Food and Nutrition Sciences
Vol. 3 No. 4 (2012) , Article ID: 18505 , 12 pages DOI:10.4236/fns.2012.34078
Effects of Dietary and Physical Activity Intervention during Pregnancy on Circulating Leptin and Adiponectin Levels
![]()
1Lombardi Cancer Center, Georgetown University, Washington DC, USA; 2UKK Institute for Health Promotion, Tampere, Finland; 3School of Health Sciences, University of Tampere, Tampere, Finland; 4National Institute for Health and Welfare, Helsinki, Finland.
Email: *riitta.luoto@uta.fi
Received January 10th, 2012; revised March 8th, 2012; accepted March 16th, 2012
Keywords: leptin; adiponectin; pregnancy; randomized trial
ABSTRACT
Objective: We investigated whether lifestyle intervention during pregnancy modifies pregnancy leptin and adiponectin levels, adipokines and their association with body mass index (BMI). Methods: Of the 126 pregnant nulliparous women recruited, 43 in the intervention and 56 in the control group completed the study. Blood samples were collected on gestation weeks 8 - 10 (baseline) and weeks 36 - 38 (end of the intervention). Statistical analyses included group-based correlations between adipokines, pre-pregnancy BMI and gestational weight gain and covariance analysis including all confounding factors. Results: Pre-pregnancy BMI correlated with baseline (p = 0.023 and p = 0.079) and end leptin levels (p = 0.128 and p < 0.001). Similarly, gestational weight gain correlated with both baseline (p = 0.009 and p = 0.046) and end leptin levels (p = 0.065 and p = 0.011). BMI at the end of pregnancy correlated with the end leptin levels, but the correlation was weaker in the control (p = 0.043) than intervention women (p < 0.001). Control women having the highest pre-pregnancy BMI tended to exhibit the lowest increase in leptin levels during pregnancy (p = 0.10), whilst in the intervention group, women who had the highest pre-pregnancy BMI exhibited the highest increase in pregnancy leptin (p = 0.058). In covariance analysis including all covariates change in leptin was associated with gestational weight gain (p = 0.036), but change in adiponectin was not (p = 0.93). Conclusion: In contrast to the control group, women in the life-style intervention group exhibited a stronger association between gestational weight gain and leptin levels, indicating that they maintained insulin sensitivity.
1. Introduction
At least 30% of pregnant women gain more weight than recommended by the Institute of Medicine (IOM); i.e., over 35 lb for a normal size woman [1]. Excessive weight gain during pregnancy has several adverse effects, including it increases the risk of developing gestational diabetes mellitus (GDM) [2,3] and having high birth weight infants [4], possibly due to impaired insulin sensitivity during pregnancy [5-7]. Therefore, it is important to develop interventions that either prevent excessive weight gain or improve insulin sensitivity. Our maternal dietary and physical activity intervention was designed to prevent excessive weight gain during pregnancy, and it was described in detail by Kinnunen et al. [8]. Although it failed to prevent excessive weight gain, it completely prevented high birth weights (>4000 g); 15% of the women who received standard maternity care had high birth weight infant [8]. Thus, our lifestyle intervention may have positively influenced insulin sensitivity.
Biological factors linked to body weight and insulin sensitivity include circulating leptin [9,10] and adiponectin levels [11,12], two adipokines secreted by fat cells [13]. Of all the adipocyte-released adipokines, adiponectin is most abundant in the human plasma, ranging from 5 to 30 ug/mL. However, adiponectin is inversely linked to body mass index (BMI), possibly because adipose tissue-derived cytokine, tumor necrosis factor a (TNF-a), and insulin suppress its release [13-16]. In pregnant women, adiponectin levels are not associated with BMI [17,18]. Leptin levels correlate directly with BMI [19,20], also during pregnancy [21-26].
Leptin regulates food intake, and in this role affects insulin sensitivity. In individuals with normal body weight, leptin acts in the hypothalamus to decrease appetite and increase energy expenditure for the purpose of maintaining adipose depots at an optimal level [27-28]. When insulin and glucose levels are elevated following a meal, they stimulate leptin secretion. Leptin, in turn, increases insulin sensitivity, but it also suppresses insulin secretion from the pancreas [10]. This adipokine has been successfully used in the treatment of insulin resistance and type 2 diabetes in an animal model [29] and in humans [10]. The feedback between body weight and leptin does not function in obese individuals, because obesity causes leptin resistance [20,30]. During pregnancy, leptin concentrations increase modestly [21,22, 24,31-33] and during the first two trimesters the increase is correlated with gestational weight gain [25]. However, during the last pregnancy trimester women become resistant to the actions of leptin [34], and this is manifested as an absence of a correlation between BMI and leptin levels [22,35,36]. It is not clear whether leptin affects GDM. High leptin have been correlated with increased risk of GDM in pregnant women [37-39], but some studies have reported an inverse relationship [25,40]. The latter is in agreement with findings in rats in which leptin prevents GDM [41]. A recent study comparing pregnancy leptin levels in women with GDM and normal controls did not find any difference [42].
Adiponectin is an insulin-sensitizing hormone [43], because it reduces hepatic glucose production, stimulates glucose uptake in skeletal muscle, and enhances insulin action in the liver. Emerging evidence links low adiponectin levels to insulin resistance, particularly in pregnant women [12,44-46]. GDM is strongly linked to low adiponectin levels [45,47-50], although in some studies no association has been found [42]. Pregnant women have higher adiponectin levels than non-pregnant women [17], but the results are conflicting as to whether adiponectin levels change during the course of pregnancy: some studies have found no changes [17], whilst some others suggest that the levels are slightly reduced [48]. In this study, we investigated whether maternal dietary and physical activity intervention which does not affect pregnancy weight gain or BMI [8], modifies circulating leptin or adiponectin levels during pregnancy, and alters the association between BMI and leptin/adiponectin.
2. Methods
2.1. Setting and General Study Design
The study was a part of a larger pilot study which aimed in preventing excessive gestational weight gain and postpartum weight retention and was carried out by the UKK Institute for Health Promotion Research in Finland. The study was approved by the Ethical Committee of the Pirkanmaa Hospital District, and each participant provided informed written consent. In Finland, the maternity health care system is available to all pregnant women in every municipality and is funded by public tax revenue. Six maternity clinics in Tampere and Hämeenlinna, Finland, participated in the study. Three maternity clinics volunteered for intervention clinics and three were control clinics. All public health nurses from the maternity clinics participated in the study, nine in the intervention clinics and six in the control clinics. In Finland, it is recommended that nulliparas make 11 - 15 visits to a nurse and three visits to a physician during pregnancy [51]. The study was carried out during five routine visits to nurses at 8 - 10, 16 - 18, 22 - 24, 32 - 34 and 36 - 38 weeks’ gestation.
2.2. Participants
Pregnant women with no earlier deliveries were eligible for the study (earlier abortions or miscarriages acceptable). Women with type 1 or 2 diabetes mellitus, twin pregnancy, physical disability that prevents from exercising, otherwise problematic pregnancy, substance abuse, treatment or clinical history of any psychiatric illness, inability to speak Finnish or intention to change place of residence within the next three months and women aged under 18 years were excluded. The nurses recruited the participants when they first contacted the maternity clinics at the beginning of their pregnancy, between August 2004 and January 2005. In total, 132 women were enrolled in the study, 69 in the intervention group and 63 in the control group.
2.3. Intervention
The intervention included individual dietary and physical activity counselling during five sessions, an individual weekly leisure time physical activity plan and an opportunity to attend supervised group exercise once a week. The participants of the intervention clinics were also given information on the recommendations for total gestational weight gain [52], take home leaflets on diet and physical activity and follow-up notebooks to keep track of their compliance. Kinnunen et al. have previously described the intervention in greater detail [8]. In the control clinics, standard maternity care practices and usual physical activity and dietary counselling were continued.
2.4. Measurements
The participants filled in a baseline questionnaire including questions on their background (e.g. socio-economic status, smoking, earlier weight development), diet, physical activity and wellbeing before the first visit to the maternity clinic at 8 - 10 weeks’ of gestation and the same questionnaire was filled in again at the end of the study on the 37th gestation week. In addition, the participants filled in a physical activity questionnaire at 16 - 18 weeks’ of gestation and a dietary questionnaire at 22 - 24 weeks’ of gestation. Each participant’s body weight and blood pressure were measured at every visit to the maternity clinic by the nurse, and the measures were written down in the participant’s maternity card from which the data was obtained. Pre-pregnancy weight and height were obtained by recall.
2.5. Blood Samples
Blood samples (4 × 10 ml) were taken of all participants at the beginning (8 - 10 weeks’ gestation, called here baseline) and at the end (36 - 38 weeks’ gestation, called here end) of the intervention by medical laboratory technologists from the UKK Institute. The samples were stored appropriately in the UKK Institute and shipped to the Clarke-Hilakivi lab at Georgetown University for analysis. Levels of circulating leptin and adiponectin were determined using human EIA kits from Assay Design, Inc. (Ann Harbor, MI) according to the manufacturer’s instructions.
2.6. Statistical Methods
All statistical analyses were performed using SPSS software, version 15.0 (SPSS Inc., Chicago IL), and statistical significance was defined as p < 0.05. The frequency distributions of maternal age, study status, smoking before pregnancy and pre-pregnancy BMI were reported in the entire study population. Age, smoking, pre-pregnancy BMI and gestational weight gain were also reported in means and percentages in the intervention and control groups.
The mean leptin and adiponectin concentrations at baseline and at the end of the intervention were examined in the entire study population as well as in the intervention and control groups. The differences between baseline and end leptin and adiponectin concentrations were tested using paired-samples t-tests, and the differences in baseline and end leptin and adiponectin concentrations between the groups were tested using independent-samples t-tests. Correlation between pre-pregnancy BMI, gestational weight gain and leptin/adiponectin was performed with Pearson correlation factors. Significance of the p-values from correlation analysis was based on sample size and 2-sided p-values were utilized.
Analysis of covariance (ANCOVA) was used to evaluate the effects of the confounding factors (maternal age, study group, smoking before or during pregnancy, pre-pregnancy BMI, gestational weight gain) on the mean baseline leptin and adiponectin concentrations. The mean change in leptin and adiponectin concentrations adjusted for baseline concentrations and other confounding factors was also examined using ANCOVA.
3. Results
Out of 132 participants enrolled to the study, 105 completed it (n = 49 in the intervention group of 69 recruited, and n = 56 in the control group of 63 recruited). Six women in the intervention group were obese (BMI > 30), but none in the control group. Since obese individuals are generally leptin resistant [20], they were removed from the analysis. On average, the participants were 28.0 years old, had pre-pregnancy BMI, determined on gestation week 8 - 10, of 22.3% and 20.8% smoked before pregnancy (Table 1). Weight gain was on average 14.6 kg during pregnancy (not shown in the table). The women in the intervention group were significantly younger than women in the control group (p < 0.035).
3.1. Leptin Levels during Pregnancy
The baseline leptin levels on gestation weeks 8 - 10 were similar in the intervention (21.2 ng/ml) and control groups (23.1 ng/ml) (p = 0.369) (Figure 1). In addition, no significant differences between the groups were seen in the leptin concentrations at the end of the intervention on gestation weeks 36 - 38 (intervention: 24.2 ng/ml; control: 24.5 ng/ml) (p = 0.927). In all subjects, mean leptin concentrations increased from 22.2 ng/ml at baseline to 24.3 ng/ml at the end of the study, but the increase was not statistically significant (p = 0.159) (Figure 1). When assessed separately in the intervention and control groups, we observed that in the intervention group the mean leptin concentrations non-significantly increased (p = 0.148). However, in the control group, no increase in
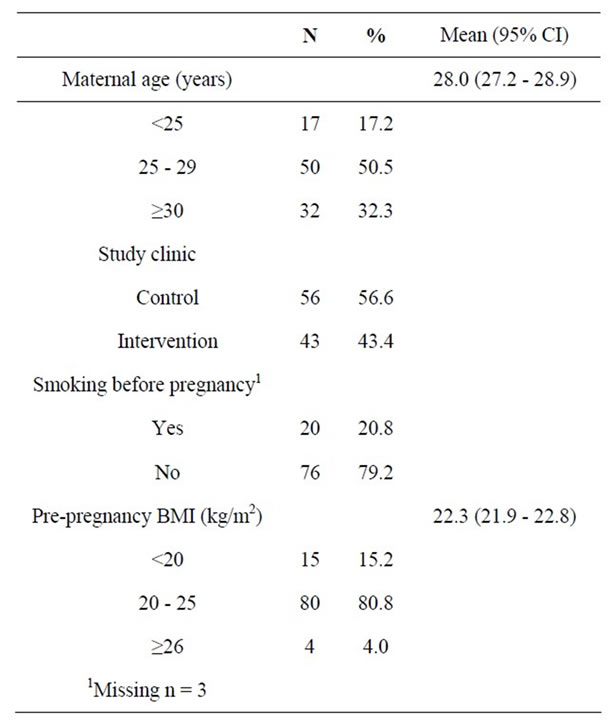
Table 1. Background characteristics.
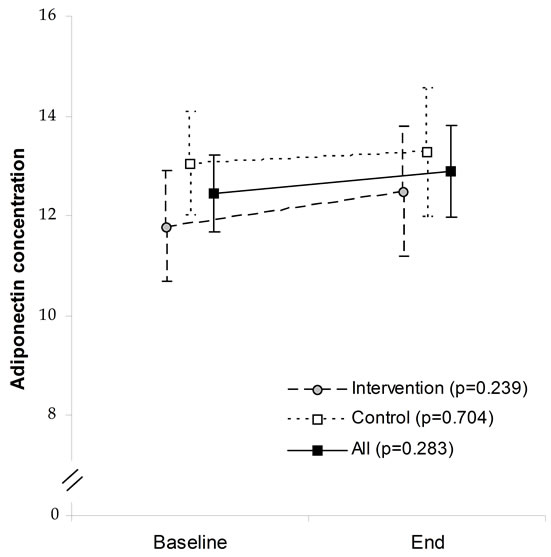

Figure 1. Baseline and end adiponectin and leptin concentrations among intervention and control groups.
mean leptin concentrations was noted during pregnancy (p = 0.527).
3.2. Adiponectin Levels during Pregnancy
Mean adiponectin levels at the baseline were slightly but non-significantly higher in the control (13.05 ng/ml) than the intervention group (11.8 ng/ml) (p = 0.105) (Figure 1).
At the end of the intervention, no between-group differences were seen (control: 13.3 vs intervention: 12.5, p = 0.404). In all subjects, adiponectin concentrations were similar at the beginning and end of the intervention (12.5 ng/ml and 12.9 ng/ml, respectively) (p = 0.283) (Figure 1). No pregnancy-induced changes in adiponectin levels were seen in the control (p = 0.704) or intervention groups (p = 0.239).
3.3. BMI and Changes in Leptin Levels during Pregnancy
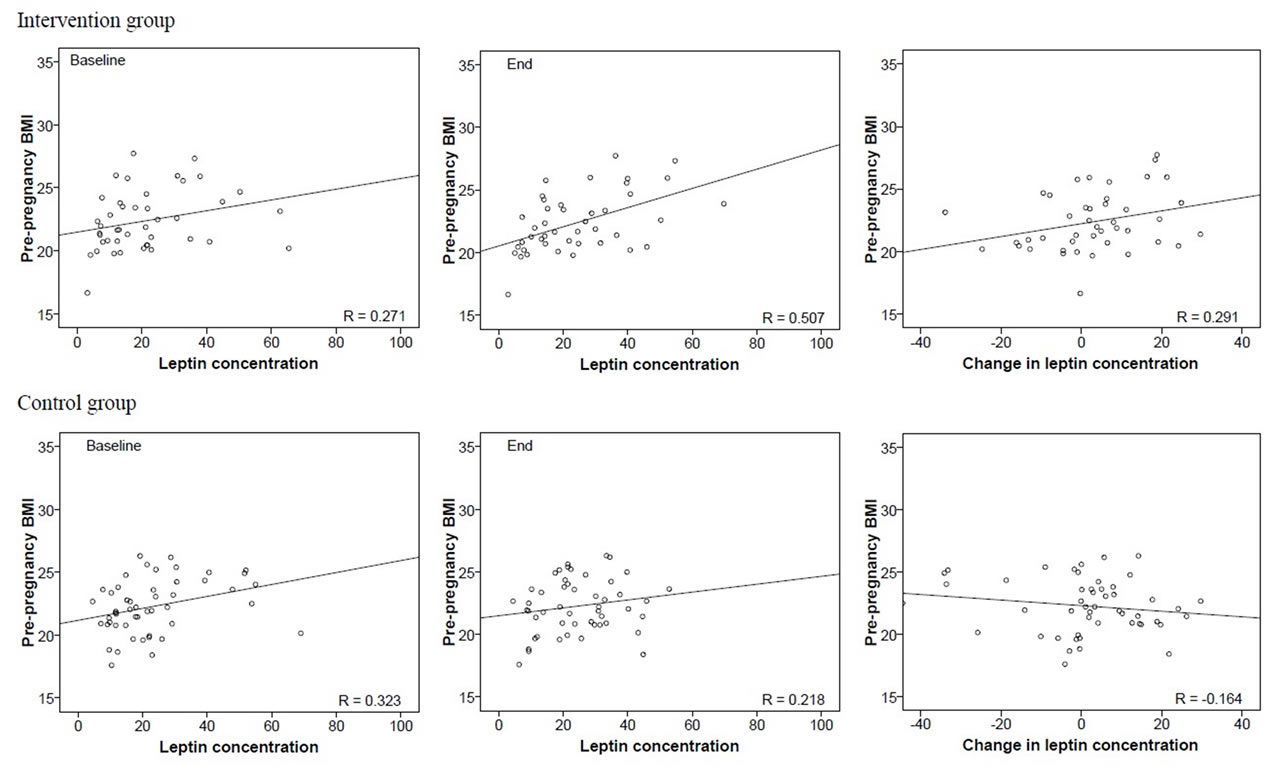
We found that in woman in the intervention group, pre-pregnancy BMI tended to correlate with the baseline leptin concentrations (r = 0.271; p = 0.079) and correlated strongly with the end leptin concentrations (r = 0.507; p < 0.001). Pre-pregnancy BMI also tended to correlate with the change in leptin concentrations (r = 0.291; p = 0.058) during pregnancy (Figure 2(a)). Among the controls, the correlation between pre-pregnancy BMI and baseline (r = 0.323; p = 0.023) and end leptin levels (r = 0.218; p = 0.13) were also seen, but pre-pregnancy BMI tended to correlate inversely with the change in leptin levels (r = −0.164; p = 0.26) (Figure 2(a)).
We also determined whether BMI at the end of intervention period correlated with end leptin levels. Since pregnant women become leptin resistant at the end of pregnancy [34,35], we expected not to see a correlation. However, as shown in Figure 2(b), a correlation was found in the intervention (r = 0.658, p < 0.001) and control groups (r = 0.287, p = 0.043). The correlation was notably stronger in the intervention than control group, indicating that in the intervention group an increase in the consumption of fruits, vegetables and whole grains promoted leptin sensitivity.
3.4. BMI and Changes in Adiponectin Levels during Pregnancy
Pre-pregnancy BMI did not correlate with the baseline adiponectin levels (intervention r = −0.075, p = 0.63; control: r = −0.17, p = 0.24), but there was a tendency for pre-pregnancy BMI to correlate inversely with end adiponectin levels (intervention: r = −0.25, p = 0.10; control: r = −0.25, p = 0.086) (Figure 2(c)). Pre-pregnancy BMI did not correlate significantly with the change in adiponectin concentrations during pregnancy in the intervention (r = −0.21; p = 0.17) or in the control group (r = −0.12; p = 0.40) (Figure 2(c)).
Figure 2(d) shows that BMI at the end of the intervention period did not correlate with end adiponectin levels in the intervention (−r = 0.159, p = 0.31) and control groups (−r = 0.178, p = 0.22).
3.5. Association between Leptin and Adiponectin Levels
Previous studies have generated conflicting data regarding possible correlation between circulating leptin and adiponectin levels in pregnant women [17,53]. In this study, baseline leptin levels were not linked to baseline adiponectin levels, but there was a tendency for inverse correlation between the end leptin and adiponectin levels both in the intervention (r = −0.258, p = 0.094) and control groups (r = −0.235, p = 0.10) (not shown in the figures).
3.6. Gestational Weight Gain and Pregnancy Leptin Levels
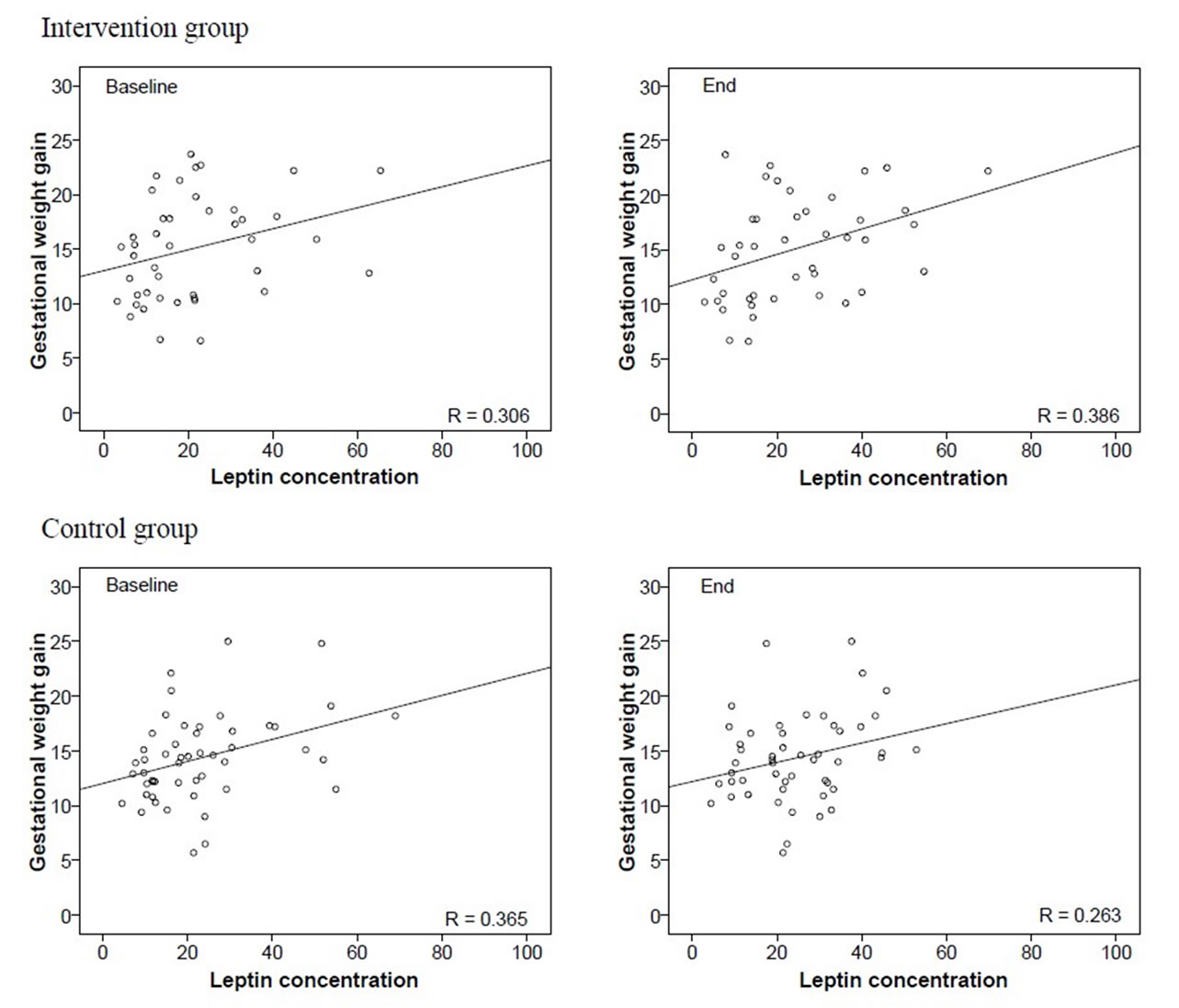
In the intervention and control groups, gestational weight gain correlated with leptin concentrations at baseline (intervention group: r = 0.306, p = 0.046; and control group: r = 0.365, p = 0.009) (Figure 3(a)).
Leptin levels at the end of intervention also correlated with gestational weight gain in the intervention group (r = 0.386, p = 0.011), and tended to do so in the control group (r = 0.263, p = 0.065). Thus, gestational weight gain is strongly associated with end leptin levels in women consuming high levels of fruits, vegetables and whole grains during pregnancy, but less so in pregnant control women.
3.7. Gestational Weight Gain and Pregnancy Adiponectin Levels
Gestational weight gain did not correlate significantly with adiponectin levels in the intervention group at baseline (r = 0.066; p = 0.67) or at end (r = 0.090; p = 0.57), and similar negative findings also were seen in the con-
 (a)
(a) (b)
(b)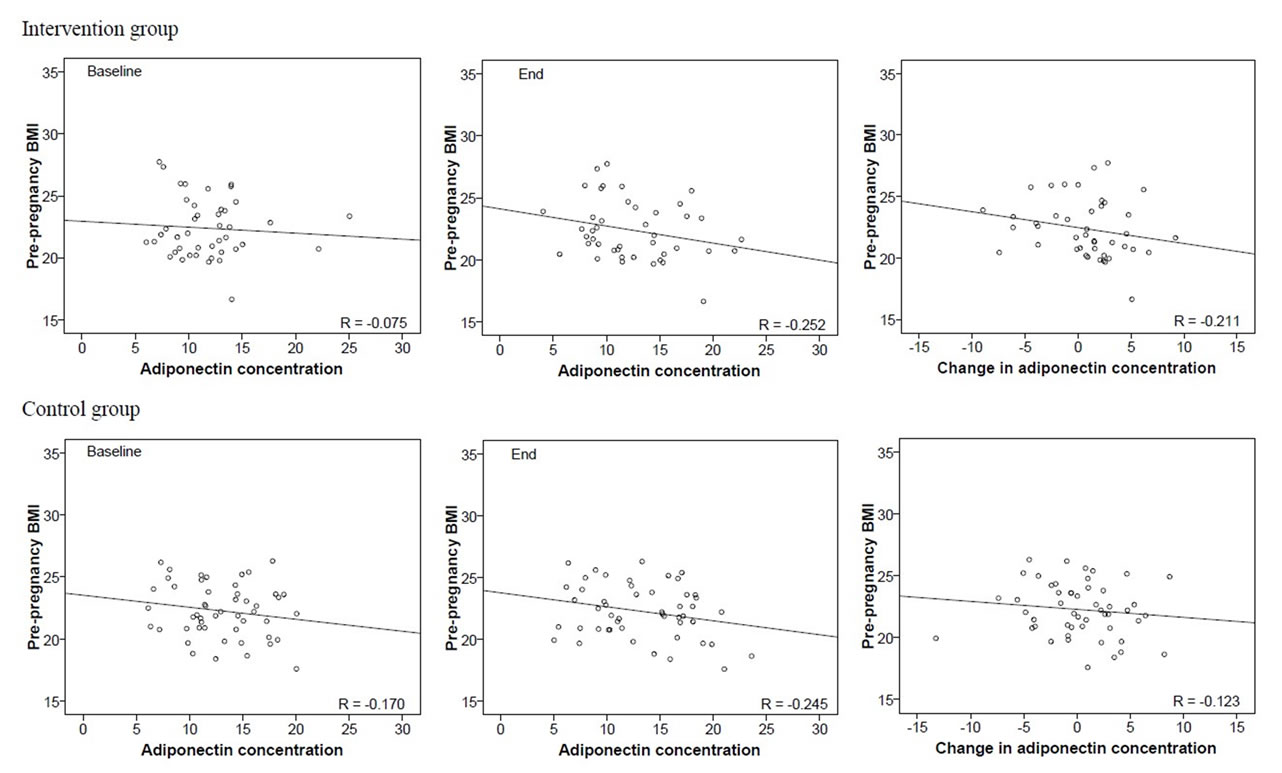 (c)
(c)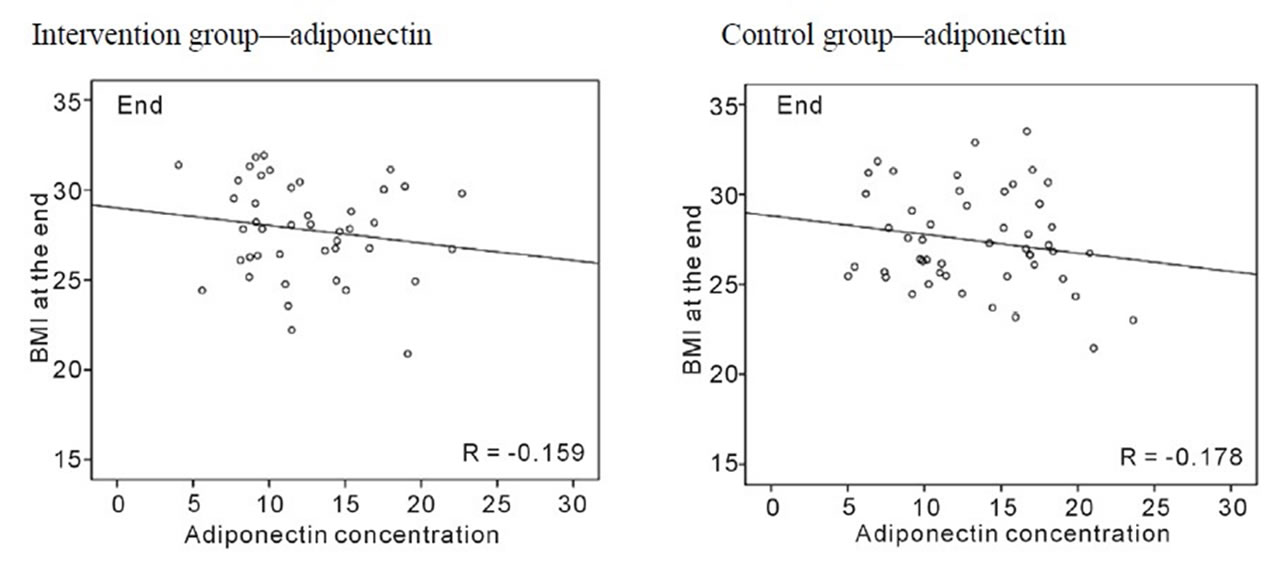 (d)
(d)
Figure 2. (a) Correlations between pre-pregnancy BMI and baseline and end leptin concentrations, and the change in leptin concentration during pregnancy by group; (b) Correlations between pre-pregnancy BMI and baseline and end adiponectin concentrations, and the change in adiponectin concentration during pregnancy by group; (c) Correlations between pre-pregnancy BMI and baseline and end adiponectin concentrations, and the change in adiponectin concentration during pregnancy by group; (d) Correlations between BMI at end and adiponectin concentrations by group.
trols (baseline: r = 0.071; p = 0.62, or end: r = 0.021; p = 0.89) (Figure 3(b)). These findings suggest that adiponectin levels during pregnancy are not influenced by gestational weight gain.
After correlation analyses which were done separately for controls and women in the intervention group, we assessed associations between the change in leptin and adiponectin levels during pregnancy and all covariates by covariate analysis. Age (p = 0.03), pre-pregnancy BMI (p = 0.005), gestational weight gain (p = 0.036) and baseline leptin concentration were linked to a change in leptin levels during pregnancy (Table 2). A change in adiponectin levels during pregnancy was inversely linked to pre-pregnancy BMI (p = 0.026), unlike in the results in correlational analyses including one group only. Change in adiponectin was also associated with baseline adiponectin concentrations (p < 0.001), but not with gestational weight gain (p = 0.93) (Table 2).
 (a)
(a)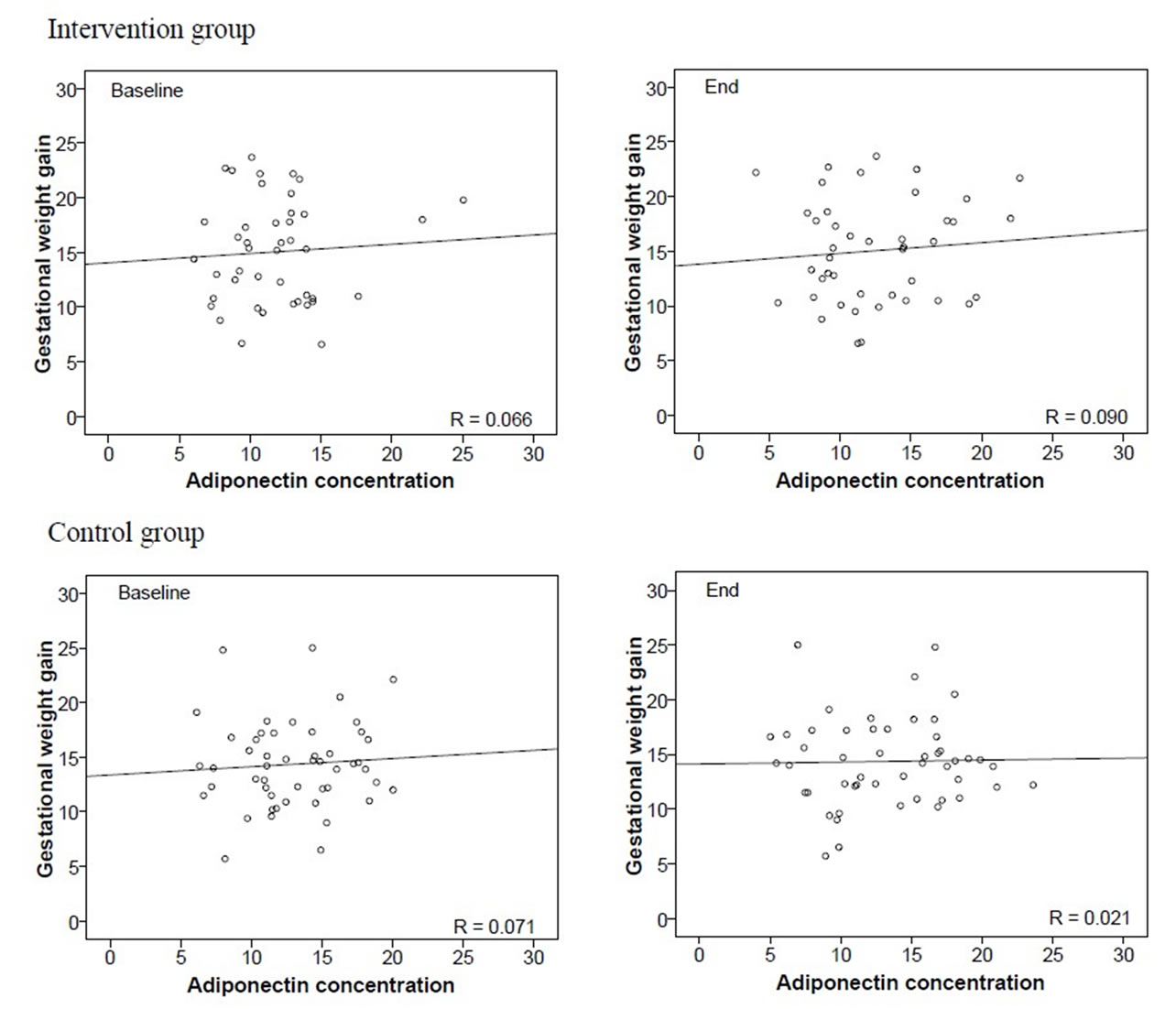 (b)
(b)
Figure 3. (a) Correlations between gestational weight gain and leptin concentrations at the beginning and end of pregnancy, by group; (b) Correlations between gestational weight gain and adiponectin concentrations at the beginning and end of pregnancy, by group.
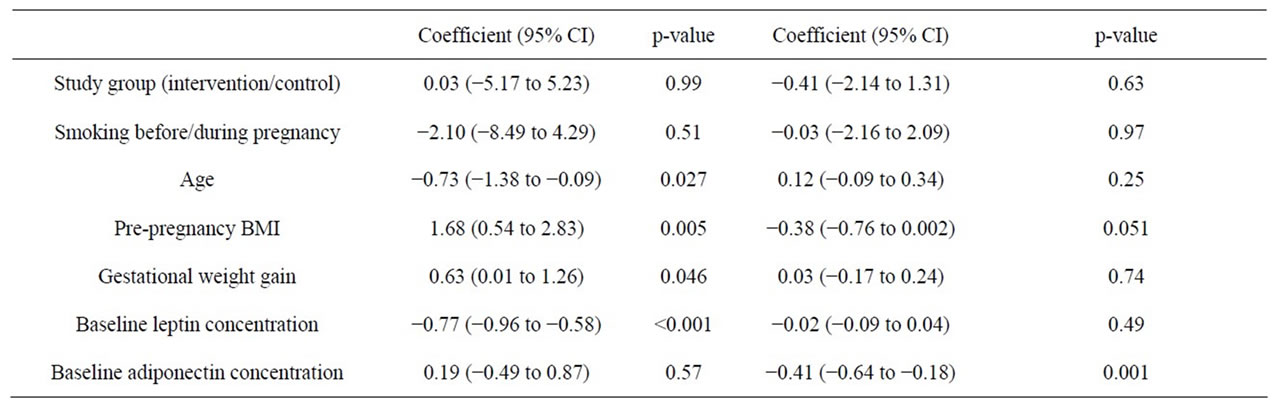
Table 2. Covariance analysis explaining covariates and changes in leptin and adiponectin in the trial. Leptin and adiponectin are dependent variables.
4. Discussion
The aim of this study was to investigate whether a lifestyle intervention during pregnancy, focused on bringing changes in the diet and physical activity, affects pregnancy leptin and adiponectin levels and their association with BMI. Although the intervention failed to modify weight gain during pregnancy, it prevented high birth weights (>4000 g) [8,54]. As high birth weight is associated with elevated pregnancy leptin levels [55] and impaired insulin sensitivity [56], we speculated that the lifestyle intervention may have affected leptin and adiponectin levels. Leptin increases insulin sensitivity and suppress insulin secretion [10], and consequently it is used to treat insulin resistance [10,29]. Adiponectin also is insulin-sensitizing adipokine [12,44-46], and low adiponectin levels are linked to insulin resistance and GDM [43,47-50].
Several studies have found that maternal leptin concentrations increase during pregnancy [21-23,33], but the increase seems to occur during the first two trimesters and then leptin levels decrease slightly during the third trimester [24,31-32,57]. This may explain why we did not see a significant increase in our mothers; their blood was collected on the 1st and 3rd trimester, but not on the 2nd. A lack of increase on the 3rd trimester is reflective of late pregnancy being a leptin-resistant stage [34,35]. During the last pregnancy trimester, leptin levels do not rise although body weight increases, indicative of pregnancy-induced leptin resistance [30], and this contributes to reduced insulin sensitivity seen during pregnancy [35, 36].
In our study, pregnancy leptin levels did not differ between the control and intervention groups during the first pregnancy trimester before intervention started, or at the end of intervention on week 36 - 38. This probably reflects the lack of effect of the intervention on gestational weight gain (Table 2). Previous studies have shown that pregnancy leptin levels correlate with prepregnancy [21,24,25,58] and pregnancy BMI [22,25,32, 40,57,58] and gestational weight gain [33]. These associations are stronger at the beginning of pregnancy than during the third trimester [22,32], consistent with development of pregnancy-linked leptin resistance [35,36]. In our study, women in the control group exhibited positive correlations between pre-pregnancy BMI and baseline and end leptin levels. However, the change in leptin concentrations during pregnancy in the controls negatively correlated with pre-pregnancy BMI, indicating that heavier women showed less increase in leptin than slimmer women during pregnancy. In contrast, in the intervention group the associations grew stronger towards the end of pregnancy, and pre-pregnancy BMI correlated positively with a change in leptin concentrations during pregnancy. Heavier women exhibited higher elevation in leptin levels during pregnancy than lean women. Since the two groups did not differ from each other regarding BMI at the beginning or end of pregnancy, and their pregnancy weight gain was similar [8], diet and physical activity intervention may have contributed in maintaining leptin sensitivity during pregnancy.
Although adiponectin levels inversely correlate with BMI [14-16], body weight during pregnancy is not be linked to adiponectin [17,59,60]. In most studies, adiponectin levels during pregnancy range between 7 and 13.5 ml/ml [17,48,59-62]. Some of these studies report a reduction in adiponectin levels as pregnancy progresses [48], and some have found no changes [17]. Results in our study indicated no changes between change in adiponectin and gestational weight gain in the control group according to correlational analyses and no changes in either group according to covariance analysis. In correlation analysis including each group separately, the intervention group exhibited a slight increase, suggesting that exposing pregnant women to a diet high in fruits, vegetables and whole grains as well as moderate physical activity may improve insulin sensitivity. Adiponectin is strongly related to insulin sensitivity [43], including during pregnancy [12,43,44,46]. Women who develop GDM exhibit reduced circulating adiponectin levels [43,47-50], and low gestational adiponectin levels have been found to predict postpartum insulin resistance and other changes leading to type 2 diabetes [61].
Our study was designed to pilot the study protocol for a larger study, a fact which elicited some limitations to this study. First, the maternal clinics volunteered for the study, and hence were not randomized. The lack of randomization may have resulted in greater baseline differences between the groups. The intervention group had significantly higher pre-pregnancy BMI, which may explain their higher leptin levels at baseline and at the end of the study, as well as the greater increase in leptin concentrations during pregnancy. The participants of the intervention group were more often smokers before pregnancy. Smoking was not significantly associated with leptin levels in the model, suggesting that smoking did not have a large effect on the results. Second, the statistical power may have been weakened by the fairly small sample size of our study. Third, the intervention may not have been effective enough to cause significant differences between the groups. However, the strength of our study was the ability to study longitudinal changes in leptin and adiponectin concentrations during pregnancy. Our study is the first to determine whether lifestyle intervention during pregnancy changes leptin and adiponectin levels or their interaction with BMI and pregnancy weight gain.
5. Conclusion
In conclusion, we found that pregnant women who increased their intake of fruits, vegetables and whole grains and were physically active, maintained sensitivity to leptin throughout pregnancy. Women in the control group appeared more resistant to leptin during the 3rd than 1st pregnancy trimester. These findings suggest that dietary and physical activity intervention helps pregnant women to maintain sensitivity to leptin throughout pregnancy, and the presence of leptin sensitivity at the end of pregnancy may explain how the intervention completely prevented high birth weight in our pilot study [8] and significantly reduced the incidence of high birth weight in the consequent study involving 399 pregnant women [54].
6. Acknowledgements
Financially supported by Competitive Research Funding of the Pirkanmaa Hospital District, Tampere University Hospital, Ministry of Education and Culture and National Cancer Institute (U54 CA000970). Medical student Minna Nummela helped in preparing the first drafts of the manuscript.
REFERENCES
- Institute of Medicine, “Weight Gain during Pregnancy: Reexamining the Guidelines,” National Academies, Washington DC, 2009.
- R. Artal, C. J. Lockwood and H. L. Brown, “Weight Gain Recommendations in Pregnancy and the Obesity Epidemic,” Obstetrics & Gynecology, Vol. 115, No. 1, 2010, pp. 152-155. doi:10.1097/AOG.0b013e3181c51908
- S. S. Huda, L. E. Brodie and N. Sattar, “Obesity in Pregnancy: Prevalence and Metabolic Consequences,” Seminars in Fetal and Neonatal Medicine, Vol. 15, No. 2, 2010, pp. 70-76. doi:10.1016/j.siny.2009.09.006
- A. M. Siega-Riz, M. Viswanathan, M. K. Moos, A. Deierlein, S. Mumford, J. Knaack, P. Thieda, L. J. Lux and K. N. Lohr, “A Systematic Review of Outcomes of Maternal Weight Gain According to the Institute of Medicine Recommendations: Birthweight, Fetal Growth, and Postpartum Weight Retention,” American Journal of Obstetrics and Gynecology, Vol. 201, No. 4, 2009, pp. 339-314. doi:10.1016/j.ajog.2009.07.002
- K. Shankar, A. Harrell, X. Liu, J. M. Gilchrist, M. J. Ronis and T. M. Badger, “Maternal Obesity at Conception Programs Obesity in the Offspring,” American Journal of Physiology—Regulatory, Integrative and Comparative Physiology, Vol. 294, No. 2, 2009, pp. R528-R538. doi:10.1152/ajpregu.00316.2007
- S. M. Nelson, P. Matthews and L. Poston, “Maternal Metabolism and Obesity: Modifiable Determinants of Pregnancy Outcome,” Human Reproduction Update, Vol. 16, No. 3, 2009, pp. 255-275.
- J. C. King, “Maternal Obesity, Metabolism, and Pregnancy Outcomes,” Annual Review of Nutrition, Vol. 26, 2006, pp. 271-291. doi:10.1146/annurev.nutr.24.012003.132249
- T. I. Kinnunen, M. Pasanen, M. Aittasalo, M. Fogelholm, L. Hilakivi-Clarke, E. Weiderpass and R. Luoto, “Preventing Excessive Weight Gain during Pregnancy—A Controlled Trial in Primary Health Care,” European Journal of Clinical Nutrition, Vol. 61, No. 7, 2007, pp. 884-891. doi:10.1038/sj.ejcn.1602602
- Y. B. Kim, S. Uotani, D. D. Pierroz, J. S. Flier and B. B. Kahn, “In Vivo Administration of Leptin Activates Signal Transduction Directly in Insulin-Sensitive Tissues: Overlapping But Distinct Pathways from Insulin,” Endocrinology, Vol. 141, No. 7, 2000, pp. 2328-2339. doi:10.1210/en.141.7.2328
- B. O. Yildiz and I. C. Haznedaroglu, “Rethinking Leptin and Insulin Action: Therapeutic Opportunities for Diabetes,” The International Journal of Biochemistry & Cell Biology, Vol. 38, No. 5-6, 2006, pp. 820-830. doi:10.1016/j.biocel.2005.09.013
- M. Matsubara, S. Maruoka and S. Katayose, “Inverse Relationship between Plasma Adiponectin and Leptin Concentrations in Normal-Weight and Obese Women,” European Journal of Endocrinology, Vol. 147, No. 2, 2002, pp. 173-180. doi:10.1530/eje.0.1470173
- J. Jurimae, T. Jurimae, S. Ring-Dimitriou, L. M. LeMura, P. J. Arciero and S. P. von Duvillard, “Plasma Adiponectin and Insulin Sensitivity in Overweight and Normal-Weight Middle-Aged Premenopausal Women,” Metabolism, Vol. 58, No. 5, 2009, pp. 638-643. doi:10.1016/j.metabol.2008.12.009
- Y. Zhang, R. Proenca, M. Maffei, M. Barone, L. Leopold and J. M. Friedman, “Positional Cloning of the Mouse Obese Gene and Its Human Homologue,” Nature, Vol. 372, No. 6505, 1994, pp. 425-432. doi:10.1038/372425a0
- M. Fasshauer, J. Klein, S. Neumann, M. Eszlinger and R. Paschke, “Hormonal Regulation of Adiponectin Gene Expression in 3T3-L1 Adipocytes,” Biochemical and Biophysical Research Communications, Vol. 290, No. 3, 2002, pp. 1084-1089. doi:10.1006/bbrc.2001.6307
- I. Kowalska, M. Straczkowski, A. Nikolajuk, A. Krukowska, I. Kinalska and M. Gorska, “Plasma Adiponectin Concentration and Tumor Necrosis Factor-Alpha System Activity in Lean Non-Diabetic Offspring of Type 2 Diabetic Subjects,” European Journal of Endocrinology, Vol. 154, No. 2, 2006, pp. 319-324. doi:10.1530/eje.1.02084
- R. M. Blumer, S. N. van der Crabben, M. E. Stegenga, M. V. V. Tanck, M. T. Ackermans, E. Endert, P. T. van der and H. P. Sauerwein, “Hyperglycemia Prevents the Suppressive Effect of Hyperinsulinemia on Plasma Adiponectin Levels in Healthy Humans,” American Journal of Physiology—Endocrinology and Metabolism, Vol. 295, No. 3, 2008, pp. E613-E617. doi:10.1152/ajpendo.90288.2008
- S. Mazaki-Tovi, H. Kanety, C. Pariente, R. Hemi, A. Wiser, E. Schiff and E. Sivan, “Maternal Serum Adiponectin Levels during Human Pregnancy,” Journal of Perinatology, Vol. 27, No. 2, 2007, pp. 77-81. doi:10.1038/sj.jp.7211639
- I. Hendler, S. C. Blackwell, S. H. Mehta, J. E. Whitty, E. Russell, Y. Sorokin and D. B. Cotton, “The Levels of Leptin, Adiponectin, and Resistin in Normal Weight, Overweight, and Obese Pregnant Women with and without Preeclampsia,” American Journal of Obstetrics and Gynecology, Vol. 193, No. 3, 2005, pp. 979-983.
- J. M. Gomez, F. J. Maravall, N. Gomez, M. A. Navarro, R. Casamitjana and J. Soler, “Interactions between Serum Leptin, the Insulin-Like Growth Factor-I System, and Sex, Age, Anthropometric and Body Composition Variables in a Healthy Population Randomly Selected,” Clinical Endocrinology, Vol. 58, No. 2, 2003, pp. 213-219. doi:10.1046/j.1365-2265.2003.01698.x
- J. M. Friedman and J. L. Halaas, “Leptin and the Regulation of Body Weight in Mammals,” Nature, Vol. 395, No. 6704, 1998, pp. 763-770. doi:10.1038/27376
- L. Hardie, P. Trayhurn, D. Abramovich and P. Fowler, “Circulating Leptin in Women: A Longitudinal Study in the Menstrual Cycle and during Pregnancy,” Clinical Endocrinology, Vol. 47, No. 1, 1997, pp. 101-106. doi:10.1046/j.1365-2265.1997.2441017.x
- C. Schubring, P. Englaro, T. Siebler, W. F. Blum, T. Demirakca, J. Kratzsch and W. Kiess, “Longitudinal Analysis of Maternal Serum Leptin Levels during Pregnancy, at Birth and up to Six Weeks after Birth: Relation to Body Mass Index, Skinfolds, Sex Steroids and Umbilical Cord Blood Leptin Levels,” Hormone Research, Vol. 50, No. 5, 1998, pp. 276-283. doi:10.1159/000023290
- T. P. Stein, T. O. Scholl and M. D. Schluter and C. M. Schroeder, “Plasma Leptin Influences Gestational Weight Gain and Postpartum Weight Retention,” The American Journal of Clinical Nutrition, Vol. 68, No. 6, 1998, pp. 1236-1240.
- T. Tamura, R. L. Goldenberg, K. E. Johnston and S. P. Cliver, “Serum Leptin Concentrations during Pregnancy and Their Relationship to Fetal Growth,” Obstetrics & Gynecology, Vol. 91, No. 3, 1998, pp. 389-395. doi:10.1016/S0029-7844(97)00670-4
- A. Festa, N. Shnawa, W. Krugluger, P. Hopmeier, G. Schernthaner and S. M. Haffner, “Relative Hypoleptinaemia in Women with Mild Gestational Diabetes Mellitus,” Diabetic Medicine, Vol. 16, No. 8, 1999, pp. 656- 662. doi:10.1046/j.1464-5491.1999.00122.x
- R. Mukherjea, T. W. Castonguay, L. W. Douglass and P. Moser-Veillon, “Elevated Leptin Concentrations in Pregnancy and Lactation: Possible Role as a Modulator of Substrate Utilization,” Life Sciences, Vol. 65, No. 11, 1999, pp. 1183-1193. doi:10.1016/S0024-3205(99)00352-5
- J. L. Halaas, K. S. Gajiwala, M. Maffei, S. L. Cohen, B. T. Chait, D. Rabinowitz, R. L. Lallone, S. K. Burley and J. M. Friedman, “Weight-Reducing Effects of the Plasma Protein Encoded by the Obese Gene,” Science, Vol. 269, No. 5223, 1995, pp. 543-546. doi:10.1126/science.7624777
- R. L. Leibel, M. Rosenbaum and J. Hirsch, “Changes in Energy Expenditure Resulting from Altered Body Weight,” The New England Journal of Medicine, Vol. 332, No. 10, 1995, pp. 621-628. doi:10.1056/NEJM199503093321001
- T. Kusakabe, H. Tanioka, K. Ebihara, M. Hirata, L. Miyamoto, F. Miyanaga, H. Hige, D. Aotani, T. Fujisawa, H. Masuzaki, K. Hosoda and K. Nakao, “Beneficial Effects of Leptin on Glycaemic and Lipid Control in a Mouse Model of Type 2 Diabetes with Increased Adiposity Induced by Streptozotocin and a High-Fat Diet,” Diabetologia, Vol. 47, No. 10, 2009, pp. 1838-1846.
- P. J. Scarpace and Y. Zhang, “Leptin Resistance: A Prediposing Factor for Diet-Induced Obesity,” American Journal of Physiology—Regulatory, Integrative Comparative Physiology, Vol. 296, No. 3, 2009, pp. R493-R500. doi:10.1152/ajpregu.90669.2008
- E. Sivan, P. G. Whittaker, D. Sinha, C. J. Homko, M. Lin, E. A. Reece and G. Boden, “Leptin in Human Pregnancy: The Relationship with Gestational Hormones,” American Journal of Obstetrics and Gynecology, Vol. 179, No. 5, 1998, pp. 1128-1132. doi:10.1016/S0002-9378(98)70118-8
- S. M. Stock, E. M. Sande and K. A. Bremme, “Leptin Levels Vary Significantly during the Menstrual Cycle, Pregnancy, and in Vitro Fertilization Treatment: Possible Relation to Estradiol,” Fertility and Sterility, Vol. 72, No. 4, 1999, pp. 657-662. doi:10.1016/S0015-0282(99)00321-0
- N. F. Butte, J. M. Hopkinson and M. A. Nicolson, “Leptin in Human Reproduction: Serum Leptin Levels in Pregnant and Lactating Women,” The Journal of Clinical Endocrinology & Metabolism, Vol. 82, No. 2, 1997, pp. 585-589. doi:10.1210/jc.82.2.585
- S. R. Ladyman, R. A. Augustine and D. R. Grattan, “Hormone Interactions Regulating Energy Balance during Pregnancy,” Journal of Neuroendocrinology, Vol. 22, No. 7, 2010, pp. 805-817.
- D. R. Grattan, S. R. Ladyman and R. A. Augustine, “Hormonal Induction of Leptin Resistance during Pregnancy,” Physiology & Behavior, Vol. 91, No. 4, 2007, pp. 366- 374. doi:10.1016/j.physbeh.2007.04.005
- R. A. Augustine, S. R. Ladyman and D. R. Grattan, “From Feeding One to Feeding Many: Hormone-Induced Changes in Bodyweight Homeostasis during Pregnancy,” The Journal of Physiology, Vol. 586, No. 2, 2008, pp. 387-397. doi:10.1113/jphysiol.2007.146316
- J. P. Kirwan, S. Hauguel-De Mouzon, J. Lepercq, J. C. Challier, L. Huston-Presley, J. E. Friedman, S. C. Kalhan and P. M. Catalano, “TNF-Alpha Is a Predictor of Insulin Resistance in Human Pregnancy,” Diabetes, Vol. 51, No. 7, 2002, pp. 2207-2213. doi:10.2337/diabetes.51.7.2207
- Z. Maghbooli, A. Hossein-Nezhad, M. Rahmani, A. R. Shafaei and B. Larijani, “Relationship between Leptin Concentration and Insulin Resistance,” Hormone and Metabolic Research, Vol. 39, No. 12, 2007, pp. 903-907. doi:10.1055/s-2007-992812
- J. M. Ategbo, O. Grissa, A. Yessoufou, A. Hichami, K. L. Dramane, K. Moutairou, A. Miled, A. Grissa, M. Jerbi, Z. Tabka and N. A. Khan, “Modulation of Adipokines and Cytokines in Gestational Diabetes and Macrosomia,” The Journal of Clinical Endocrinology & Metabolism, Vol. 91, No. 10, 2006, pp. 4137-4143. doi:10.1210/jc.2006-0980
- K. A. McLachlan, D. O’Neal, A. Jenkins and F. P. Alford, “Do Adiponectin, TNFalpha, Leptin and CRP Relate to Insulin Resistance in Pregnancy? Studies in Women with and without Gestational Diabetes, during and after Pregnancy,” Diabetes/Metabolism Research and Reviews, Vol. 22, No. 2, 2006, pp. 131-138. doi:10.1002/dmrr.591
- H. Yamashita, J. Shao, T. Ishizuka, P. J. Klepcyk, P. Muhlenkamp, L. Qiao, N. Hoggard and J. E. Friedman, “Leptin Administration Prevents Spontaneous Gestational Diabetes in Heterozygous Lepr(db/+) Mice: Effects on Placental Leptin and Fetal Growth,” Endocrinology, Vol. 142, No. 7, 2001, pp. 2888-2897. doi:10.1210/en.142.7.2888
- R. Saucedo, A. Zarate, L. Basurto, M. Hernandez, E. Puello, R. Galvan and S. Campos, “Relationship between Circulating Adipokines and Insulin Resistance during Pregnancy and Postpartum in Women with Gestational Diabetes,” Archives of Medical Research, Vol. 42, No. 4, 2011, pp. 318-323. doi:10.1016/j.arcmed.2011.06.009
- T. Kadowaki, T. Yamauchi, N. Kubota, K. Hara, K. Ueki and K. Tobe, “Adiponectin and Adiponectin Receptors in Insulin Resistance, Diabetes, and the Metabolic Syndrome,” The Journal of Clinical Investigation, Vol. 116, No. 7, 2006, pp. 1784-1792. doi:10.1172/JCI29126
- A. H. Berg, T. P. Combs and P. E. Scherer, “ACRP30/ Adiponectin: An Adipokine Regulating Glucose and Lipid Metabolism,” Trends in Endocrinology & Metabolism, Vol. 13, No. 2, 2002, pp. 84-89. doi:10.1016/S1043-2760(01)00524-0
- T. Kadowaki, T. Yamauchi, N. Kubota, K. Hara and K. Ueki, “Adiponectin and Adiponectin Receptors in Obesity-Linked Insulin Resistance,” Novartis Foundation Symposium, Vol. 286, 2007, pp. 164-176. doi:10.1002/9780470985571.ch15
- K. J. Mather, T. Funahashi, Y. Matsuzawa, S. Edelstein, G. A. Bray, S. E. Kahn, J. Crandall, S. Marcovina, B. Goldstein and R. Goldberg, “Adiponectin, Change in Adiponectin, and Progression to Diabetes in the Diabetes Prevention Program,” Diabetes, Vol. 57, No. 4, 2008, pp. 980-986. doi:10.2337/db07-1419
- A. E. Altinova, F. Toruner, N. Bozkurt, N. Bukan, A. Karakoc, I. Yetkin, G. Ayvaz, N. Cakir and M. Arslan, “Circulating Concentrations of Adiponectin and Tumor Necrosis Factor-Alpha in Gestational Diabetes Mellitus,” Gynecological Endocrinology, Vol. 23, No. 3, 2007, pp. 161-165. doi:10.1080/09513590701227960
- P. M. Catalano, M. Hoegh, J. Minium, L. Huston-Presley, S. Bernard, S. Kalhan and S. Hauguel-De Mouzon, “Adiponectin in Human Pregnancy: Implications for Regulation of Glucose and Lipid Metabolism,” Diabetologia, Vol. 49, No. 7, 2006, pp. 1677-1685. doi:10.1007/s00125-006-0264-x
- S. Soheilykhah, M. Mohammadi, M. Mojibian, S. Rahimi-Saghand, M. Rashidi, H. Hadinedoushan and M. fkhami-Ardekani, “Maternal Serum Adiponectin Concentration in Gestational Diabetes,” Gynecological Endocrinology, Vol. 25, No. 9, 2009, pp. 593-596. doi:10.1080/09513590902972109
- S. Weerakiet, K. Lertnarkorn, P. Panburana, S. Pitakitronakorn, K. Vesathada and S. Wansumrith, “Can Adiponectin Predict Gestational Diabetes?” Gynecological Endocrinology, Vol. 22, No. 7, 2006, pp. 362-368. doi:10.1080/09513590600818919
- Suositukset, “Seulontatutkimukset ja Yhteistyo Aitiyshuollossa,” Gummerus, Jyvaskyla, 1999.
- Institute of Medicine, “Nutrition during Pregnancy, Weight Gain and Nutrient Supplements,” National Academic Press, Washington DC, 1990.
- K. Cseh, E. Baranyi, Z. Melczer, E. Kaszas, E. Palik and G. Winkler, “Plasma Adiponectin and Pregnancy-Induced Insulin Resistance,” Diabetes Care, Vol. 27, No. 1, 2004, pp. 274-275. doi:10.2337/diacare.27.1.274
- R. Luoto, T. I. Kinnunen, M. Aittasalo, P. Kolu, J. Raitanen, K. Ojala, K. Mansikkamaki, S. Lamberg, T. Vasankari, T. Komulainen and S. Tulokas, “Primary Prevention of Gestational Diabetes Mellitus and Large-for-Gestational-Age Newborns by Lifestyle Counseling: A ClusterRandomized Controlled Trial,” PLoS Medicine, Vol. 8, No. 5, 2011, p. e1001036. doi:10.1371/journal.pmed.1001036
- A. Lagiou, P. Lagiou, D. S. Vassilarou, M. Stoikidou and D. Trichopoulos, “Comparison of Age at First Full-Term Pregnancy between Women with Breast Cancer and Women with Benign Breast Diseases,” International Journal of Cancer, Vol. 107, No. 5, 2003, pp. 817-821. doi:10.1002/ijc.11476
- G. K. Ong, J. K. Hamilton, M. Sermer, P. W. Connelly, G. Maguire, B. Zinman, A. J. Hanley and R. Retnakaran, “Maternal Serum Adiponectin and Infant Birthweight: The Role of Adiponectin Isoform Distribution,” Clinical Endocrinology, Vol. 67, No. 1, 2007, pp. 108-114. doi:10.1111/j.1365-2265.2007.02846.x
- F. S. Al Atawi, M. H. Addar, A. S. Warsy and Z. A. Babay, “Leptin Concentration during Different Trimesters of Pregnancy and Its Relation to Other Pregnancy Hormones,” Saudi Medical Journal, Vol. 25, No. 11, 2004, pp. 1617-1622.
- A. Kautzky-Willer, G. Pacini, A. Tura, C. Bieglmayer, B. Schneider, B. Ludvik, R. Prager and W. Waldhausl, “Increased Plasma Leptin in Gestational Diabetes,” Diabetologia, Vol. l 44, No. 2, 2001, pp. 164-172.
- J. Fuglsang, C. Skjaerbaek, J. Frystyk, A. Flyvbjerg and P. Ovesen, “A Longitudinal Study of Serum Adiponectin during Normal Pregnancy,” BJOG, Vol. 113, No. 1, 2006, pp. 110-113. doi:10.1111/j.1471-0528.2005.00792.x
- B. Eriksson, M. Lof, H. Olausson and E. Forsum, “Body Fat, Insulin Resistance, Energy Expenditure and Serum Concentrations of Leptin, Adiponectin and Resistin before, during and after Pregnancy in Healthy Swedish Women,” British Journal of Nutrition, Vol. 103, No. 1, 2010, pp. 50-57. doi:10.1017/S0007114509991371
- R. Retnakaran, Y. Qi, P. W. Connelly, M. Sermer, A. J. Hanley and B. Zinman, “Low Adiponectin Concentration during Pregnancy Predicts Postpartum Insulin Resistance, Beta Cell Dysfunction and Fasting Glycaemia,” Diabetologia, Vol. 53, No. 2, 2009, pp. 268-276.
- J. K. Nien, S. Mazaki-Tovi, R. Romero, O. Erez, J. P. Kusanovic, F. Gotsch, B. L. Pineles, R. Gomez, S. Edwin, M. Mazor, J. Espinoza, B. H. Yoon and S. S. Hassan, “Plasma Adiponectin Concentrations in Non-Pregnant, Normal and Overweight Pregnant Women,” Journal of Perinatal Medicine, Vol. 35, No. 6, 2007, pp. 522-531. doi:10.1515/JPM.2007.123
NOTES
*Corresponding author.

