American Journal of Plant Sciences
Vol.07 No.14(2016), Article ID:71357,15 pages
10.4236/ajps.2016.714186
Alleviation of Drought Stress in Arabidopsis thaliana by 17β-Estradiol Application
Pallavi Upadhyay, Camelia Maier
Department of Biological Sciences, Texas Woman’s University, Denton, Texas, USA

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: September 21, 2016; Accepted: October 17, 2016; Published: October 20, 2016
ABSTRACT
Animal steroidal hormones, including estrogens, are being introduced into the agricultural soil and water supply from increased pharmaceutical and farm waste. Considering the current levels of xenoestrogen contamination of plant environments in view of the climate change induced drought conditions, this study was designed to understand the effect of estradiol (ES) application on Arabidopsis drought stress responses. Estradiol treatment (10 nM, 100 nM) of plants subjected to drought stress conditions by withholding water for 7 days resulted in increased tolerance to drought stress reflected in the significantly higher plant survival rates of 74% and 78%, respectively compared to control plants’ survival rates of 36% (no treatment) and 40% (mock treatment). Estradiol application significantly increased the content of glutathione, proline and H2O2 and significantly enhanced the transcription of the stress responsive genes GSTU3, GER5, HSP101, and HSP70b. A high concentration of ES (10 µM) did not protect plants against drought stress and proved to be toxic. These results provide new insight into the effect of ES on drought-stress responses in Arabidopsis with possible practical agricultural applications regarding the effect of environmental estrogens on crop plants.
Keywords:
Arabidopsis, Drought Stress, Glutathione, H2O2, Proline, Stress Genes, Xenoestrogens

1. Introduction
Environmental contamination with mammalian sex hormones (MSHs) is an environmental concern. Increasing levels of MSHs are being introduced into the agricultural soil and water supply through farm and pharmaceutical wastes [1] [2] . Despite the fact that MSHs act as endocrine disruptors in aquatic animals, a number of studies have demonstrated their beneficial effects on plants coping with abiotic stresses [3] - [9] . Progesterone, 17β-estradiol and androsterone applications mitigated the effects of salinity stress by enhancing germination rate, and root and shoot length in maize [6] and wheat seedlings [7] . The protective effect of MSHs has also been reported in the case of chilling and heavy metal stresses [8] [9] . Foliar application of progesterone to chickpea plants exposed to chilling stress resulted in enhanced antioxidant enzyme activity, chlorophyll content and relative leaf water content [8] . Estradiol supplementation of the seed germination medium protected lentil seedlings from the negative effects of heavy metal stress [9] .
Drought not only limits the distribution of plants, but also affects crop productivity and quality since it is the primary production-limiting factor in plants. Models of climate change predict increases of ambient temperatures accompanied by drought conditions, which will enhance the effects of other biotic and abiotic plant stresses, thus affecting agricultural production. The worldwide crop production is impacted by drought and therefore, increased drought tolerance is a priority for many breeding programs.
Plants respond to drought stress and acquire resistance through complex and integrated biochemical and physiological processes that include changes in gene expression and cross talk between different stress signaling networks. A common consequence of various abiotic stresses, such as drought, salt, heavy metal and other stresses, is the production of reactive oxygen species (ROS) in plant cells and oxidative damage to tissues [10] . Studies have shown a direct correlation between the enhanced antioxidant activities and drought tolerance in various plant species [10] [11] [12] . Treatment of germinating bean (Phaseolus vulgaris) seeds with various concentrations of progesterone, β-estradiol and androsterone resulted in increased activity of antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POX) and catalase (CAT) in plants grown under drought stress. At the same time, decreased lipid peroxidation and hydrogen peroxide (H2O2) levels were observed in the MSH-treated bean seedlings and attributed to an increase in antioxidant enzyme activity, which correlated to increased plant resistance to stress [3] . Another study reported that progesterone-treated bean plants under salt stress had higher SOD, POX and CAT activities and displayed lower levels of lipid peroxidation as compared to the non MSH-treated plants [5] . The radical-scavenging enzymes together with the antioxidants ascorbic acid and glutathione (GSH) constitute an effective system for detoxification of ROS. Moreover, plants accumulate osmolytes, such as proline, organic acids, sugar alcohols and sugars that act as compatible solutes leading to a decrease of the osmotic potential, thus resulting in drought tolerance [13] . Under stress conditions that cause water deficit within the cell, high concentrations of proline protect membrane structures, stabilize the quaternary structure of proteins and scavenge hydroxyl radicals [14] .
Various transcriptomic studies identified genes that respond to drought stress, some of which facilitate coordination of multiple plant responses at the cellular and whole metabolism levels. The dehydration-responsive transcriptional factor DREB2A, for example, has a dual function by regulating Arabidopsis responses to drought and heat stresses [15] . Thousands of genes were shown to be differentially regulated upon drought stress. Among those, drought stress-related transcription factors were classified in ten different gene families (DREB, bZIP, MYC, MYB, NAC, AP2-domain, NF-Y, ERF, WRKY and zinc fingers) [16] .
Considering the current levels of xenoestrogen contamination of plant environments in view of the climate change induced drought conditions as a challenge facing agriculture today and in the future, this study was designed to understand the effect of ES application on Arabidopsis drought stress responses. We demonstrate that application of low concentrations of ES to Arabidopsis plants alleviated drought stress via increased levels of glutathione, H2O2 and proline and upregulated stress-related gene expression.
2. Materials and Methods
2.1. Plant Material and Growth Conditions
Arabidopsis thaliana ecotype Columbia wild type (WT) seeds (Lehle Seeds, TX, USA) were surface sterilized according to the procedure described previously [17] [18] and grown in Murashige and Skoog [19] nutrient medium solidified with 1% agar and increasing concentrations of ES (10 nM, 100 nM, 10 μM). The plates were maintained under long-day conditions (16 hours light and 8 hours dark) at 22˚C, 50% humidity, and 200 μmol/m2/s. The experimental design of simulated plant exposure to ES followed the method previously described [20] . Arabidopsis seedlings germinated on MS plates without ES or with 0.01% ethanol were used as controls. At day 14, the seedlings were transferred from MS plates into pots in a Percival growth chamber under long-day conditions (16 hours light and 8 hours dark) at 22˚C, 50% humidity, and 200 μmol/m2/s and were sprayed with ES once a week for the following two weeks.
2.2. Drought Stress Induction
Drought stress experiments were performed according to the procedure employed by Kagale et al. [18] with slight modifications. ES-treated and untreated, non-stressed plants were watered every third day by subirrigation. Drought conditions in the 21-day- old plants were introduced by withholding water for 7 days. Leaf tissue was collected for biochemical and gene expression analysis at the beginning (0 days post treatment) and at the end (7 days post treatment) of the induced drought stress period. Following the drought stress, watering plants every third day was resumed for a week. Plants that survived were counted, and the percentage survival rate for each treatment was calculated as an indicator of drought stress tolerance. These experiments were repeated thrice, each time with 30 plants.
2.3. Glutathione Estimation
Total glutathione was estimated in leaves using the Glutathione Assay Kit (Sigma Aldrich, USA) according to the manufacturer’s specifications and was expressed as µmoles/g fresh weight.
2.4. Hydrogen Peroxide Estimation
Hydrogen peroxide was estimated in leaves using the Amplex® Red Hydrogen Peroxide/ Peroxidase Kit (ThermofischerSci, USA) following the manufacturer’s specifications and expressed as µmoles/g fresh weight.
2.5. Proline Estimation
Estimation of leaf proline was conducted according to a previously published protocol by Bates et al. [21] and expressed as µmoles/g fresh weight.
2.6. RNA Isolation and qRT-PCR
RNA was isolated from leaves of 21-day-old ES-treated and untreated, drought stressed and unstressed Arabidopsis plants using the Plant RNA isolation reagent (Life Technologies, USA) according to the manufacturer’s protocol. RT-PCR from the total RNA extracted was performed using the RETROscript reverse transcription kit (Thermo Fisher Sci, USA). Primers were designed using the Primer-3 software. The following primer sequences were used for the PCR analysis: (GSTU, GLUTATHIONE S TRANSFERASE) GSTU3F: 5'CAATGGCCGAGAAAGAAGAG3´, GSTU3R: 5'AAGTAGCAACGGGCTCTTGA3', (GEr5, GEM-RELATED 5) GER5F: 5'CATCGGAATGTTCCATACCTGGAGT3', GER5R: 5'TTGGCTCTGTTCCGAAAATCTGTCT3', (HSP70, HEAT SHOCK PROTEIN 70) HSP70bF: 5'AGGATAAAACCGCTGGTGTG3´, HSP70bR: 5´ATTCTTGGCCTCCACCTTCT3', (HSP101, HEAT SHOCK PROTEIN 101) HSP101F: 5'GCCAAGTGTGCCTGACACCATTAGT3', HSP101R: 5'GCTTTATCCGGTAAATGCCGACCA3', (EF1α, EUKARYOTIC ELONGATION FACTOR α) EF1αF: 5'TTCACCCTTGGTGTCAAGCAGATG3', EF1αR: 5'TCAGGGTTGTATCCGACCTTCTTCA3'. The RT-PCR experiments were carried out using the iQ SYBR® Green supermix and the BioRAD CFX96 RT-PCR detection system (BioRad, USA) according to the manufacturers’ protocol. Reaction parameters were set as follows: initial denaturation at 95˚C for 5 mins followed by 35 cycles of 30 s at 95˚C, 30 s at 55˚C (annealing) and 30 s at 72˚C (extension). The amplification sizes are as follows: GSTU3―129 bp, GER5―117 bp, HSP70b―29 bp, HSP101―126 bp, EF1α―100 bp. The relative RNA levels in each sample were calibrated and normalized against EF1α expression that was used as an internal control.
2.7. Statistical Analysis
Data are the means ± SD of three independent replicates. Data were subjected to a one-way analysis of variance (ANOVA) and the mean differences were compared using Tukey’s test. Comparisons with P < 0.05 were considered significantly different.
3. Results
3.1. Estradiol Application Alleviated Drought Stress in Arabidopsis
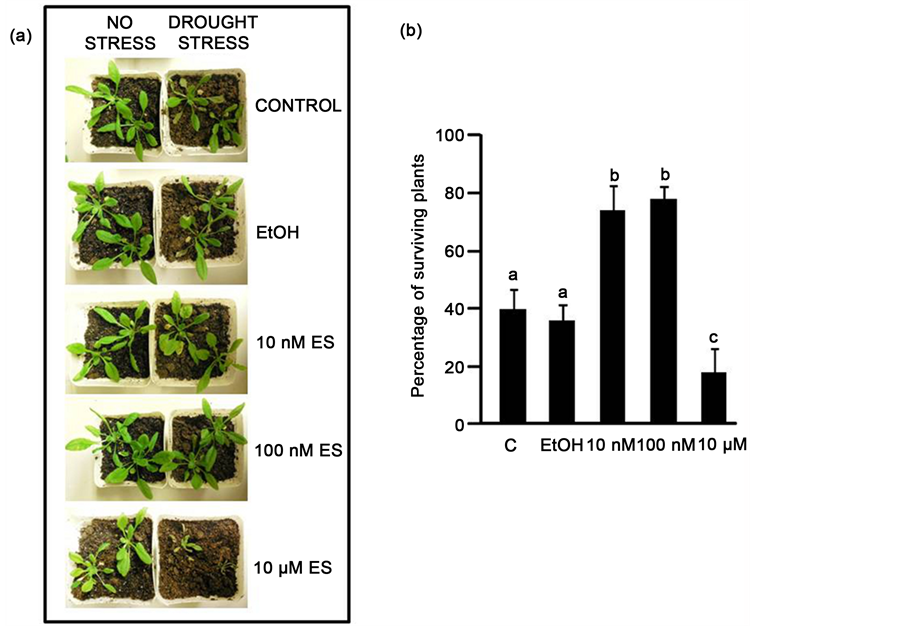
After seven days of induced drought by withholding irrigation, both ES-treated and control plants developed characteristic drought stress symptoms of wilting, chlorosis and tissue senescence, although the 10 nM and 100 nM ES-treated plants showed relatively less symptoms (Figure 1(a)). At the end of the recovery week, during which the watering schedule was resumed, it was observed that the 10 and 100 nM ES-treated plants coped better than controls and 10 µM ES-treated plants with the induced drought conditions in terms of tissue senescence and chlorosis. Significantly higher tolerance to drought stress was observed in 10 and 100 nM ES-treated plants with 74% and 78% of the plants surviving, respectively, as compared to 40% surviving regularly watered control plants and to 36% surviving EtOH control plants. The lowest tolerance to drought stress was recorded for 10 µM ES-treated plants with an 18% survival rate (Figure 1(b)).
3.2. Estradiol Application Increased Glutathione Levels in Arabidopsis during Drought Stress
Under induced drought conditions, an increase in glutathione levels was observed in all Arabidopsis plants irrespective of the treatment. However, significantly higher levels of glutathione were found in Arabidopsis plants treated with 10 nM ES (81% increase)
Figure 1. Estradiol application alleviates drought stress in Arabidopsis. (a) Representative image of ES-treated and untreated plants following 7 days of drought stress; (b) Plant survival (%) after induced drought stress and recovery period. The results are means ± SD (n = 30). Different alphabets represent statistically significant difference (P < 0.05, ANOVA). C = no treatment, EtOH = 0.01% ethanol treated, 10 nM, 100 nM, 10 µM = applied estradiol concentrations.
and 100 nM ES (48% increase) than in control plants. The lowest levels of glutathione were estimated in leaves of plants treated with 10 µM ES under drought stress and even under non-stress conditions (Figure 2). As compared to the control plants and the other ES treatments, 10 µM ES treated plants showed a 30% decrease in glutathione levels under no stress conditions, indicating ES effect. Under drought conditions, the level of glutathione in 10 µM ES treated plants increased with approximately 21% but was significantly lower than in the 10 nM and 100 nM ES treated plants (Figure 2), showing an accumulated drought effect on top of the ES effect.
3.3. Estradiol Treatment Increased Hydrogen Peroxide Levels in Arabidopsis Leaves during Drought Stress
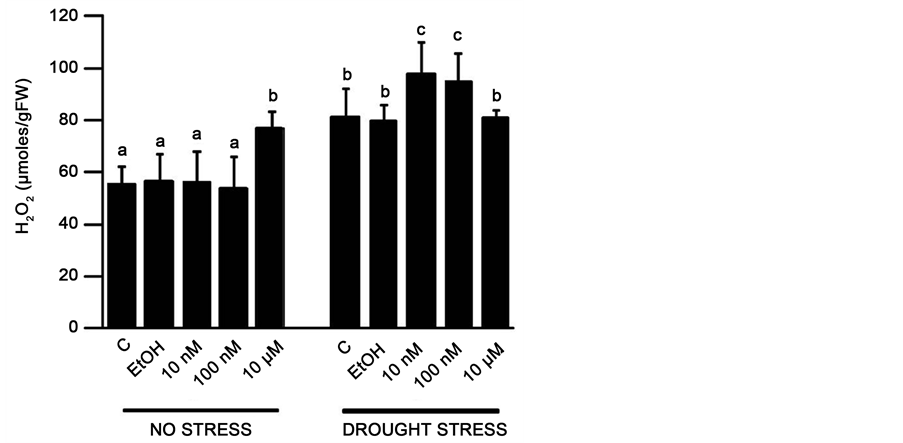
H2O2 can scavenge high energy electrons generated during abiotic stresses and thus protect plant cells, however high levels of H2O2 can contribute towards oxidative stress [22] . It was observed that, under no-stress conditions, the Arabidopsis plants treated with 10 µM ES accumulated significantly higher levels of H2O2, a 38% increase from the levels found in the controls and the other ES treatments. The levels of H2O2 in control and 10 and 100 nM ES-treated plants under no stress were not significantly different indicating that ES treatment is responsible for the high concentration of H2O2 in the 10 µM ES treated plants (Figure 3). Under induced drought stress, H2O2 concentration in 10 µM ES treated plants was no different than in no stressed plants and no significant different than the levels in drought-stressed control plants. With approximately 23% increase in H2O2 levels as compared to the non ES-treated controls, plants treated with 10 and 100 nM ES accumulated the highest levels of H2O2 under drought conditions (Figure 3).
Figure 2. Glutathione levels in Arabidopsis leaves at the end of induced drought period. Different alphabets represent statistically significant difference (P < 0.05, ANOVA). The results are means ± SD (n = 9). C = no treatment, EtOH = 0.01% ethanol treated, 10 nM, 100 nM, 10 µM = applied estradiol concentrations.
Figure 3. H2O2 levels in Arabidopsis leaves at the end of induced drought period. Different alphabets represent statistically significant difference (P < 0.05, ANOVA). The results are means ± SD (n = 9). C = no treatment, EtOH = 0.01% ethanol treated, 10 nM, 100 nM, 10 µM = applied estradiol concentrations.
3.4. Estradiol Application Increased Proline Levels in Arabidopsis during Drought Stress
Proline, an essential amino acid, is a compatible osmolyte, which protects cellular structures during drought stress. Leaves of both 10 and 100 nM ES-treated Arabidopsis plants accumulated significantly higher levels of proline under drought stress than the unstressed plants. Although the proline levels accumulated in drought stressed leaves were significantly higher for all the plants, the 10 and 100 nM ES treated leaves displayed an 80% increase in proline levels as compared to the control plants. As observed with glutathione levels, the lowest proline levels were estimated in plants treated with 10 µM ES (Figure 4). Although the proline levels were significantly higher than in the non-stressed plants, the drought-stressed 10 µM ES treated plants had a 25% decrease in proline levels as compared to the non ES-treated plants (Figure 4).
3.5. Estradiol Application Upregulated Stress-Related Gene Expression
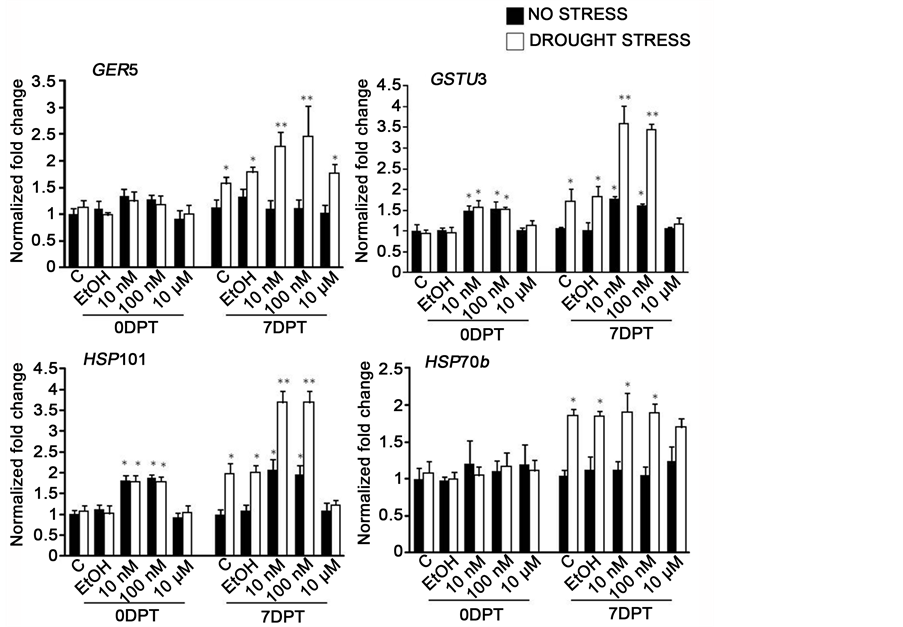
Real time-PCR was performed to assess the expression levels of four stress responsive genes that are involved in providing plant cells protection during drought conditions. Enhanced expression of GLUTATHIONE S-TRANSFERASEU3 (GSTU3), GEM-RELATED5 (GER5), HEAT SHOCK PROTEIN101 (HSP101) and HSP70b was observed 7 days post drought stress (Figure 5). The expression of GSTU3 was approximately 3.5 fold higher in the leaves of drought-stressed 10 and 100 nM ES-treated plants than in those of non-stressed control plants. Expression levels of GER5 in drought-stressed 10 and 100 nM ES-treated plants was determined to be approximately 3.6 folds higher than in the control plants. The application of 10 and 100 nM ES, under
Figure 4. Proline levels in Arabidopsis plants at the end of induced drought period. Different alphabets represent statistically significant difference (P < 0.05, ANOVA). The results are means ± SD (n = 9). C = no treatment, EtOH = 0.01% ethanol treated, 10 nM, 100 nM, 10 µM = applied estradiol concentrations.
Figure 5. Quantitative PCR analysis of stress-related gene expression in Arabidopsis in response to ES treatment and induced drought stress. ES upregulates GSTU3, GER5, HSP70b and HSP101 expression. Asterisks represent statistically significant differences (P < 0.05, ANOVA). The results are means ± SD (n = 9). C = no treatment, EtOH = 0.01% ethanol treated, 10 nM, 100 nM, 10 µM = applied estradiol concentrations. White bars (drought stress), Black bars (no stress).
non-drought conditions, also resulted in enhanced expressions of GSTU3 and GER5 in the corresponding plants but they were significantly higher (more than twofold) in the corresponding drought-stressed plants (Figure 5).
Expression of both HSP101 and HSP70b was upregulated by drought stress and not by ES (Figure 5). Drought enhanced the expression of HSP70b in both ES-treated and untreated plants. The increase in HSP70b expression in drought-stressed 10 µM ES treated plants was significantly lower than the gene expression in 10 and 100 nM ES-treated plants control plants.
A similar increase in HSP101 expression by drought also was observed in the drought-stressed 10 and 100 nM ES-treated plants (Figure 5). The increase in HSP101 expression in drought-stressed 10 µM ES treated plants was not significantly different than the gene expression in control plants.
4. Discussion
4.1. Effects of 17β-Estradiol Application on Stress-Related Biochemical Responses
The results of this study provide evidence for a protective role of 17β-estradiol against drought stress in Arabidopsis when applied at low concentrations (10 and 100 nM) in the range of the ES concentrations found in contaminated soil [1] [2] . Application of ES at a higher concentration (10 µM) proved to be toxic to the plants, thus further compromising their ability to cope with drought stress. In response to drought stress, significantly higher accumulation of GSH, H2O2 and proline was observed in 10 and 100 nM ES-treated plants as compared to control plants, which could explain the increased stress tolerance of these plants.
A number of studies have demonstrated an important role for glutathione in drought stress tolerance in various plant species. During abiotic stresses, upon reaction with oxidizing agents, GSH, a tripeptide composed of the amino acids glutamic acid, cystine and glycine (γ-L-Glutamyl-L-cystine glycine), is converted to GSSG [11] . It has been reported that glutathione levels (GSH/GSSG ratio) changes rapidly in response to water deficit, thus helping maintain the redox homeostasis of the cell [12] . In wheat (Triticum sp.) plants grown under drought conditions, GSH concentration increased in the flag leaves of both drought resistant and sensitive varieties [23] . Induction of drought stress by the application of PEG-6000 to the seedlings of Brassica campestris and B. juncea resulted in increased GSSG levels. B. juncea seedlings, in the presence of PEG-6000, displayed elevated levels of GSH as well [24] [25] . It is possible that ES alleviates the detrimental effects of drought stress in Arabidopsis by influencing the GSH-GSSG cycle.
Similarly, an increase in proline contents was observed in plants that were exposed to drought conditions [26] . Studies with Arabidopsis and rice plants showed that accumulation of proline, one of the most powerful osmoprotectant, was an essential response of the plant cells to drought stress [27] [28] . The beneficial drought-coping effect of ES treatment is evident from the observation that 10 and 100 nM ES-treated plants accumulated the highest levels of both GSH and proline when subjected to drought stress. Proline is an extremely efficient ROS scavenger, thus protecting proteins and membranes by reducing free radical levels and providing a source of carbon during rehydration [29] . It could be possible that ES directly affects proline metabolism or interacts with plant hormones or through other mechanisms in elevating proline levels in plants under drought stress.
Hydrogen peroxide is a unique ROS that plays a dual role within the plant cells. High levels of H2O2 generated during abiotic stress, including drought stress, can damage cellular structures but a low concentration of H2O2 is maintained within the cells and functions as a signal, mediator or effector involved in developmental processes and stress responses [22] [30] . High levels of H2O2 can by itself result in oxidative damage to the cells [21] but it has been demonstrated in a number of plants species, including maize [31] , mustard [32] , tall fescue and rye grass [33] , that an increase in H2O2 levels primes the plants for enhanced drought stress resistance. Significant increases in H2O2 levels in 10 and 100 nM ES-treated Arabidopsis subjected to drought conditions were responses to drought stress and not to the ES treatment and it indicates that H2O2 contributed to the enhanced drought stress tolerance of these plants. Plants exposed to higher ES concentrations (10 µM ES) showed an increase in H2O2 level even under no drought conditions, which remain unaltered in response to drought stress, indicating ES toxicity. These plants displayed the lowest survival rate at the end of the recovery period and their GSH and proline levels at the end of the drought stress period were the lowest among all treatments, which could explain their poor performance in coping with the stress conditions.
4.2. Effects of 17β-Estradiol Application on Stress-Related Gene Expression
To the best of our knowledge this is the first report on changes in stress related gene expression in ES-treated plants. A previous study in our laboratory on the effects of ES treatment on gene expression in Arabidopsis seedlings employing microarray analysis revealed that expression of stress responsive genes were upregulated [34] . Quantitative PCR analysis of GSTU3, GER5, HSP70b and HSP101 expression in ES-treated and untreated plants in the current study showed that the expression of these stress responsive genes is enhanced in response to drought stress.
Significantly higher expression of both GSTU3 and GER5 was observed in drought- stressed 10 and 100 nM ES-treated plants, which may have contributed to their enhanced drought stress tolerance. The treatments may have primed the plants for increased tolerance as the non-stressed plants displayed elevated expression of GSTU3 when compared to the other treatments.
The glutathione S-transferase enzymes play an important role in plant stress resistance as they facilitate the binding of GSH to xenobiotic substrates [35] [36] . Transgenic expression of a tomato GST, LeGSTU2, resulted in enhanced drought stress tolerance in Arabidopsis [36] . GSTU3 displays increased expression in response to abiotic stress conditions [37] . Similarly, the expression of GER5 is induced by ABA and abiotic stress and is required for growth and development in Arabidopsis [38] .
Heat Shock Proteins belong to a specialized class of chaperone proteins whose expression is induced in response to a number of abiotic stresses [39] . In Arabidopsis, both HSP70b and HSP101 are required for developing thermotolerance [40] [41] . HSP70 in wheat was upregulated by more than tenfold and in Arabidopsis by almost eightfold under drought conditions [42] [43] . Overexpression of Erianthus arundinaceus HSP70 increased drought and salinity tolerance of sugarcane [44] . HSP101 is involved in protecting protein and membrane integrity [45] [46] . In our study, even in the absence of heat stress, expression of both HSP70b and HSP101 was elevated in response to drought stress. In a manner similar to the expression of GSTU3, the expression of HSP101 was elevated in non-stressed 10 and 100 nM ES-treated plants. These results are similar to those of brassinosteroid application to Arabidopsis plants, where treatment with the hormone resulted in increased tolerance to drought stress and elevated expression of HSP genes [18] . Since HSPs are required for proper protein folding and functioning during normal and stress conditions, it can be suggested that application of ES can potentially enhance the ability of plants to cope with other forms of abiotic stress. Since ES is the ligand for estrogen receptors, which contain zinc finger domains, it is also possible that ES interacts with plant zinc-fingers transcriptional factors, some of which are activated by drought [47] [48] , in inducing its beneficial effects on drought-stressed plants.
5. Conclusion
This study focused on the impact of ES treatment on Arabidopsis responses to drought stress both at the gene expression and biochemical levels. The results indicate that ES effects on Arabidopsis plants, specifically enhancing the drought tolerance, are both genomic and nongenomic. ES treatment at the 10 and 100 nM concentrations can be beneficial for plant survival under drought stress; however, at higher concentrations (10 µM), ES can be detrimental to plant growth and survival under stress conditions. The concentration range of ES applied in this study is relevant with respect to the environmental concentrations of the hormone and our observations open new avenues for understanding the impact of xenoestrogens on plant stress responses with potential applications in agriculture.
Acknowledgements
This research was supported by Texas Woman’s University, Research Enhancement Program and College of Arts and Sciences, Research Development Funds. This article was published with support from Texas Woman’s University Libraries’ Open Access Fund.
Cite this paper
Upadhyay, P. and Maier, C. (2016) Alleviation of Drought Stress in Arabidopsis thaliana by 17β-Estradiol Application. American Journal of Plant Sciences, 7, 2072-2086. http://dx.doi.org/10.4236/ajps.2016.714186
References
- 1. Finlay-Moore, O., Hartel, P. and Cabrera, M. (2000) 17β-Estradiol and Testosterone in Soil and Runoff from Grasslands Amended with Broiler Litter. Journal of Environmental Quality, 29, 1604-1611.
http://dx.doi.org/10.2134/jeq2000.00472425002900050030x - 2. Ying, G. and Kookana, R.S. (2005) Sorption and Degradation of Estrogen-Like-Endocrine Disrupting Chemicals in Soil. Environmental Toxicology and Chemistry, 24, 2640-2645.
http://dx.doi.org/10.1897/05-074R.1 - 3. Erdal, S. (2009) Effects of Mammalian Sex Hormones on Antioxidant Enzyme Activities, H2O2Content and Lipid Peroxidation in Germinating Bean Seeds. Journal of the Faculty of Agriculture, 40, 79-85.
- 4. Dumlupinar, R., Genisel, M., Erdal, S., Korkut, T., Taspinar, M.S. and Taskin, M. (2011) Effects of Progesterone, β-Estradiol, and Androsterone on the Changes of Inorganic Element Content in Barley Leaves. Biological Trace Element Research, 143, 1740-1745.
http://dx.doi.org/10.1007/s12011-011-8980-6 - 5. Erdal, S., Genisel, M., Turk, H. and Gorcek, Z. (2012) Effects of Progesterone Application on Antioxidant Enzyme Activities and K+/Na+ Ratio in Bean Seeds Exposed to Salt Stress. Toxicology and Industrial Health, 28, 942-946.
http://dx.doi.org/10.1177/0748233711430975 - 6. Erdal, S. (2012) Exogenous Mammalian Sex Hormones Mitigate Inhibition in Growth by Enhancing Antioxidant Activity and Synthesis Reactions in Germinating Maize Seeds Under Salt Stress. Journal of the Science of Food and Agriculture, 92, 839-843.
http://dx.doi.org/10.1002/jsfa.4655 - 7. Erdal, S. (2012) Alleviation of Salt Stress in Wheat Seedlings by Mammalian Sex Hormones. Journal of the Science of Food and Agriculture, 92, 1411-1416.
http://dx.doi.org/10.1002/jsfa.4716 - 8. Genisel, M., Turk, H. and Erdal, S. (2013) Exogenous Progesterone Application Protects Chickpea Seedlings against Chilling-Induced Oxidative Stress. Acta Physiologiae Plantarum, 35, 241-251.
http://dx.doi.org/10.1007/s11738-012-1070-3 - 9. Chaoui, A. and El Ferjani, E. (2013) β-Estradiol Protects Embryo Growth from Heavy-Metal Toxicity in Germinating Lentil Seeds. Journal of Plant Growth Regulation, 32, 636-645.
http://dx.doi.org/10.1007/s00344-013-9332-x - 10. Das, K. and Roychoudhury, A. (2014) Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Frontiers in Environmental Science, 2, 53.
- 11. Pastori, G.M. and Trippi, V.S. (1992) Oxidative Stress Induces High Rate of Glutathione Reductase Synthesis in a Drought Resistant Maize Strain. Plant and Cell Physiology, 33, 957-961.
- 12. Contour-Ansel, D., Torres-Franklin, M.L., Cruz de Carvalho, M.H., D’Arcy-Lameta, A. and Zuily-Fodil, Y. (2006) Glutathione Reductase in Leaves of Cowpea: Cloning of two cDNAs, Expression and Enzymatic Activity under Progressive Drought Stress, Desiccation and Abscisic Acid Treatment. Annals of Botany, 98, 1279-1287.
http://dx.doi.org/10.1093/aob/mcl217 - 13. Blum, A. (2011) Plant Breeding for Water-Limited Environments. Vol. XIV, Springer, New York, 258 p.
http://dx.doi.org/10.1007/978-1-4419-7491-4 - 14. Verbruggen, N. and Hermans, C. (2008) Proline Accumulation in Plants: A Review. Amino Acids, 35, 753-759.
http://dx.doi.org/10.1007/s00726-008-0061-6 - 15. Sakuma, Y., Maruyama, K., Osakabe, Y., Qin, F., Seki, M., Shinozaki, K. and Yamaguchi-Shinozaki, K. (2006) Functional Analysis of an Arabidopsis Transcription Factor, DREB2A, Involved in Drought-Responsive Gene Expression. The Plant Cell, 18, 1292-1309.
http://dx.doi.org/10.1105/tpc.105.035881 - 16. Alter, S., Bader, K.C., Spannagl, M., Wang, Y., Bauer, E., Schon, C.C. and Mayer, K.F. (2015) Drought DB: An Expert-Curated Compilation of Plant Drought Stress Genes and their Homologs in Nine Species. Database, 2015.
- 17. Abrahám, E., Rigó, G., Székely, G., Nagy, R., Koncz, C. and Szabados, L. (2003) Light-Dependent Induction of Proline Biosynthesis by Abscisic Acid and Salt Stress Is Inhibited by Brassinosteroid in Arabidopsis. Plant Molecular Biology, 51, 363-372.
http://dx.doi.org/10.1023/A:1022043000516 - 18. Kagale, S., Divi, U.K., Krochko, J.E., Keller, W.A. and Krishna, P. (2007) Brassinosteroid Confers Tolerance in Arabidopsis thaliana and Brassica napus to a Range of Abiotic Stresses. Planta, 225, 353-364.
http://dx.doi.org/10.1007/s00425-006-0361-6 - 19. Murashige, T. and Skoog, F. (1962) A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiologia Plantarum, 15, 473-497.
http://dx.doi.org/10.1111/j.1399-3054.1962.tb08052.x - 20. Upadhyay, P. and Maier, C. (2016) Effects of 17β-Estradiol on Growth, Primary Metabolism, Phenylpropanoid-Flavonoid Pathways and Pathogen Resistance in Arabidopsis thaliana. American Journal of Plant Sciences, 7, 1693-1710.
http://dx.doi.org/10.4236/ajps.2016.713160 - 21. Bates, L., Waldren, R. and Teare, I. (1973) Rapid Determination of Free Proline for Water-Stress Studies. Plant and Soil, 39, 205-207.
http://dx.doi.org/10.1007/BF00018060 - 22. Petrov, V.D. and Van Breusegem, F. (2012) Hydrogen Peroxide—A Central Hub for Information Flow in Plant Cells. AoB Plants, 2012, pls014.
http://dx.doi.org/10.1093/aobpla/pls014 - 23. Herbinger, K., Tausz, M., Wonisch, A., Soja, G., Sorger, A. and Grill, D. (2002) Complex Interactive Effects of Drought and Ozone Stress on the Antioxidant Defence Systems of Two Wheat Cultivars. Plant Physiology and Biochemistry, 40, 691-696.
http://dx.doi.org/10.1016/S0981-9428(02)01410-9 - 24. Hossain, M.A., Mostofa, M.G. and Fujita, M. (2013) Heat-Shock Positively Modulates Oxidative Protection of Salt and Drought-Stressed Mustard (Brassica campestris L.) Seedlings. Journal of Plant Science & Molecular Breeding, 2, 1-14.
http://dx.doi.org/10.7243/2050-2389-2-2 - 25. Alam, M.M., Hasanuzzaman, M., Nahar, K. and Fujita, M. (2013) Exogenous Salicylic Acid Ameliorates Short-Term Drought Stress in Mustard (Brassica juncea L.) Seedlings by Up-Regulating the Antioxidant Defense and Glyoxalase System. Australian Journal of Crop Science, 7, 1053-1063.
- 26. Bhaskara, G.B., Yang, T. and Verslues, P.E. (2015) Dynamic Proline Metabolism: Importance and Regulation in Water Limited Environments. Frontiers in Plant Science, 6, 484.
http://dx.doi.org/10.3389/fpls.2015.00484 - 27. Todaka, D., Shinozaki, K. and Yamaguchi-Shinozaki, K. (2015) Recent Advances in the Dissection of Drought-Stress Regulatory Networks and Strategies for Development of Drought-Tolerant Transgenic Rice Plants. Frontiers in Plant Science, 6, 84.
http://dx.doi.org/10.3389/fpls.2015.00084 - 28. Rejeb, K.B., Jdey, A., Abdelly, C. and Savoure, A. (2015) Hydrogen Peroxide Produced by NADPH Oxidases Increases Proline Accumulation during Salt or Mannitol Stress in Arabidopsis thaliana. New Phytologist, 208, 1138-1148.
http://dx.doi.org/10.1111/nph.13550 - 29. Szabados, L. and Savoure, A. (2010) Proline: A Multifunctional Amino Acid. Trends in Plant Science, 15, 89-97.
http://dx.doi.org/10.1016/j.tplants.2009.11.009 - 30. Bhattacharjee, S. (2012) An Inductive Pulse of Hydrogen Peroxide Pretreatment Restores Redox-Homeostasis and Oxidative Membrane Damage under Extremes of Temperature in Two Rice Cultivars. Plant Growth Regulation, 68, 395-410.
http://dx.doi.org/10.1007/s10725-012-9728-9 - 31. Gondim, F.A., de Souza Miranda, R., Gomes-Filho, E. and Prisco, J.T. (2013) Enhanced Salt Tolerance in Maize Plants Induced by H2O2 Leaf Spraying Is Associated with Improved Gas Exchange Rather than with Non-Enzymatic Antioxidant System. Theoretical and Experimental Plant Physiology, 25, 251-260.
http://dx.doi.org/10.1590/S2197-00252013000400003 - 32. Hossain, M.A. and Fujita, M. (2013) Hydrogen Peroxide Priming Stimulates Drought Tolerance in Mustard (Brassica juncea L.) Seedlings. Plant Gene & Trait, 4, 109-123.
http://dx.doi.org/10.5376/pgt.2013.04.0020 - 33. Wang, Y., Zhang, J., Li, J. and Ma, X. (2014) Exogenous Hydrogen Peroxide Enhanced the Thermotolerance of Festuca arundinacea and Lolium perenne by Increasing the Antioxidative Capacity. Acta Physiologiae Plantarum, 36, 2915-2924.
http://dx.doi.org/10.1007/s11738-014-1661-2 - 34. Adetunji, K. (2012) Microarray Analysis of Estradiol Regulated Gene Expression in Arabidopsis Seedlings. MS Thesis, Texas Woman’s University, Denton, Texas.
- 35. Edwards, R., Dixon, D.P. and Walbot, V. (2000) Plant Glutathione S-Transferases: Enzymes with Multiple Functions in Sickness and in Health. Trends in Plant Science, 5, 193-198.
http://dx.doi.org/10.1016/S1360-1385(00)01601-0 - 36. Xu, J., Xing, X., Tian, Y., Peng, R., Xue, Y., Zhao, W. and Yao, Q.-H. (2015) Transgenic Arabidopsis Plants Expressing Tomato Glutathione S-Transferase Showed Enhanced Resistance to Salt and Drought Stress. PLoS ONE, 10, e0136960.
http://dx.doi.org/10.1371/journal.pone.0136960 - 37. Sappl, P.G., Carroll, A.J., Clifton, R., Lister, R., Whelan, J., Harvey Millar, A. and Singh, K.B. (2009) The Arabidopsis Glutathione Transferase Gene Family Displays Complex Stress Regulation and Co-Silencing Multiple Genes Results in Altered Metabolic Sensitivity to Oxidative Stress. The Plant Journal, 58, 53-68.
http://dx.doi.org/10.1111/j.1365-313X.2008.03761.x - 38. Baron, K.N., Schroeder, D.F. and Stasolla, C. (2014) Gem-Related 5 (GER5), an ABA and Stress-Responsive GRAM Domain Protein Regulating Seed Development and Inflorescence Architecture. Plant Science, 223, 153-166.
http://dx.doi.org/10.1016/j.plantsci.2014.03.017 - 39. Yu, A., Li, P., Tang, T., Wang, J., Chen, Y. and Liu, L. (2015) Roles of Hsp70s in Stress Responses of Microorganisms, Plants, and Animals. BioMed Research International, 2015, Article ID: 510319.
http://dx.doi.org/10.1155/2015/510319 - 40. Sung, D.Y., Vierling, E. and Guy, C.L. (2001) Comprehensive Expression Profile Analysis of the Arabidopsis Hsp70 Gene Family. Plant Physiology, 126, 789-800.
http://dx.doi.org/10.1104/pp.126.2.789 - 41. Hong, S. and Vierling, E. (2001) Hsp101 Is Necessary for Heat Tolerance but Dispensable for Development and Germination in the Absence of Stress. The Plant Journal, 27, 25-35.
http://dx.doi.org/10.1046/j.1365-313x.2001.01066.x - 42. Grigorova, B., Vaseva, I.I., Demirevska, K. and Feller, U. (2011) Expression of Selected Heat Shock Proteins after Individually Applied and Combined Drought and Heat Stress. Acta Physiologiae Plantarum, 33, 2041-2049.
http://dx.doi.org/10.1007/s11738-011-0733-9 - 43. Sung, D.Y. and Guy, C.L. (2003) Physiological and Molecular Assessment of Altered Expression of Hsc70-1 in Arabidopsis. Evidence for Pleiotropic Consequences. Plant Physiology, 132, 979-987.
http://dx.doi.org/10.1104/pp.102.019398 - 44. Augustine, S.M., Narayan, J.A., Syamaladevi, D.P., Appunu, C., Chakravarthi, M., Ravichandran, V. and Subramonian, N. (2015) Erianthus arundinaceus HSP70 (EaHSP70) Overexpression Increases Drought and Salinity Tolerance in Sugarcane (Saccharum spp. Hybrid). Plant Science, 232, 23-34.
http://dx.doi.org/10.1016/j.plantsci.2014.12.012 - 45. Gullì, M., Corradi, M., Rampino, P., Marmiroli, N. and Perrotta, C. (2007) Four Members of the HSP101 Gene Family Are Differently Regulated in Triticum durum Desf. FEBS Letters, 581, 4841-4849.
http://dx.doi.org/10.1016/j.febslet.2007.09.010 - 46. Hong, S.W., Lee, U. and Vierling, E. (2003) Arabidopsis Hot Mutants Define Multiple Functions Required for Acclimation to High Temperatures. Plant Physiology, 132, 757-767.
http://dx.doi.org/10.1104/pp.102.017145 - 47. Wang, F., Tong, W., Zhu, H., Kong, W., Peng, R., Liu, Q. and Yao, Q. (2016) A Novel Cys2/His2 Zinc Finger Protein Gene from Sweet Potato, IbZFP1, Is Involved in Salt and Drought Tolerance in Transgenic Arabidopsis. Planta, 243, 783-797.
http://dx.doi.org/10.1007/s00425-015-2443-9 - 48. Luo, X., Bai, X., Zhu, D., Li, Y., Ji, W., Cai, H., Wu, J., Liu, B. and Zhu, Y. (2012) GsZFP1, a New Cys2/His2-Type Zinc-Finger Protein, Is a Positive Regulator of Plant Tolerance to Cold and Drought Stress. Planta, 235, 1141-1155.
http://dx.doi.org/10.1007/s00425-011-1563-0