. Some of the tested compounds showed activities which was higher than the reference doxorubicin. Keywords: 1,2,4-Triazine; 1,3,4-Oxadiazine; Pyrazole; Antitumor 1. Introduction It is well known that the hydrazone group plays an im- portant role for the antimicrobial activity. Furthermore, a number of hydrazide-hydrazone claimed to possess in- teresting antibacterial antifungal [1-3] anticonvulsant [4-6] anti-inflammatory [7-9] antimalarial [10] and anti- tuberculosis activities [11-15] With the aim of obtaining new hydrazide-hydrazones with such wide spectrum of pharamaceutical applications, we report here the synthe- sis of a series of hydrazide-hydrzones via the reaction of cyanoacetylhydrazine (1) with -haloketones [16,17]. All synthesized compounds have been screened for anti- tumor activity against breast adenocarcinoma (MCF-7), non-small cell lung cancer (NCI-H460) and CNS cancer (SF-268). 2. Results and Discussions The reaction of 1 with chloroacetone (2) in 1,4-dioxane gave the hydrazide-hydrazon derivative 3. The structure of compound 3 was based on the analytical and spectral data. Thus, the 1H NMR spectrum of the reaction product showed the presence of a singlet at δ 2.36 ppm corre- sponding to CH3 group, two singlets at δ 3.37, 4.02 ppm corresponding to two CH2 groups and a singlet at δ 8.09 ppm for the NH group. Further confirmation for the stru- cture of compound 3 was obtained through studying its reactivity towards some chemical reagents. Thus, the rea- ction of 3 with either hydrazine hydrate (4a) or phenyl- hydrazine (4b) gave the 1,2,4 triazine derivatives 6a and 6b, respectively. Formation of the latter products is ex- plained through the intermediate formation of 5a,b fol- lowed by water elimination. The reaction of 6b with benzenediazonium chloride (7) gave the phenyl hydrazone derivative 9 (Scheme 1). The reaction took place through the first coupling of 6 with 7 to give the phenylhydrazone derivative 8 followed by Micheal addition of the N-NH group present in the triaz- ine ring to the cyano group to give the pyrazolo[5-1-c] [1,2,4]triazine derivative 9. On the other hand, the reac- tion of 6b with either malononitrile (10a) or ethyl cyan- oacetate (10b) gave the tricyclic products 11a and 11b, respectively. Analytical and spectral data of 11a,b are consistent with the proposed structures (see experimental section). The reaction of compound 3 with either ben- zenediazonium chloride 7 or p-chlorobenzenediazonium chloride 12 gave 1,3,4-oxadiazine derivatives 13a and 13b respectively. On the other hand, the reaction of com- pound 3 with potassium cyanide (14) gave the 3-amino *Corresponding author. C opyright © 2012 SciRes. OJMC  R. M. MOHAREB ET AL. 2 Scheme 1. Synthesis of compounds 3 - 9. pyrazole derivative 15. Formation of the pyrazole deriva- tive 15 took place in analogous with our recent reported work [18]. The reaction of compound 3 with benzaldehyde (16) gave the 2-α-benzal acetonitryl-1,3,4-oxadiazine deriva- tives 18 (Scheme 2) its formation occured through the first formation of the benzal derivative followed by HCl liberation. The 1HNMR spectrum of the latter product showed the presence of a singlet at δ 2.68 for CH3 group, a singlet at δ 5.26 for oxadiazine CH2 group, a singlet at δ 6.05 for CH = C and a multiplet at δ 7.22 - 7.35 for C6H5 group. The reaction of compound 18 with either malononitrile (10a) or ethyl cyanoacetate (10b) gave the pyrido [2,1-b] 1,3,4-oxadiazine derivatives 20a and 20b. The structure elucidation of the latter products was based on analytical and spectral data (see experimental section). Next, we studied the reaction of cyanoacetylhydrazine (1) with 2- bromocyclohexanone (21) in 1,4-dioxane at room tem- perature. The reaction leads to the formation of the hy- drazide-hydrazone derivative 22. The analytical and spe- ctral and spectral data of the latter product are in agree- ment with the proposed structure (see experimental sec- tion). Compound 22 reacted with either hydrazine hydrate (4a) or phenylhydrazine (4b) to give the tricyclic prod- ucts 24a and 24b, respectively. The formation of 24a,b was explained in terms of intermediate formation of hy- drazono derivatives followed by cyclization to give the intermediates 23a,b, respectively. The latter intermedi- ates underwent further cyclization to give 24a,b. The reaction of 22 with either benzenediazonium chloride (7) Copyright © 2012 SciRes. OJMC 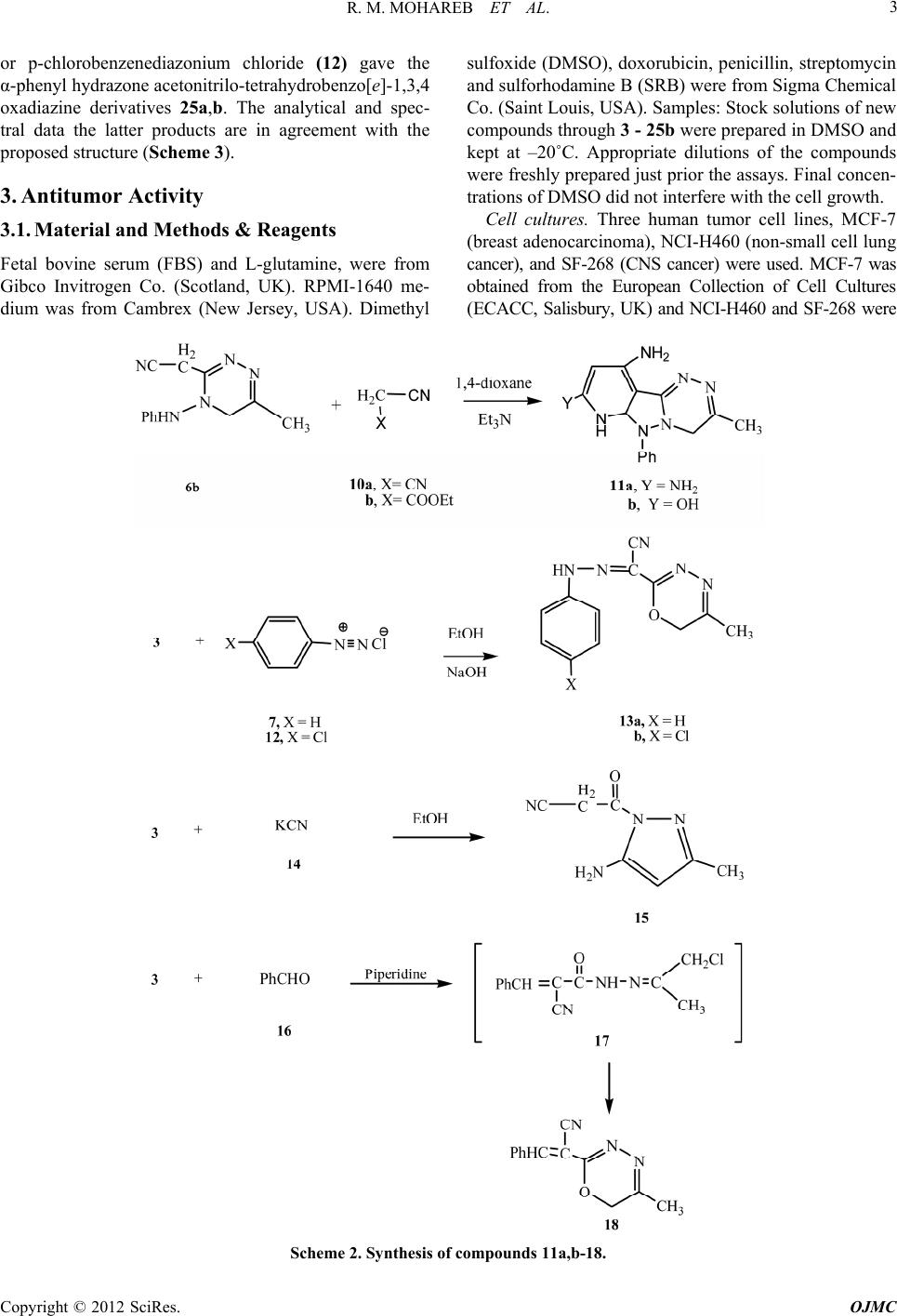 R. M. MOHAREB ET AL. 3 or p-chlorobenzenediazonium chloride (12) gave the α-phenyl hydrazone acetonitrilo-tetrahydrobenzo[e]-1,3,4 oxadiazine derivatives 25a,b. The analytical and spec- tral data the latter products are in agreement with the proposed structure (Scheme 3). 3. Antitumor Activity 3.1. Material and Methods & Reagents Fetal bovine serum (FBS) and L-glutamine, were from Gibco Invitrogen Co. (Scotland, UK). RPMI-1640 me- dium was from Cambrex (New Jersey, USA). Dimethyl sulfoxide (DMSO), doxorubicin, penicillin, streptomycin and sulforhodamine B (SRB) were from Sigma Chemical Co. (Saint Louis, USA). Samples: Stock solutions of new compounds through 3 - 25b were prepared in DMSO and kept at –20˚C. Appropriate dilutions of the compounds were freshly prepared just prior the assays. Final concen- trations of DMSO did not interfere with the cell growth. Cell cultures. Three human tumor cell lines, MCF-7 (breast adenocarcinoma), NCI-H460 (non-small cell lung cancer), and SF-268 (CNS cancer) were used. MCF-7 was obtained from the European Collection of Cell Cultures (ECACC, Salisbury, UK) and NCI-H460 and SF-268 were Scheme 2. Synthesis of compounds 11a,b-18. Copyright © 2012 SciRes. OJMC 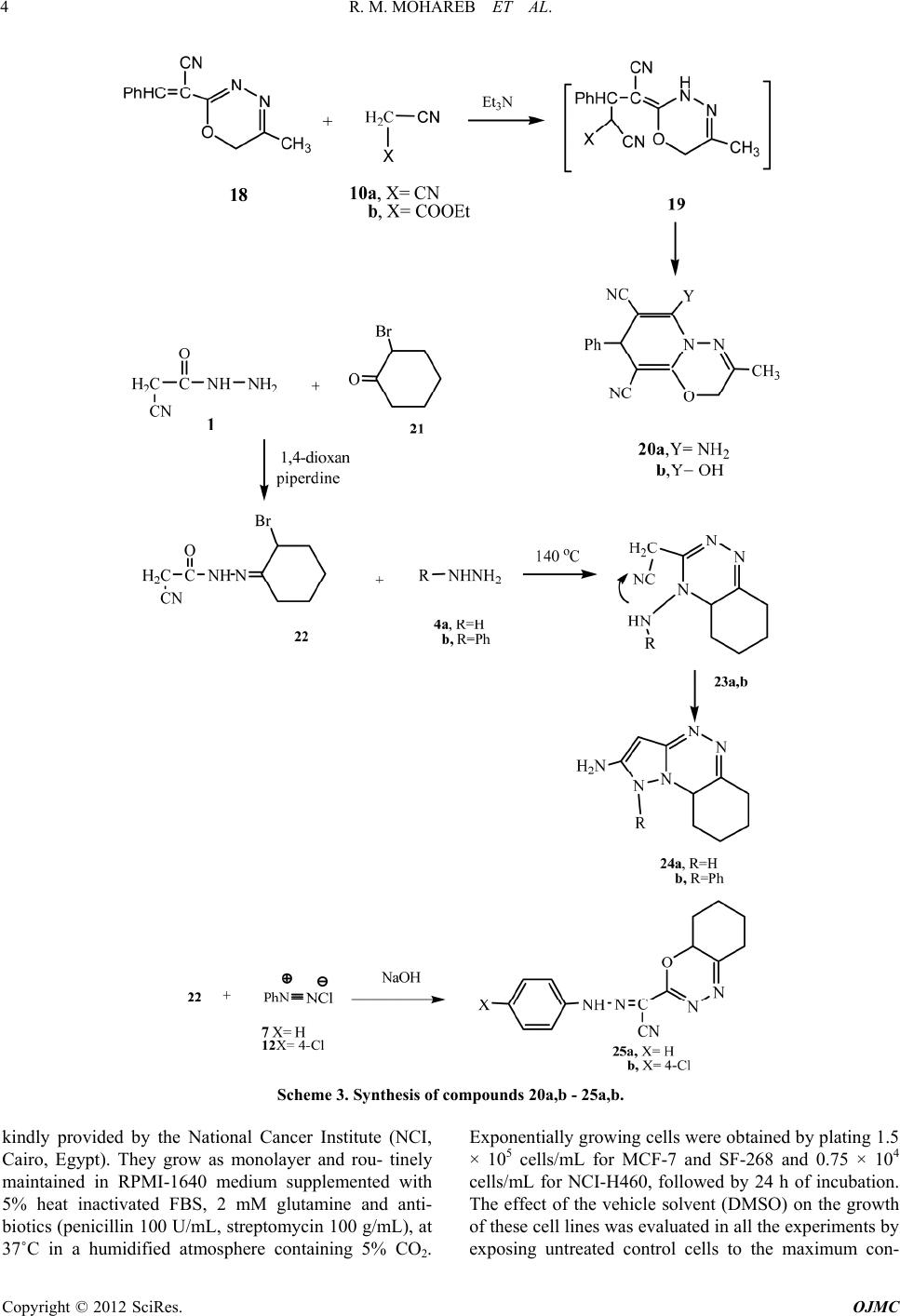 R. M. MOHAREB ET AL. 4 Scheme 3. Synthesis of compounds 20a,b - 25a,b. kindly provided by the National Cancer Institute (NCI, Cairo, Egypt). They grow as monolayer and rou- tinely maintained in RPMI-1640 medium supplemented with 5% heat inactivated FBS, 2 mM glutamine and anti- biotics (penicillin 100 U/mL, streptomycin 100 g/mL), at 37˚C in a humidified atmosphere containing 5% CO2. Exponentially growing cells were obtained by plating 1.5 × 105 cells/mL for MCF-7 and SF-268 and 0.75 × 104 cells/mL for NCI-H460, followed by 24 h of incubation. The effect of the vehicle solvent (DMSO) on the growth of these cell lines was evaluated in all the experiments by exposing untreated control cells to the maximum con- Copyright © 2012 SciRes. OJMC  R. M. MOHAREB ET AL. 5 centration (0.5%) of DMSO used in each assay. 3.2. Effect on the Growth of Human Tumor Cell Lines The effect of compounds 3 - 25b was evaluated on the in vitro growth of three human tumor cell lines representing different tumor types, namely, breast adenocarcinoma (MCF-7), non-small cell lung cancer (NCI-H460) and CNS cancer (SF-268), after a continuous exposure of 48 h. The results are summarized in Table 1. All the com- pounds were able to inhibit the growth of the human tu- mor cell lines in a dose dependent manner (data not shown). Compound 11a (7-methyl-9-phenyl-8,9-dihdro-1, 5,6,8a,9-pentaaza-fluorene-2,4-diamine) and 20b (2,8- dihydro-6-hydro xy-3-methyl-8-phenylp yrido[ 2,1-b][1,3, 4]oxadiazine-7,9-dicarbonitrile) showed the best results, exhibiting an equivalent potency in all the three tumor cell lines; these values are much lower than that of the gram positive control doxorubicin. Compounds 20b and 24b are the following in relation to the anticancer activity. On the other hand, compounds 3, 6a, 9, 11a, 18, 20a, 22, 24a and 25b showed moderated growth inhibitory effect which showed GI50 < 30 mol·L–1. Comparing the activities of 20a and 20b it is observed that the 2-amino group in 20a showed a stronger growth inhibitory effect than the 2-hydroxy substituent in 20b although the results in NCI-H460 cell line are compara- ble. Also comparing compounds 24a and 24b, it is obvious Table 1. Effect of compounds 3 - 25b on the grow th of three human tumor cell lines. GI50 (mol·L–1) Compound No. MCF-7 NCI-H460 SF-268 3 8.0 ± 0.3 12.6 ± 1.8 12.3 ±1.0 6a 12.0 ± 2.2 22.3 ± 3.6 33 ± 0.4 6b 66.2 ± 12.7 30.9 ± 8.2 42.8 ± 8.3 9 10.2 ± 0.8 8.6 ± 1.4 6.2 ± 1.6 11a 0.02 ± 0.002 0.03 ± 0.01 0.01 ± 0.002 11b 30.5 + 12.0 20.1 ± 12.8 20.0 ± 4. 31 13a 30.2 ± 2.2 22.2 ± 6.2 12.6 ± 2.6 13b 16.2 ± 2.2 20.6 ± 1.6 33.2 ± 3.7 15 30.0 ± 2.5 22.0 ± 4.6 20.5 ± 2.8 18 14.0 ± 0.4 12.3 ± 0.6 26.5 ± 4.3 20a 8.4 ± 2.8 14.1 ± 0.6 20.3 ± 0.5 20b 0.02 ± 0.002 0.01 ± 0.008 0.02 ± 0.008 22 22.8 ± 10.0 30.2 ± 6.4 18.2 ± 10.6 24a 20.2 ± 8.2 16.0 ± 2.6 12.2 ± 4.8 24b 0.4 ± 0.08 0.2 ± 0.04 0.1 ± 0.01 25a 40.6 ± 10.0 32.6 ± 8.0 62.4 ± 14.8 25b 12.2 ± 4.0 16.0 ± 2.8 14.2 ± 4.6 Doxorubicin0.04 ± 0.008 0.09 ± 0.008 0.09 ± 0.007 Results are given in concentrations that were able to cause 50% of cell growth inhibition (GI50) after a continuous exposure of 48 h and show means ± SEM of three-independent experiments performed in duplicate. that compound 24b which is the (1-phenyl-1,6,7,8,9a- hexahydro-benzo[e]pyrazolo[5,1-c][1,2,4]triazin-2-ylami ne) with its N-H group is much higher that 24b with its N-Ph group. It is obvious that compounds 6b, 11b, 13a and 25a showed the lowest inhibitory effect towards the three cell line. The relative inhibitory effect of the newly synthesized and the reference doxorubicin, towards the three cancer cell lines, is indicated through Figure 1. 4. Experimental Materials The requisite starting materials such as chloroacetone, hydrazine hydrate, phenyl hydrazine, sodium nitrite, malononitrile, ethyl cyanoacetate, aniline, p-chloro ani- line, potassium cyanide, benzaldehyde, bromocyclohex- anone were produced from Aldrich Company and used without any further purification. All the solvents were purified and dried by standard method. All melting points were determined in open capillaries and are uncorrected. IR spectra were measured using KBr discs on a Pye Unicam SP-1000 spectrophotometer. 1HNMR spectra was measured on a varian EM390-200 MHz instrument in DMSO as solvent using tetramethylsilane (TMS) as internal reference standard. The chemical shifts are quoted as δ ppm. N-(1-Chloropropan-2-ylidene)-2-cyanoacetohydrazi de (3). Equimolecular amounts of 1 (0.99 g, 0.01 mol) and chloroacetone (2) (0.92 g, 0.01 mol) in 1,4-dioxane (25 mL) and drops of dimethylformamide (5 mL) were stirred at room temperature for 1 h. The formed solid product was collected by filtration. White crystals from ethanol in a yield 88% (1.52) g and with m.p. 160˚C; Calculated for C6H8N3OCl (173.59): C, 41.51; H, 4.64; N, 24.20. Found: C, 40.89; H, 4.10; N, 23.75. IR (U/cm–1) = 3450 - 3329 (NH), 2892, 2877 (CH3, CH2), 2260 (CN), 1688 (C=O). 1HNMR (δ ppm): 2.36 (s, 3H, CH3), 3.37, 0 10 20 30 40 50 60 70 doxorubicin 3 6a 6b 9 11a 11b 13a 13b 15 18 20a 20b 22 24a 24b 25a 25b MCF -7 NCI-H460 SF-268 Figure 1. Inhibition of cell growth of the newly synthesized products towards the three cancer cell lines. Copyright © 2012 SciRes. OJMC  R. M. MOHAREB ET AL. 6 4.02 (2s, 4H, 2CH2), 8.09 (s, 1H, NH); MS: m/z 173 (M+, 10%). 3[(4-Amino-6-methyl-4,5-dihydro-[1,2,4]triazin-3-yl )]-acetonitrile (6a) and [6-Methyl-4-(N’-phenyl-hydra zino)-4,5-dihydro-[1,2,4]triazin-3-yl]-acetonitrile (6b). General procedure: To a solution of 3 (1.73 g, 0.01 mol) in 1,4-dioxane (30 mL), and either hydrazine hy- drate (4a) (0.50 g, 0.01 mol) or phenyl hydrazine (4b) (1.08 g, 0.01 mol) were added. The reaction mixture was kept on ice bath for 10 min. The solid product formed upon dilution with ice/water containing hydrochloric acid (till pH6), was collected by filtration. Compound 6a: Brown crystals from ethanol, yield, 60% (0.9) g, m.p. >300˚C. Calculated for C 6H9N5 (151.16): C, 47.67; H, 6.00; N, 46.33. Found: C, 47.00; H, 5.55; N, 45.86. IR (U/cm–1) = 3466 - 3328 (NH2, NH), 3061 (CH aromatic), 2988 (CH3), 2226 (CN), 1658 (C=N), 1643 (C=C). 1HNMR (δ ppm): 2.86 (s, 3H, CH3), 4.23 (s, 2H, CH2), 5.08 (s, 2H, NH2), 5.21 (s, 2H, triazine CH2); MS: m/z 151 (M+, 60%). Compound 6b: Buff crystals from 1,4-dioxane, yield, 72% (1.63) g, m.p. 180˚C. Calculated for C 12H13N5 (227.26): C, 63.42; H, 5.70; N, 30.80. Found: C, 62.89; H, 4.88; N, 29.99. IR (U/cm–1) = 3476 - 3331 (NH), 3055 (CH aromatic), 2982 (CH3), 2261 (CN), 1655 (C=N), 1648 (C=C). 1HNMR (δ ppm): 2.83 (s, 3H, CH3), 4.19 (s, 2H, CH2), 5.28 (s, 2H, triazine CH2) , 7.28 - 7.39 (m, 5H, C6H5), 8.78 (s, 1H, NH); MS: m/z 227 (M+, 40%). 8-(2-Phenylhydrazono)-3-methyl-6-phenylpyrazolo[ 5,1-c][1,2,4]triazin-7(4H,6H,8H)-imine (9). To a cold solution (0˚C - 5˚C) of 6b (2.27 g, 0.01 mol) in ethanol (30 mL) containing sodium hydroxide (10%, 6 mL) benzenediazonium chloride (7) (0.01 mol) [obtained via the addition of sodium nitrite solution (0.70 g, 0.01 mol) to a cold solution (0˚C - 5˚C) of aniline (0.93 g, 0.01 mol) in hydrochloric acid (8 mL) was added with continuous stirring ] was added with continuous stirring. The reaction mixture was stirred for an additional 2 h then the formed solid product was collected by filtration. Orange crystals from ethanol in a yield, 80%, (2.65) g, with m.p. 236˚C - 240˚C. Calculated for C 18H17N7 (331.15): C, 65.22; H, 5.13; N, 29.59, Found: C, 64.66; H,4.76; N, 28.91. IR (U/cm–1) = 3455 - 3323 (2NH), 3052 (CH aromatic), 2980 (CH3), 1655 (C=N), 1648 (C= C). 1HNMR (δ ppm): 2.83 (s, 3H, CH3), 4.65 (s, 1H, D2Oexchangeable, NH), 5.26 (s, 2H, triazine CH2), 7.29 - 7.37 (m, 10H, 2C6H5), 8.82 (s, 1H, D2Oexchangeable, NH); MS: m/z 331 (M+, 100%). 7-Methyl-9-phenyl-8,9-dihydro-1,5,6,8a,9-pentaaza- fluorene-2,4-diamine (11a) and 4-Amino-7-methyl-9- phenyl-8,9-dihydro-1,5,6,8a,9-pentaaza-fluoren-2-ol (11b). General procedure: Equimolecular amounts of 6b (2.27 g, 0.01 mol) and either malononitrile (10a) (0.66 g, 0.01 mol) or ethyl cyanoacetate (10b) (1.13 g, 0.01 mol) were added in 1,4-dioxane (25 mL) containing triethy- lamine (1.00 mL) were heated under reflux for 4 h. The formed solid product upon dilution with ice/water con- taining hydrochloric acid (till pH6), was collected by fil- tration. Compound (11a): Red crystals from ethanol, yield 76% (2.22) g, m.p. 160˚C. Calculated for C 15H17N7 (293.33) C, 61.00; H, 5.80; N, 33.20. Found: C, 61.27; H, 5.53; N,33.44. IR (U/cm–1) = 3473 - 3344 (2NH2), 3057 (CH aromatic), 2980 (CH3), 1662 (C=N), 1640 (C= C).1HNMR (δ ppm): 2.79 (s, 3H, CH3), 4.43, 5.18 (2s, 4H, 2NH2), 5.20 (s, 2H, triazine CH2), 7.23 - 7.36 (m, 8H, C6H5, pyridine 3H); MS: m/z 295 (M+, 65%). Compound (11b): Red crystals from ethanol, yield 77% (2.26) g, m.p. 140˚C. Calculated for C 15H15N6O (296.33): C, 61.80; H, 5.44; N, 28.36. Found: C, 60.82; H, 5.64; N, 28.49. IR (U/cm–1) = 3563 - 3324 (OH, NH2), 3053 (CH aromatic), 2980 (CH3), 1657 (C=N), 1637 (C = C). 1HNMR (δ ppm): 2.82 (s, 3H, CH3), 4.32 (s, 2H, D2O exchangeable, NH2), 5.18 (s, 2H, triazine CH2), 7.16 - 7.39 (m, 8H, C6H5, pyridine 3 h), 10.21 (s, 1H, OH); MS: m/z 296 (M+, 30%). 2-(2-Phenylhydrazono)-2-(5-methyl-6 H-1,3,4-oxadi azin-2-yl)acetonitrile (13a) and 2-(2-p-chlorolhydra- zono)-2-(5-methyl-6H-1,3,4-oxadiazin-2-yl)acetonitril e (13b). General procedure: To a cold solution (0˚C - 5˚C) of 3 (1.73 g, 0.01 mol) in ethanol (30 mL) containing sodium hydroxide (10%, 6 mL) either benzenediazonium chlo- ride (7) (0.01 mol) or P-chloro benzenediazonium chlo- ride (12) obtained via the addition of sodium nitrite solu- tion (0.70 g, 0.01 mol) to a cold solution (0˚C - 5˚C) of aniline or p-chloro aniline (1.27 g, 0.01 mol) (0.01 mol) (0.93 g, 0.01 mol) in hydrochloric acid (8 mL) was added with continuous stirring was added with continuous stir- ring. The reaction mixture was stirred for an additional 2 h then the formed solid product was collected by filtra- tion. Compound 13a: Red crystals from ethanol, yield, 71% (1.71) g, m.p. 236˚C - 240˚C. Calculated for C12H11N5O (241.23): C, 59.74 ; H, 4.59; N, 29.03. Found: C, 60.01; H, 4.93; N, 28.7 0. IR (U/cm–1) = 3467 - 3322 (NH), 3055 (CH aromatic), 2974 (CH3), 2227 (CN), 1656 (C=N), 1637 (C=C). 1HNMR (δ ppm): 2.66 (s, 3H, CH3), 5.26 (s, 2H, triazine CH2), 7.23 - 7.36 (m, 5H, C6H5), 8.26 (s, 1H, NH); MS: m/z 241 (M+, 70%). Compound 13b: Red crystals from ethanol, yield, 70% (1.92 g), m.p. 100˚C. Calculated for C12H10N5OCl (275.68): Calcd: C, 52.28; H, 3. 66; N, 25. 40. Found: C, 51.97; H, 3.45; N, 25.23. IR (U/cm–1) = 3573 - 3312 (NH), 3053 (CH aromatic), 2983 (CH3), 1650 (C=N), 1634 (C=C). 1HNMR (δ ppm): 2.80 (s, 3H, CH3), 5.16 (s, 2H, triazine CH2), 7.28 - 7.37 (m, 4H, C6H4), 8.24 (s, 1H, Copyright © 2012 SciRes. OJMC  R. M. MOHAREB ET AL. 7 NH); MS: m/z 275 (M+, 80%). 3-(5-Amino-3-methyl-1H-pyrazol- 1-y l)-3 -oxo- pro pa nenitrile (15). To a solution compound 3 (1.73 g, 0.01 mol) in etha- nol (25 mL) in a water bath at 60˚C potassium cyanide (14) (0.65 g, 0.01 mol) was added with continues stirring. The reaction mixture was left in water bath for 30 min at 60˚C then poured onto a beaker containing ice/water mixture and drops of hydrochloric acid. The formed solid product was collected by filtration and dried. Orange crystals from ethanol in a yield 59% (0.96 g), with m.p. 148˚C - 150˚C. Calculated for C7H8N4O (164.15): C, 51.21; H, 4.90; N, 34.12. Found : C, 51.41; H, 4.82; N, 34.10. IR (U/cm–1) = 3468 - 3348 (NH2), 3055 (CH aromatic), 2984 (CH3), 2227 (CN), 1644 (C= N), 1633 (C=C). 1HNMR (δ ppm): 2.74 (s, 3H, CH3), 4.11 (s, 2H, CH2), 4.84 (s, 2H, NH2), 5.53 (s, 1H, pyrazole); MS: m/z 164 (M+, 33%). 2-(5-Methyl-6H-[1,3,4]oxadiazin-2-yl)-3-phenyl-acr ylonitrile (18) Equimolecular amounts of 3 (1.73 g, 0.01 mol) and benzaldehyde (16) (1.06 g, 0.01 mol) in 1,4-dioxane (30 mL) containing piperidine (1.00 mL) were heated under reflux for 3 h. The solid product formed upon dilution with ice/water containing hydrochloric acid (till pH6), was collected by filtration. Red crystals from ethanol in a yield 76% (1.71 g) with m.p. 100˚C. Calculated for C13H11N3O (225.24): C, 69.32; H, 4.92; N,18.66. Found: C, 68.84; H, 5.23; N,18.55. IR (U/cm–1) = 3057 (CH aromatic), 2985 (CH3), 2234 (CN), 1641(C=N), 1633 (C=C). 1HNMR (δppm): 2.68 (s, 3H, CH3), 5.26 (s, 2H, oxadiazine-CH2), 6.05 (s, 1H, CH=C), 7.22 - 7.35 (m, 5H, C6H5); MS: m/z 255 (M+, 80%). 6-Amino-2,8-dihydro-3-methyl-8-phenylpyrido[2,1- b][1,3,4]oxadiazine-7,9-dicarbonitrile (20a) and 2,8- Di-hydro-6-hydroxy-3-methyl-8 -pheny lpy r i do [ 2, 1-b][ 1,3,4]oxadiazine-7,9-dicarbonitrile (20b). General procedure: Equimolecular amounts of 18 (2.25 g, 0.01 mol) either malononitrile (10a) (0.66 g, 0.01 mol) or ethyl cyanoactate (10b) were added(1.13 g, 0.01 mol) in 1,4-dioxane (25 mL) containing triethyl- amine (1.00 mL) were heated under reflux for 4 h. The solid product formed upon dilution with ice/water contain- ing hydrochloric acid (till pH6), was collected by filtration. Compound (20a): Brown crystals from ethanol, yield 71 % (2.06 g), m.p. 110˚C. Calculated for C 16H13N5O (291.26): C, 65.97; H, 4.50; N, 24.04. Found: C, 65.79; H, 4.36; N, 23.98. IR (U/cm–1) = 3473 - 3344 (NH2), 3057 (CH aromatic), 2980 (CH3), 2222, 2220 (2CN), 1662 (C=N), 1640 (C=C). 1HNMR (δ ppm): 2.79 (s, 3H, CH3), 4.43 (1s, 2H, D2O exchangeable, NH2), 5.20 (s, 2H, Oxadiazine CH2), 5.52 (s, 1H, pyridine H-4), 7.23 - 7.36 (m, 5H, C6H5); MS: m/z 291 (M+, 40%). Compound (20b): Orange crystals from 1,4-dioxane, yield 69%, (2 g), m.p. 80˚C. Calculated for C16H12N4O2 (292.25): C, 65.75; H, 4.13; N,19.16. Found: C, 66.10; H, 4.43; N,19.58. IR (U/cm–1) = 3852 - 3480 (OH), 2980 (CH3), 2228, 2218 (2 CN), 1644 (C=N), 1636 (C=C). 1HNMR (δ ppm): 2.78 (s, 3H, CH3), 5.21 (s, 2H, oxa- diazine-CH2), 5.51 (s, 1H, pyridine H-4), 7.21 - 7.33 (m, 5H, C6H5), 10.22 (s, 1H, D2O exchangeable, OH); MS: m/z 292 (M+, 32%). N’-(2-bromocyclohexylidene)-2-cyanoacetohydrazi de (22). To a hot solution of cyanoacetylhydrazine (1) (0.99 g, 0.01mol), in 1,4-dioxane (40 mL), α-bromocyclohex- anone (21) (1.76 g, 0.01 mol) was added and drops of piperidine (1 mL). The reaction mixture was kept at room temperature with stirring for 1 h and the formed solid product was filtrated off. Orange crystals from 1,4-dioxane in a yield 88% (2.27 g) with m.p. 137˚C - 140˚C. Calculated for C9H12BrN3O (258.12): C, 41.88; H, 4.69; Br, 30.96; N, 16.27. Found: C, 41.67; H, 4.33; Br, 31.05; N, 16.34. IR (U/cm–1) = 3466 - 3329 (NH), 3055 (CH aromatic), 2260 (CN), 1640 (C=N), 1683 (C=O), 1630 (C=C). 1HNMR (δ ppm): 2.21 - 2.27 (m, 8H, 4CH2), 3.88 (s, 2H, CH2), 4.22 (s, 1H, CH), 8.30 (s, 1H, D2O exchangeable, NH); MS: m/z 258 (M+, 90%). 1-amino-5,6,7,8-tetrahydro-4aHbenzo[e]pyrazolo[5, 1-c][1,2,4]triazine (24a) and 1-Phenyl-5,6,7,8-tetrahy- dro-4aHbenzo [e]pyrazolo[5,1- c][1,2,4]triazine (24b). A mixture of 22 (2.58 8 g, 0.01 mol) and hydrazine hydrate (4a) (0.99 g, 0.01 mol) was heated in an oil bath 140˚C for 1 h, then left to cool. The remaining product was heated in ethanol then poured into ice/water mixture and the formed solid product was collected by filtration. Compound (24a): Brown crystals from 1,4-dioxane, yield 74% (1.41 g), m.p. > 300˚C. Calculated for C9H13N5 (191.23): C, 56.52; H, 6.85; N, 36.62. Found: C, 56.85; H, 6.60; N, 36.98. IR (U/cm–1) = 3478 - 3321 (NH2, NH), 3058 (CH aromatic), 1643 (C=N), 1636 (C= C).1HNMR (δ ppm): 2.20 - 2.25 (m, 4H, 2CH2), 2.33 - 2.41 (m, 5H, 2CH2, CH), 4.82 (s, 2H, D2O exchangeable, NH2), 5.32 (s, 1H, pyrazole H-4), 8.25 (s, 1H, D2O exchangeable, NH); MS: m/z 191 (M+, 100%). Compound (24b): Equimolecular amounts of 22 (2.58 g, 0.01 mol) and phenylhydrazine (4b) (1.08 g, 0.01 mol) in 1,4-dioxane (40 mL) were heated under reflux for 4 h. The solid product formed upon dilution with ice/water contain- ing hydrochloric acid (till pH6), was collected by filtration. Compound (24b): Brown crystals from 1,4- dioxane, yield 78% (2.08 g), m.p. 217˚C - 220˚C. Calculated for C15H17N5 (267.31): C, 67.39; H, 6.41; N, 26.19. Found: C, 67.69; H,6.32; N, 26.74. IR (U/cm–1) = 3465 - 3330 (NH2), 3056 (CH aromatic), 1640 (C=N), 1634 (C=C). 1H NMR (δ ppm): 2.23 - 2.28 (m, 4H, 2CH2), 2.32 - 2.39 (m, 5H, 2CH2, CH), 4.80 (s, 2H, D2O exchangeable, NH2), 5.38(s,1H, pyrazole H-4), 7.28 - 7.36 (m, 5H, Copyright © 2012 SciRes. OJMC  R. M. MOHAREB ET AL. 8 C6H5); MS: m/z 267 (M+, 100%). 2-(2-Phenylhydrazono)-2-(5,6,7,8-tetrahydro-4aH-b enzo[e][1,3,4]oxadiazin-3-yl)acetonitrile (25a) and 2- (2-4-chlorophenylhydrazono)-2-(5,6,7,8-tetrahydro-4a H-benzo [ e][1,3,4]oxadiazin-3-yl)acetonitrile (25b). General procedure: To a cold solution (0˚C - 5˚C) of 22 (2.58 g, 0.01 mol) in ethanol (35 mL) containing so- dium hydroxide (6 mL, 10%) and a solution of benze- nediazonium chloride (0.01 mol) or p-chlorobenzene- diazonium chloride (0.01 mol) obtained via the addition of sodium nitrite solution (0.70 g, 0.01 mol) to a cold solution (0˚C - 5˚C) of either aniline (0.93 g, 0.01 mol) or p-chloroaniline (1.27 g, 0.01 mol) containing the ap- propriate amounts of hydrochloric acid (10 mL) was added with continuous stirring] was added with continu- ous stirring. The reaction mixture was stirred for an addi- tional 2 h then the formed solid product was collected by filtration. Compound (25a): Red crystals from ethanol, yield, 82% (2.31 g), m.p. 110˚C. Calculated for C 15H15N5O (281.30): C, 64.04; H, 5.37; N, 24.89. Found: C, 64.44; H, 5.00; N, 24.66. IR (U/cm–1) = 3466 - 3316 (NH), 3053 (CH aromatic), 2890 (CH2), 2246 (CN), 1646 (C=N), 1632 (C=C). 1HNMR (δ ppm): 2.22 - 2.27 (m, 4H, 2CH2), 2.30 - 2.43 (m, 5H, 2CH2, CH), 7.26 - 7.38 (m, 5H, C6H5), 8.28 (s, 1H, NH); MS: m/z 281 (M+, 20%). Compound (25b): Orange crystals from ethanol, yield 85% (2.72 g), m.p. 120˚C. Calculated for C15H14N5OCl (315.74): C, 57.06; H, 4.47; Cl, 11.23; N, 22.18. Found: C, 57.45; H, 4.52; Cl, 11.09; N, 21.98. IR(U/cm–1) = 3475 - 3322 (NH), 3059 (CH aromatic), 2251 (CN), 1649 (C=N), 1630 (C=C). 1HNMR (δ ppm): 2.25 - 2.29 (m, 4H, 2CH2), 2.34 - 2.40 (m, 5H, 2CH2, CH), 7.29 - 7.42 (m, 4H, C6H4), 8.24 (s, 1H, NH); MS: m/z 315 (M+,70%). 5. Conclusion The present work describes the synthesis of the new hy- drazide-hydrazone derivative 3. The latter was used for the synthesis of a series of heterocyclic products with antitumor activities. Compounds 11a and 20b showed the highest inhibitor effect towards the tested three can- cer cells. The work opened the door towards pharmaceu- tical uses of other hydrazide-hydrazones that could be obtained by the sequence described in this article. 6. Acknowledgements R. M. Mohareb thanks the Alexander von Humboldt Foun- dation for the financial support during summer, 2009 during his fellowship in Germany (Erlangen) for doing research and completing this work. REFERENCES [1] C. Loncle, J. M. Brunel, N. Vidal, M. Dherbomez and Y. Letourneux, “Synthesis and Antifungal Activity of Cho- lesterol-Hydrazone Derivatives,” European Journal of Medicinal Chemistry, Vol. 39, No. 12, 2004, pp. 1067- 1071. doi:10.1016/j.ejmech.2004.07.005 [2] S. P. Garoufalias, N. Pouli, P. A. Marakos and G. Ladas, “Synthesis Antimicrobial and Antifungal Activity of Some New 3-Substituted Derivatives of 4-(2,4-Dichlorophenyl)- 5-Adamantyl-1 H-1,2,4-Triazole,” II Farmaco, 2002, Vol. 57, No. 12, pp. 973-977. doi:10.1016/S0014-827X(02)01227-2 [3] P. Vicini, F. Zani, P. Cozzini and I. Doytchinova, “Hy- drazones of 1,2-Benzisothiazole Hydrazides: Synthesis, Antimicrobial Activity and QSAR Investigations,” Euro- pean Journal of Medicinal Chemistry, Vol. 37, No. 7, 2002, pp. 553-564. doi:10.1016/S0223-5234(02)01378-8 [4] F. D. Popp, “Potential Anticonvulsant XII. Anticonvul- sant Activity of Some Aldehyd Derivatives,” European Journal of Medicinal Chemistry, Vol. 24, No. 3, 1989, pp. 313-315. doi:10.1016/0223-5234(89)90016-0 [5] S. K. Sridhar, S. N. Pandeya, J. P. Stables and A. Ramesh, “Anti-Convulsant Activity of Hydrazones, Schiff and Mann Basis of Istatin Derivatives,” European Journal of Pharmaceutical Science, Vol. 16, No. 3, 2002, pp. 129- 132. doi:1016/S0928-0987(02)00077-5 [6] S. G. Küçükgüzel, A. Mazi, F. Sahin, S. Öztürk and J. P. Stables, “Synthesis and Biological Activities of Diflunisal Hydrazide-Hydrazones,” European Journal of Medicinal Chemistry, Vol. 38, No. 11, 2003, pp. 1005-1013. doi:10.1016/j.ejmech.2003.08.004 [7] M. A. Gaston, L. R. Dias, A. C. Freitas, A. L. Miranda and P. Barreiro, “Synthesis and Analgesic Properties of New 4-Arylhydrazone 1-H Pyrazole [3,4-b] Pyridine De- rivatives,” Pharmaceutica Acta Helvetiae, Vol. 71, No. 2, 1996, pp. 213-219. doi:10.1016/0031-6865(96)00012-X [8] S. Gemma, G. Kukreja, C. Fattorusso, M. Persico, M. Romano, M. Altarelli, L. Savini, G. Campiani, E. Fatto- russo and N. Basilico, “Synthesis of N1-Arylidene-N2- Quinolyl- and N2-Acrydinylhydrazones as Potent Anti- malarial Agents Active against CQ-Resistant P. falcipa- rumstrains,” Bioorganic and Medicinal Chemistry Letters, Vol. 16, No. 20, 2006, pp. 5384-5388. doi:10.1016/j.bmcl.2006.07.060 [9] A. D. Pilla, S. Rani, P. D. Rathod, F. P. Xanvier, K. Vasu, K. H. Padh and V. Sudarsanam, “QSAR Studies on Some Thiophene Analogs as Anti-Inflammatory Agents: En- hancement of Activity by Electronic Parameters and Its Utilization for Chemical Lead Optimization,” Bioorganic and Medicinal Chemistry, Vol. 13, No. 4, 2005, pp. 1275- 1283. doi:10.1016/j.bmc.2004.11.016 [10] P. Melnyk, V. Leroux, C. Sergheraert and P. Grellier, “Design, Synthesis and in Vitro Antimalarial Activity of an Acylhydrazone Library,” Bioorganic and Medicinal Chemistry Letters, Vol. 16, No. 1, 2006, pp. 31-35. doi:10.1016/j.bmcl.2005.09.058 [11] S. G. Küçükgüzel, S. Rollas, I. Küçükgüzel and M. Kiraz, “Synthesis and Antimycobacterial Activity of Some Cou- pling Products from 4-Aminobenzoic Acid Hydrazones,” European Journal of Medicinal Chemistry, Vol. 34, No. 12, 1999, pp. 1093-1100. Copyright © 2012 SciRes. OJMC  R. M. MOHAREB ET AL. Copyright © 2012 SciRes. OJMC 9 doi:10.1016/S0223-5234(99)00129-4 [12] J. Patole, U. Sandbhor, S. Padhye, D. N. Deobagkar, C. Anson and A. E. Powell, “Structural Chemistry and in Vitro Antitubercular Activity of Acetylpyridine Benzoyl Hydrazone and Its Copper Complex against Mycobacte- rium smegmatis,” Bioorganic and Medicinal Chemistry Letters, Vol. 13, No. 1, 2003, pp. 51-55. doi:10.1016/S0960-894X(02)00855-7 [13] R. Maccari, R. Ottana and M. G. Vigorita, “In Vitro Ad- vanced Antimycobacterial Screening of Isoniazid-Related Hydrazones, Hydrazides and Cyanoboranes: Part 14,” Bioorganic and Medicinal Chemistry Letters, Vol. 15, No. 10, 2005, pp. 2509-2513. doi:10.1016/j.bmcl.2005.03.065 [14] M. T. Cocco, C. Congiu, V. Onnis, M. C. Pusceddu, M. L. Schivo, A. Logu, E. Gürsoy and N. U. Güzeldemirci, “Synthesis and Primary Cytotoxicity Evaluation of New Imidazo[2,1-b]Thiazole Derivatives,” European Journal of Medicinal Chemistry, Vol. 42, No. 3, 2007, pp. 320- 326. doi:10.1016/j.ejmech.2006.10.012 [15] N. Karal, A. Kocabalkanl, A. Gürsoy and O. Ateş, “Syn- thesis and Antitubercular Activity of 4-(3-Coumarinyl)- 3-Cyclohexyl-4-Thiazolin-2-One Benzylidenehydrazon,” II Farmaco, Vol. 57, No. 7, 2002, pp. 589-593. doi:10.1016/S0014-827X(02)01254-5 [16] V. Suni, M. R. Kurup and M. Nethaji, “Unusual Isolation of a Hemiaminal Product from 4-Cyclohexyl-3-Thio- semicarbazide and Di-2-Pyridyl Ketone: Structural and Spectral Investigations,” Journal of Molecular Structure, Vol. 749, No. 1-3, 2005, pp. 177-182. doi:10.1016/j.ejmech.2006.10.012 [17] G. Kuecuekguezel, A. Kocatepe, E. D. Clercq, F. Şahin and M. Guelluece, “Synthesis and Biological Activity of 4-Thiazolidinones, Thiosemicarbazides Derived from Di- flunisal Hydrazide,” Cheminform, Vol. 37, No. 34, 2006, pp. 353-359. doi:10.1002/chin.200634124 [18] A. P. Hoener, H. B. Frutos and J. C. Gauvin, “Novel One- Pot Microwave Assisted Gewald Synthesis of 2-Acy- lamino Thiophenes on Solid Support,” Synlett, Vol. 1, No. 1, 2003, pp. 63-67. doi:10.1055/s-2003-36229
|