Journal of Cancer Therapy
Vol.2 No.2(2011), Article ID:5480,6 pages DOI:10.4236/jct.2011.22026
Clinical Outcome of Topical Interferon Alpha-2b Cream in Phase II Trial for LSIL/CIN 1 Patients
![]()
1Department of Gynecology, Charite Universitätsmedizin, Berlin, Germany;
2Gynecologic Practice, Wagner and Stibbe, Bad Münder, Germany.
Email: {Roberto.kurzeja, achim.Schneider}@charite.de, Gboehmer@gmx.net
Received January 22nd, 2011; revised March 31st, 2011; accepted April 7th, 2011.
Keywords: Cervical Cancer, HPV Infections; BiphasixTM, Interferon, Cervical Intraepithelial Neoplasia
ABSTRACT
Objectives: Interferon alpha-2b possesses variable activity against human papillomavirus (HPV) associated cervical intraepithelial neoplasia (CIN). No topical therapy is currently available for treatment of early stage CIN. We evaluated a new patented drug delivery technology in order to achieve topical efficacy. Methods: Two separate studies were conducted in parallel. IFN002 (treatment group) was an open label study. Twenty patients with Pap IIW, III and IIID (CIN1) were treated with intravaginal application of Interferon alpha-2b cream (5 g, 2 MIU/g) three times a week (alternate days) for 6 weeks with 6 weeks of follow up to determine its effect on cytologic and colposcopic assessment. HPV001 (control group) was a 12 week observational study. Both studies had similar inclusion/exclusion criteria and patient population. Results: In IFN002, 8 of 20 patients (40%) in the ITT population showed resolution of abnormal Pap smear during the 12 weeks following start of treatment (responders). In HPV001, 7 of 21 patients (33.3%) were regressors (p = 0.45, one-sided FET). In the PP population, 7 of 12 (58.3%) patients in IFN002 were regressors compared to 7 regressors of 19 patients (36.8%) in HPV001 patients (p = 0.21, one-sided FET). Among patients with Pap IIID, 8 of 14 patients in IFN002 showed resolution of abnormal Pap smear, while 4 of 14 patients resolved in HPV001 (one-sided FET, p = 0.13). Conclusions: Interferon alpha-2b cream (5 g, 2 MIU/g) may be an effective treatment for CIN 1 patients, and future investigation is warranted.
1. Introduction
Human papillomavirus (HPV) infection is one of the most commonly acquired sexually transmitted infections (STI). More than 6 million people acquire HPV infections annually [1]. It is well known that infection with one or more of several oncogenic subtypes of HPV can lead to development of cervical intraepithelial neoplasia (CIN).
CIN ranges in severity from CIN 1, which is more benign and often regresses by itself, to CIN 3, which is classified as a true precursor of cancer of the cervix and has strong malignant potential. In the US, an estimated 1.5 million women present annually with low-grade squamous intraepithelial lesions (LSIL) [2], which is roughly equivalent to CIN 1 [3]. Although these LSIL/ CIN1 lesions may regress without treatment, they could progress to invasive vaginal, vulvar, and anal cancer, especially in women with persistent presence of high-risk HPV types.
At present, no immediate pharmaceutical therapy is available for HPV-infected women with LSIL/CIN 1. If LSIL/CIN 1 persists for up to two years, surgical ablative or excisional treatments are usually performed, possibly resulting in increased risk of fertility and pregnancy complications [4].
A safe, non-invasive pharmaceutical therapeutic alternative to conventional surgical treatment modalities that could resolve abnormal cytology in early stages of disease and diminish or eradicate HPV presence would provide a significant benefit to the healthcare system and the physical and emotional well-being of many young women.
Recombinant interferon alpha-2b (IFNα2b) is known to be active against a variety of HPV-induced lesions. All currently approved interferon alpha-2b products are injectables. To date, no topical dosage form of interferon alpha-2b has been approved for delivery via the skin or mucosa in humans.
Using BiphasixTM, a patented drug delivery technology, we have developed a specialized topical cream containing micro-encapsulated IFNα2b (Interferon alpha-2b Cream, 2 MIU/g; Topical Interferon Alpha-2b), for vaginal administration in HPV-positive patients with LSIL/CIN 1. The formulation has previously been shown in animal studies (unpublished data) to deliver sustained levels of interferon alpha-2b into the viable epidermis. This favored its use as a therapeutic against HPV-induced lesions as the infection generally manifests exclusively in the epidermis and mucosa [1]. The aim of the present study was to demonstrate the safety and efficacy of topically applied Interferon alpha-2b Cream in resolving abnormal Pap smears in HPV-positive patients.
2. Materials and Methods
Two independent studies were conducted in parallel. IFN002 was an open label study with a 4-week screening phase, 6 weeks of treatment and 6 weeks of follow up. HPV001 was a single cohort observational study conducted over 12 weeks. The studies were carried out at multiple sites in Germany with adherence to GCP guidelines.
2.1. IFN002 Study
Twenty otherwise healthy females ages 18 years or older were enrolled into the IFN002 study and represented Pap groups IIW, III and IIID (CIN1) of no more than twelve months duration and a HPV-positive status. Study inclusion criteria were LSIL (condyloma or CIN 1) of no more than 12 months duration, negative pregnancy, use of effective methods of birth control during the study, and confirmation of HPV-positive status by PCR. Exclusion criteria were previous treatment of cervical lesions, history of abnormal Pap smears or HPV-positive status for more than 12 months CIN 3, urogenital warts, known HIV+ status, known autoimmune diseases, use of immunomodulatory/immunosuppressant drugs, pregnancy or lactation, concomitant STI and/or known alcohol, drug or substance dependence.
The study drug Interferon alpha-2b Cream (2 MIU/g) was manufactured under GMP conditions and filled into single-use polypropylene tubes designed to expel 5 g of cream into an accompanying metered low density polyethylene (LDPE) applicator device for intravaginal use.
Patients were instructed to apply the entire contents of a single tube of study cream high into the vagina in the evening just prior to bedtime 3 times a week (on alternate days) for 6 weeks. Subjects were advised not to use the cream while menstruating and to apply the cream following sexual intercourse should the timing of intercourse have coincided with cream application. All other topical preparations applied to the genital area were to be avoided. A flow chart of the investigation and clinical assessment for IFN002 is included in Table 1.
To ensure patient compliance, the used and unused tubes were weighed. Tubes with a tare weight of 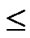 4g were considered to have been fully administered.
4g were considered to have been fully administered.
Patients were informed that they were free to withdraw from the study at any time and for any reason. The investigator was allowed to remove a patient from the study if it was not in the best medical interest of the patient to continue in the study.
Efficacy in the treatment study (IFN002) was assessed by evaluating the primary end point, which was defined
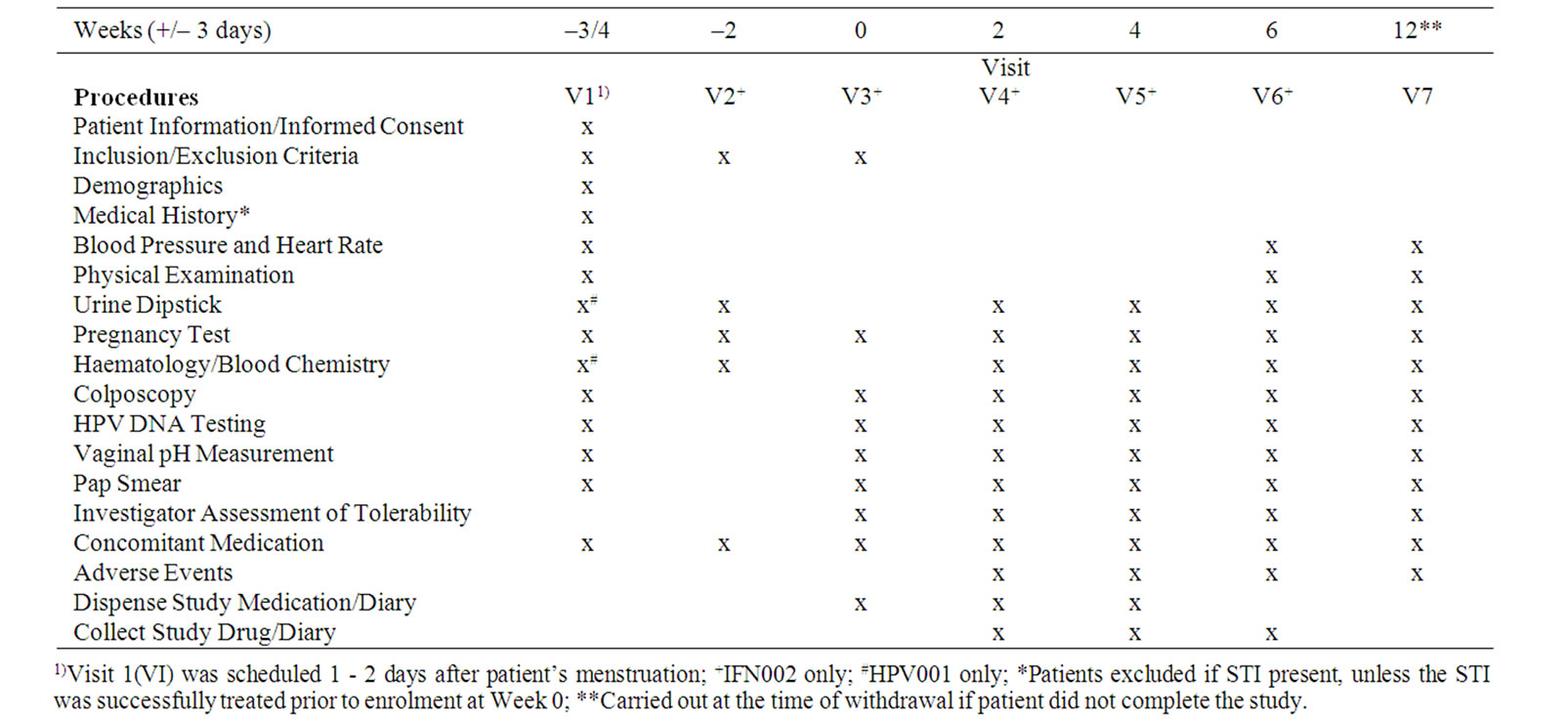
Table 1. Study flow chart for IFN002.
as the proportion of patients in the Intent-To-Treat (ITT) population with reversion of abnormal cytology to normal (Pap grouping I or II) during 12 weeks after the start of the treatment period (i.e. week 2, 4, 6 or 12, corresponding to visit V04, V05, V06 and V07, respectively).
A secondary end point was identical to the primary end point for ITT, except as observed in the Per Protocol (PP) population. An additional secondary endpoint included the proportion of patients in the PP and ITT populations who reverted from HPV-positive to HPVnegative status within 12 weeks after the start of the observation period using a GP5+/6+ PCR-EIA method.
2.2. HPV001 Study
Twenty one otherwise healthy females aged 18 years or older were enrolled into HPV001. Inclusion/exclusion criteria for HPV001 were the same as for IFN002. The following assessments were conducted at visit 1(V1): colposcopy (PAP smear and HPV status included), vaginal pH, blood pressure/heart rate, physical examination, urinalysis, blood chemistry/haematology, pregnancy status, concomitant medication, and adverse effects.
Subjects returned for a final visit 12 weeks after V1 where colposcopy (PAP smear and HPV status included), vaginal pH, blood pressure/heart rate, physical examination, urinalysis, blood chemistry/haematology, pregnancy status, concomitant medication, and adverse effects were evaluated.
In the HPV001 control study, the primary endpoint was the proportion of patients with a resolution of an abnormal Pap smear at the end of the 12 weeks observation period.
2.3. Statistical Analysis
Statistical analysis of the efficacy parameters was performed using FET p = 0.05 two sided or p = 0.025 one sided. The difference in response rates between both groups was determined via one-sided Fisher’s Exact Test (FET).
Safety parameters of a quantitative nature (laboratory results) were analyzed by using the asymptotic MannWhitney-U-test for differences between groups and the asymptotic Wilcoxon-test for dependent samples (within group differences).
3. Results
Twenty patients entered the treatment phase and received study medication at least once. These patients constitute the “safety” sample.
All 20 “safety” patients were assessed to be part of the ITT sample. Twelve of the 20 ITT were included in the PP population (Table 2).
Table 2. Reasons for exclusion from PP population for IFN002.
For the HPV001 study, the 21 patients who entered the observation period and had a second visit after about 12 weeks constitute the “safety” sample. All 21 “safety” patients were assessed to be part of the ITT sample while 19 patients were regarded to be “per protocol” (Table 3).
In the IFN002 study, the primary study endpoint was defined as the proportion of patients with resolution of an abnormal Pap smear during the 12 weeks after the start of the treatment period in the ITT population. In the treatment group, 8 out of 20 patients (40.00%) in the ITT population were responders in contrast to 7 out of 21 patients (33.3%) in the control group (Table 4). This result was not statistically significant (p = 0.45, one-sided FET). Furthermore, the Pap smear results of two patients in the treatment group worsened after a preliminary PAP response at earlier visits.
A secondary endpoint for the IFN002 study was defined as the proportion of patients in the PP population with resolution of an abnormal Pap smear during the 12 weeks after the start of the treatment period. In the treatment group, 7 of 12 patients (58.3%) were responders in contrast to 7 of 19 patients (36.8%) in the control group. This result was not statistically significant (p = 0.21, one-sided FET).
In patients who presented with PAP IIID at enrollment, there was a trend, though not statistically significant, toward a higher proportion of resolution in treated patients (8 of 14) than in controls (4 of 14); (one-sided FET, p = 0.13). Enrollment is defined as the randomization visit (V3).

Table 3. Reasons for exclusion from PP population for HPV001.
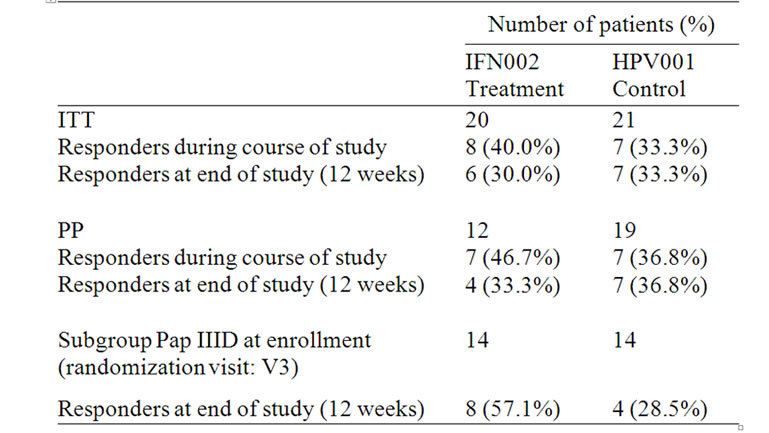
Table 4. Summary of responding patients as defined by study population group.
Another secondary endpoint was the proportion of patients in the ITT and PP populations with resolution of HPV-positive status during the 12 weeks after the start of the treatment period. An HPV-negative response was defined as an occurrence of an HPV-negative finding at least one time after the start of treatment. In the treatment group, 3 out of 20 patients (15.00%) in the ITT population were HPV-responders in contrast to 2 out of 21 patients (9.52%) in the control group. In the PP population of the treatment group, 1 out of 12 patients (8.3%) were HPV-responders versus 2 out of 19 patients (10.53%) in the control group. Thus, the differences in secondary end point responses observed in both the treatment and control groups were not statistically significant.
Results of cervical lesion assessments by colposcopy showed no between-group differences and no relevant change over time within groups. Colposcopy at screening (V01 and V03 in the treatment group) was suggestive of normal findings or CIN 1, as demanded by the in-/exclusion criteria. At final individual observations in the ITT population, 14 patients (66.67%) in the control group were unchanged, 5 patients (23.80%) had worsened and 2 patients (9.52%) had improved to normal. In the ITT population in the treatment group 12 patients (60.0%) improved towards “normal” or “atypical”. Six patients (30.00%) remained CIN 1 however one patient did show colposcopic response at V05 but at visit V07 reverted back to CIN I. Two patients (10.00%) worsened to CIN 2 or even CIN 3.
According to the investigator’s tolerability assessments of the cervical, vaginal, introital and vulval sites, erythema was observed at the cervical site in a small number (ten) of treatment group patients and in one control group patient. Mild vesicles were documented in one control group patient. A mild erosion/ulceration was observed in one treatment group patient and in one control group patient. No patient from either group showed a visible reaction at the vaginal site. A mild erythema was seen in only one patient at the introital site and a moderate erosion/ulceration was seen in one patient of the treatment group at the vulval site.
In the IFN002 study, 17 of 20 patients used between 15 and 25 tubes with tare weight ≤ 4 g and were thus compliant according to protocol. The extent of exposure of these patients was therefore 150 - 250 MIU IFNα2b over the study duration.
Twenty seven AEs in the treatment group occurred after the start of treatment in 14 of the 20 patients (70.00%). Two of these AEs were listed as serious (accident by swimming with concussion of the brain and compression of the cervical cord), 11 were moderate, and 14 were mild (Table 5).
In 6 of the 27 AEs (22.22%) was the causal relationship to the study drug assessed by the physician as “possible”, including temperature (fever), bleeding/spotting, abdominal pain, diarrhea, vaginal irritation, and perianal erosion. In all other patients, the causal relationship was assessed as “unlikely” or “unrelated”.
4. Discussion
This is the first reported clinical trial evaluating the use of a BiphasixTM-based Interferon alpha-2b Cream as a therapy for HPV-related LSIL. In this study, 10 MIU formulation of recombinant IFNα2b cream was administered intravaginally three times a week for 6 weeks, for a total study dose of 180 MIU.


Table 5. Incidence of adverse events.
Overall patient compliance was very good and the product was well tolerated. Mild erosion/ulceration was observed in only one patient. Local irritation was observed in a very small number (ten) of patients in the treatment group, but none of the patients showed a visible reaction at the vaginal site. No serious adverse events related to product use were reported.
Although the Pap response differences in the nonstratified ITT populations of the treatment and control groups were not statistically significant, Interferon alpha-2b Cream showed a clear response in 58% of patients in the PP population compared to about 37% in the control group. In addition, 8 of 14 patients presenting with Pap IIID on cytology from the ITT population of IFN002 were responders, which was higher than the 4 responders out of 14 Pap IIID patients in the control population (one-sided FET p = 0.13). Colposcopic findings revealed that 60% of patients in the treatment group had improved towards “normal or atypical” versus just under 10% in the control group.
In the IFN002 study, the primary study endpoint was defined as the proportion of patients with resolution of an abnormal Pap smear during the 12 weeks after the start of the treatment period in the ITT population. In the HPV001 study, the primary endpoint was defined as the proportion of patients with resolution of an abnormal Pap smear at the end of the 12 weeks observation period. Thus, the two definitions of response were not completely identical, and the probability of a response in the treatment group could have been higher due to the greater frequency of Pap smear investigations.
Other clinical trials that have found interferons to be effective against a variety of HPV-related cervical infections have reported cure rates between 0 and 100%. In an open study, Penna et al. [5] reported 80% lesion regression and 51% reversion of HPV type 16/18 to normal following daily intra-perilesional application of 3 MIU IFN beta for 3 weeks into the cervix of women with CIN associated with HPV infection. Similarly, in an open pilot study, Katesmark et al. [6] showed a 73% (histology) complete response rate when IFN was injected into the transformation zone in patients with CIN I and II.
Zarcone et al. [7] reported a 12-patient study with combined intramuscular and topical alpha-IFN therapy in the treatment of CIN 1 and 2 in HPV-positive women. The administration of intramuscular doses of up to 3 MIU IFN daily for 3 weeks combined with intravaginal application of an unspecified dose of IFN cream during the last two weeks of treatment resulted in a complete response in 7 patients, partial response in 4 patients, and no response in 1 patient The variability in results most likely reflects differences in dosage, duration of treatment, mode of application, study design, severity of disease, and measures of efficacy.
While previously reported studies showed that interferon therapy can be an effective treatment for CIN-associated HPV infections, none examined the HPV status of patients post therapy. In the study reported here, HPV DNA testing was performed throughout the treatment and follow-up period. All patients who responded to the Interferon alpha-2b Cream treatment had high-risk strains of HPV DNA at the time of enrollment. Thus, the present treatment appears to be effective in patients with LSIL/CIN1 associated with oncogenic HPV types.
In summary, this study demonstrated statistically nonsignificant findings comparing treatment to an untreated control population both in terms of Pap response and colposcopic assessment. An insignificant result was observed for patients with Pap IIW or Pap III upon enrollment. A trend towards clinical effect, although not significant, was observed in the stratified subgroup of patients who showed Pap IIID on cytology enrollment. The combination of the excellent safety profile exhibited by Interferon alpha-2b Cream together with the tendency in favour of the treatment indicates that further investigation into the efficacy of this therapeutic candidate in CIN 1/2 patients is warranted.
5. Acknowledgments
We acknowledge the support of our nursing staff in the colposcopy clinics, the investigative and molecular biology staff at the Friedrich-Schiller-Universität Jena, the pathology staff at the Deutsche Klinik/Ärztepartnerschaft in Bad Münder, our biometrician Dipl.math. Dipl.psych. Klaus Rettig, the staff of Medical Consulting Dr. Schlichtiger GmbH and the sponsor of this research, Helix BioPharma Corp.
No conflict of interests is reported for RC, GB and AS.
REFERENCES
- K. A. Ault, “Epidemiology and Natural History of Human Papillomavirus Infections in the Female Genital Tract,” Infectious Diseases in Obstetrics and Gynecology, Vol. 2006, No.1, January 2006, pp. 1-5.
- M. Schiffman and D. Solomon, “Findings to Date from the ASCUS-LSIL Triage Study (ALTS),” Archives of Pathology and Laboratory Medicine, Vol. 127, No. 8, August 2003, pp. 946-949.
- The ASCUS-LSIL Triage Study (ALTS) Group, “A Randomized Trial on the Management of Low-Grade Squamous Intraepithelial Lesions Cytology Interpretations,” American Journal of Obstetrics and Gynecology, Vol. 188, No. 6, June 2003, pp. 1393-1400.
- M. Kyrgiou, G. Koliopoulos, P. Martin-Hirsch, M. Arbyn, W. Prendiville and E. Paraskeviadis, “Obstetric Outcomes after Conservative Treatment for Intraepithelial or Early Invasive Cervical Lesions: Systematic Review and Metaanalysis,” The Lancet, Vol. 367, No. 9509, February 2006, pp. 489-498. doi:10.1016/S0140-6736(06)68181-6
- C. Penna, M. G. Fallani, R. Gordigiani, L. Sonni, G. L. Taddei and M. Marchionni, “Intralesional Beta-Interferon Treatment of Cervical Intraepithelial Neoplasia Associated with Human Papillomavirus Infection,” Tumori, Vol. 80, No. 2, February 1994, pp. 146-150.
- M. Katesmark, S. Coulter-Smith, K. Reynolds and F. Lawton, “A Pilot Study of the Efficacy and Tolerability of Intralesional Recombinant Human Beta Interferons in Cervical Intraepithelial Neoplasia,” Annals Academy of Medicine Singapore, Vol. 28, No. 6, November 1999, pp. 775-777.
- R. Zarcone, P. Bellini, G. Cardone and A Cardone, “Treatment of Cervix Condylomata with Alpha-IFN Leucocytar,” Clinical and Experimental Obstetrics and Gynecology, Vol. 22, No. 4, March 1995, pp. 326-329.

