American Journal of Plant Sciences
Vol.4 No.2(2013), Article ID:28321,7 pages DOI:10.4236/ajps.2013.42042
Acclimatization of in Vitro Propagated Pineapple (Ananas comosuss (L.), var. Smooth cayenne) Plantlets to ex Vitro Condition in Ethiopia
![]()
Ethiopia Institute of Agricultural Research, Jimma Agricultural Research Center, Plant Biotechnology Research, Jimma, Ethiopia.
Email: *ayelign@gmail.com
Received November 27th, 2012; revised December 30th, 2012; accepted January 7th, 2013
Keywords: Acclimatization; Ananas comosuss; ex Vitro; in Vitro; Pots; Substrates
ABSTRACT
Pineapple (Ananas comosuss, var. Smooth cayenne), which is a popular tropical fruit, is propagated vegetatively. Conventional propagation alone does not provide clean and adequate planting material demanded in Ethiopia. Recently, in vitro multiplication has become a promising technique for large-scale production. However, the acclimatization to the external environment procedure impedes the efficiency, which needs carefully optimized acclimatization techniques. We report optimized acclimatization procedures following firstand second-stage hardening methods for in vitro pineapple plantlets. Primarily, Jiffy-7 peat pellet allowed growing plants vigorously and provided above 8% survival rate over soil mix. Nevertheless, in Ethiopia, soil mix is cheaper and locally accessible. The primarily acclimatized plantlets are needed to be hardened further for better establishment and survival in the field. Black polybag and polysleeve pots filled with soil mix were evaluated in the greenhouse. A significant difference was obtained between pots for number of roots and substrate weight. Polybags had higher root number than polysleeves and saved about 27% of substrates per plant, which is a reduction of 25% of total transportation cost. Hence, the soil mix and polybags were found to be preferable over substrates and pots, for subsequent in vitro pineapple acclimatization.
1. Introduction
Pineapple (Ananas comosuss L.) is one of the most popular and delicious triploid fruit. It is esteemed for its pronounced flavor and nutritive elements. Pineapple is propagated vegetatively through suckers, slips or crowns [1]. However, these planting materials have there limitations including transmission of diseases, less uniformity and inadequacy for commercial production.
In Ethiopia, the major pineapple production sites are located in the southern and southwestern part of the country owned by private farmers and state farm. The farmers produce in small-scale on fragments of lands, whereas the state farm of Coffee Plantation Development Enterprise produces pineapple var. smooth cayenne along with their coffee and/or maize plantation [2]. Existing of severe shortage of planting materials is the key bottleneck for expanding pineapple cultivation in the country. Efforts have been exerted on conventional pineapple production through suckers, slips and crowns. However, the demand and availability of planting materials during planting period are not well synchronized since the planting time in the country relies on the rain fed season alone. Hence, in vitro culture techniques have been applied worldwide to address these problems [3,4].
In vitro propagation is a crucial technique for disease free, rapid, uniform and mass production of pineapple plantlets [3-5]. Nevertheless, ultimate success of in vitro produced plantlets depends upon the successful transfer and establishment of plants in ex vitro conditions. High loss or damage of in vitro raised plants can occurred when transferred to ex vitro conditions because of the transfer shock [6]. This is due to the exposure of plants to many new ex vitro situations such as low humidity, high level of irradiation, water deficit because of the poor hydraulic conductivity of the roots and low root-stem connection [7]. Therefore, plants under in vitro condition need to be acclimatized by different options. The threats for survival in ex vitro can be overcome by acclimatizing the plants with gradual lowering air humidity temperature, air flow and irradiation level [6].
The successful establishment of in vitro raised plants on the soil and later on field is the major success of in vitro propagation [3,5,6]. Thus, in vitro pineapple plantlets need to be acclimatized carefully. The acclimatization period varies from 6 - 8 months until the plants reach an appropriate size (200 - 300 mm height) for field transfer [5]. The losses during acclimatization and the lengthy period are the major limitations of a widespread use of micropropagated pineapple plantlets. To acclimatize in vitro plants, various approaches are employed by different researchers towards successful establishment of in vitro plants under ex vitro condition. For instance, substrates [8] and the type of pots used during acclimatization are crucial factors determining the survival rate and performance of plants under ex vitro conditions [9]. The lengthy growth period of pineapple plantlets during greenhouse acclimatization is also cut down by application of humic acids and plant growth-promoting bacteria [10].
Large-scale in vitro pineapple plantlets production has been started in Ethiopia a few years ago [11]. In line with this, the primarily acclimatization has been optimized on seedling trays filled with soil mix (2 soil, 1 coffee husk and 1 sand ratio) in greenhouses with above 85% of survival rate [4]. Other commercially available substrate (Jiffy-7 peat pellets), which is usually used in the developed world, has also a better survival and growth performance for small-scale applications. However, the survival rate on soil mix as well as the costs and accessibility of Jiffy-7 peat pellets are bottlenecks for large-scale acclimatization of in vitro pineapple plantlets. Hence, the primary aims of this paper was to evaluate modified soil mix like 1 soil, 2 coffee husk and 1 sand ratio, and Jiffy- 7 peat pellets for a better survival rate, growth performance and cost of acclimatization.
On the other hand, acclimatized plants do not have the appropriate size for direct planting into the field, which results in poor survival rate [6] when they are transferred. To attain a better survival rate and establishment on field, these plants need to be acclimatized further in greenhouses using pots such as black polysleeves and/or polybags until plants reach the appropriate size. Although pots allow further growth, it requires substrates (soil mix), root development and labor to fill it. Therefore, this paper also evaluated a suitable and cost effective pot type for better growth performance of in vitro raised pineapple plants in greenhouses.
2. Materials and Methods
2.1. Plant Materials
Pineapple (Ananas comosuss var. Smooth cayenne) plantlets were multiplied under in vitro condition according to the protocol established previously [4,11] in the plant biotechnology laboratory, Jimma Agricultural Research Center, Ethiopia. Well elongated, rooted and uniform plantlets were selected and taken out from culture Jam Jars (Figure 1(a)). The plantlets were thoroughly washed with running tap water to remove the gelling agent or medium sticking to the roots. Then, they were transplanted into primary acclimatization substrates.
The media were prepared using full strength Murashige and Skoog (MS) (1962) basal salts amended 3% (w/v) table sugar (local shop, Jimma, Ethiopia) and 2 mg/l BA for multiplication. Half strength MS medium supplemented with 3 mg/l IBA and 3% (w/v) table sugar
 (a)
(a)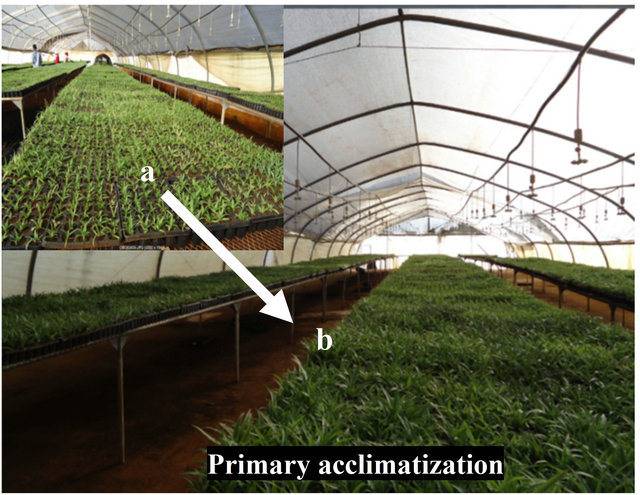 (b)
(b) (c)
(c)
Figure 1. In vitro raised pineapple plantlets prior to and after acclimatization in the greenhouse. (a) Rooted in vitro plantlets in Jam Jars; (b) Primary acclimatization in seedling trays filled with substrates. (a) Early after planting and (b) prior to the secondary acclimatization; (c) Secondary acclimatization in black poly pots filled with soil mix placed on the floor of the greenhouse.
was also prepared for rooting. The pH of the medium was adjusted to 5.8 using 1 N NaOH or 0.1 N HCl. The media were then solidified with 0.8% (w/v) agar (Sigma Chemical Co. Germany) for multiplication and with composite of enset starch (local shop, Jimma, Ethiopia) (60 g/l) and agar (2 g/l) for rooting [12]. It was later dispensed into Jam jars (~40 ml) followed by autoclaving at 1.06 kg/cm2 and 121˚C for 20 minutes.
2.2. First-Stage Acclimatization
Two acclimatization media treatments were arranged in the greenhouse prior to planting. This was replicated 10 times. The first treatment medium was soil mix composing of soil, coffee husk and sand in 1:2:1 ratio, respectively according previous report [4]. Forest soil and sand were collected from Jimma Agricultural Research Center, whereas coffee husk was purchased from Coffee Huller Private Company, Jimma, Ethiopia. These substrates were mixed and disinfected with formaldehyde (3%) for 72 h after mixing. Then, the soil mix was filled into seedling trays and plantlets were planted according to the treatments layout in complete randomized design. For the second treatment, Jiffy-7 peat pellets were obtained from Denmark (Jiffy A/S, Ryomgaard, Denmark), which were donated and transported by European Union. The pellets contain compressed peat surrounded by a biodegradable net. They were prepared for planting by soaking then into tap water for 10 min and were then squeezed to remove over soaked water and later placed in seedling trays. Trays filled with soil mixes and pellets were placed on a wire mesh bed inside the greenhouse. Selected plantlets were planted into those arranged seedling trays (Figure 1(b)) and kept for 2.5 months. Ten plants were allocated along every replication. The plants were watered once a day along with two days interval NPK (5 g/l) foliar fertilizer. The relative humidity in the greenhouse was maintained to 70% - 80% through misting (1 - 3 times/ day) coupled with wet sheath cloth depending on the external environment condition.
2.3. Second-Stage Acclimatization
In this experiment, two poly pot types such as black polysleeves (8 cm diameter) and polybags (8 cm diameter) were employed. The pots were easily accessed from a local market. Small holes were made at the bottom of polybags using a paper punch to allow aeration and drainage of excess water. The pots were filled with soil mix, which was similar substrate as first acclimatization treatments. The filling was done by hand with different pressure intensities, a bit compact for polysleeves to avoid loss of soil at the bottom and a loose filling for polybags. Then, pots were arranged properly and placed on the flour of the greenhouse. Later, the plants acclimatized during first-stage acclimatization were transplanted into both pot types and kept on the greenhouse floor (Figure 1(c)) for 4 months. This experiment was done in a complete randomized design with ten replications. Watering and NPK (5 g/l) foliar fertilizer were applied within two days interval along with misting (1 - 3 times/ day) depending on the external environment condition. Through misting the relative humidity was reduced to 60% - 70%.
2.4. Data Collection and Statistical Analysis
Non-destructive and destructive data were collected from first and second stage-acclimatization after 2.5 and 4 months, respectively. Non-destructive parameters collected were survival rate (%), number of leaves and roots, length of shoot and root (cm) as well as girth (mm). On the other hand, fresh and dry weight of shoot and root (g) as well as soil weight (g) were collected through destructive method. Fresh weight was measured immediately after removing the soil and separating shoots from roots. Dry matter of shoots and roots were taken after drying in an oven at 70˚C for 48 h following procedures [13]. The data were analyzed according to reference [14] using SAS, statistical software package (Version 8.01) [15] and significant mean values were compared using the procedure of REGWQ test (Ryan-Einot-Gabriel-Welsch Multiple Range Test).
3. Results
In vitro multiplied pineapple plantlets need to be acclimatized in the greenhouse prior to transferring into the field. Since acclimatization of in vitro plantlets in ex vitro conditions is a critical step for survival and growth performance, two major hardening stages have been evaluated.
3.1. Primary Acclimatization
In vitro raised pineapple plantlets were planted into seedling trays filled with locally prepared soil mix and commercially available Jiffy-7 pellets. The survival rate and growth performance of plantlets were evaluated. The result revealed that plantlets grown in Jiffy-7 peat pellet showed above 98% of survival followed by soil mix (1 soil, 2 coffee husk and 1 sand) with above 90% of survival (Figure 2(a)). In line with this, highly significant differences (p < 0.01) for leaf number, girth, shoot fresh and dry weight were obtained between substrates. The results also showed that highly significant differences (p < 0.01) for root number, root fresh and dry weights (Table 1) were found. Apparently, Jiffy-7 pellet was a good substrate that showed higher mean values of almost all growth parameters except root length. Therefore, a significant difference (p < 0.05) for root length was observed, in which soil mix showed higher mean root length (5.78 cm) (Figure 2(b)) on average than Jiffy-7 pellets, which is a crucial factor for survival.
3.2. Secondary Acclimatization
Because of the small size and weakness of the first-stage acclimatized pineapple plantlets, further hardening of these plantlets is required to increase the strength and growth performance. After transplanting those plantlets into different pot types, the plant performance and costs of substrates and transportation were evaluated. A significant difference (p < 0.05) for number of root was observed between pot types. Higher mean root number (19.22) was obtained from polybags than polysleeves (16.11) (Table 2). However, other growth parameters were showed non-significant differences between substrates.
Highly significant difference (p < 0.01) for the amount of substrates (1 soil, 2 coffee husk and 1 sand) in each pot types was observed during this experiment. The highest mean value (1225.05 g) were measured from polysleeves in contrary to polybags that had a mean value of 892.14 g. Using polybag for this stage of acclimatization reduced about 27% of the substrates needed per plant compared to polysleeves (Figure 3(a)). As a result, polybags provide benefits for secondary acclimatization by improving the amount of roots (Figure 3(b))
along with substrate costs reduction over polysleeves. Evidently, replacing polysleeves with polybags, transportation cost of potting plants into growing field is reduced by about 25 percent.
 (a)
(a) (b)
(b)
Figure 2. First-stage acclimatization of pineapple plantlets (a) after 2.5 months and their (b) root structure grown on both substrates.
Table 1. Substrate types influenced the growth performance of in vitro raised pineapple plantlets during the first-stage acclimatization. Data were collected after 2.5 months hardening in the greenhouse.
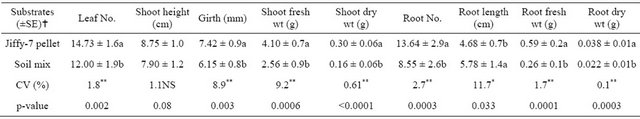
Means followed by the same letter within the same column are not significantly different. All growth parameters per substrate are mentioned as mean value. No. = number, Wt = weight, … = (±SE) standard error, CV = coefficient of variation, NS = Non-significant; * = significant at p < 0.05; ** = highly significant at p < 0.01.
Table 2. The effect of pot types on the secondary acclimatization of in vitro raised pineapple plantlets. Data for every growth parameters were collected after 4 months of growing in pots.
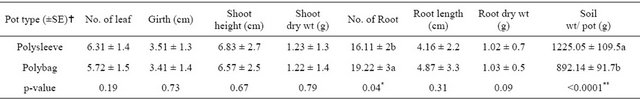
Means followed by the same letter within the same column are not significantly different at 0.05 probability level. All growth parameters are as mean value/plant. No. = Number, Wt = weight, … = ± (SE) standard error, * = significant different; ** = highly significant different.
 (a)
(a) (b)
(b)
Figure 3. In vitro raised pineapple plants grown in pots in the greenhouse for the secondary acclimatization after (a) 4 months and their (b) root structure.
4. Discussion
Pineapple (Ananas comosuss var. Smooth cayenne) is one of the vegetatively propagated tropical fruit crops. In vitro propagation is implemented widely for large-scale pineapple planting materials production. Acclimatization or hardening is a crucial step prior to transplantation of plantlets into the field. Pineapple requires a long acclimatization period until it reaches an appropriate size [5] because of its slow growth nature. It adds costs for labor, substrates, transportation and other facilities. Although the growth period can be reduced by applying humic acid and plant growth-promoting bacteria during acclimatization [10], the type of growing media remains a challenge for successful acclimatization. The growing media and pot types are known to affect the plant survival rate and growth performances in the greenhouse or nursery [9,16]. Thus, we report here two subsequent stages of acclimatization procedures.
4.1. Soil Mix Is a Cheap and Locally Available Substrate for Primary Acclimatization
In vitro pineapple plantlets were primarily acclimatized on different substrates. As the result showed, the substrates influenced the survival rate in the greenhouse, which was about 90% in soil mix and 98% in commercially available substrate called Jiffy-7 peat pellet. This result shows that the survival rate on soil mix was improved by 5% as compared to previous report [4], which is above 85% on 2 soil, 1 coffee husk and 1 sand.
It is suggested that modified soil mixture, which has more organic matter and equal proportion of soil and sand, improves aeration and reduces water retention leading to root growth, which comprises improved root and shoot-root connection for hydraulic conductivity [7]. This led to a better survival rate and plant performance than previously reported for soil mix [4]. However, soil mix showed a lower survival rate (about 8%) as well as plant performance than Jiffy-7 pellet. It is a composition of very cheap, ecological sound and locally accessible substrates for large-scale in vitro pineapple plantlets acclimatization as compared to Jiffy-7 peat pellet.
In contrast, Jiffy-7 peat pellet showed greatest survival rate and good plant performance, suggests that its relatively low pH facilitates nutrient uptake [8,17] and its high water holding capacity [18] plays an important role in plant growth. This result was in agreement to reference [19], who reported greater growth performance of commercial sweet corn on peat pellets. The pellets have a good porosity and allow the roots to penetrate quickly, suggesting more mobilization and absorption of nutrients in the pellet [8]. Although the Jiffy 7 pellets showed a higher root dry and fresh weight and root number, indicating promoted root formation, it is produced from expensive (~0.1 $/pellet) and less available moss [20]. Due to this fact, it is inaccessible for large-scale acclimatization of pineapple in Ethiopia. Peat production has ecological impacts that are creating concerns for the longterm availability of peat as the primary acclimatization substrate [21]. Thus, due to locally availability, cheapness, re-usability and ecologically sound, soil mix is found to be a good substrate for large-scale preliminary acclimatization of in vitro pineapple plantlets in Ethiopia. In contrast, peat pellets are better for plant performance with respect to root development even if they are expensive and inaccessible.
4.2. Polybag Is a Preferable Pot for Secondary Acclimatization
Since pineapple growing fields are located throughout the country, the plantlets should be strong, vigorous, well acclimatized and simple for handling to tolerate transportation and field shocks [6]. Although first-stage acclimatized plantlets are simple for handling and require less cost of transportation, they are weak to withstand ex vivo stresses due to poor root-stem connection, poor cuticle development, high stomatal conductance and poor photosynthesis ability [6,7]. When primarily acclimatized plants are directly transferred into the field, they are exposed to external environment conditions, which affect plant survival rate and performance [6]. As a result, primarily acclimatized plants need to be further acclimatized in the greenhouse or nursery for better growth and root development. Further hardening in the greenhouse requires high transportation cost as compared to the nursery where located near to the growing field. Nevertheless, the management in the greenhouse is as simple as the nursery because it can be done on existing facilities and has a close follow up for any further improvements. To do this, the choice of pot type along with growing medium is one of the critical decisions for secondary acclimatization.
Different types of pots are employed for growing plants in the nursery or the greenhouse. Similar to media types, the pot type and size affect the plant growth performance [9]. Bearing this in mind, hardening of pineapple plantlets was evaluated on different pot types and the results show that polybags were found to be good for potting in order to obtain a better plant performance and required less substrates. This result was in agreement with reports of reference [9] on secondary hardening of banana plantlets using polybags filled with fortified soil substrates. Enhanced root structures in polybags suggested that the plantlets can access water and nutrients leading to a quick establishment in the field. The reason is that root growth is critical for planted seedlings to withstand stresses [22]. Polybags also reduces the need of substrates that result in reduction of total cost of acclimatization and transportation of in vitro pineapple plantlets. In future, a detail physiological response of primary and secondary acclimatized plantlets, field performance, yield and quality of the fruit are essential to be evaluated.
5. Conclusion
Two subsequent hardening stages for in vitro raised pineapple plantlets were optimized prior to transferring them into the field for Ethiopian condition. Soil mix (1 soil, 2 coffee husk and 1 sand) was found as a cheap and locally available material, making it a good substrate for primary acclimatization in the greenhouse although Jiffy-7 peat pellet improved growth performance and survival rate slightly more. Using polybags was also the preferable pot in terms of cost and usefulness to obtain a better plantlet performance during secondary acclimatization.
6. Acknowledgements
The authors would like to appreciate all staff of Plant biotechnology research laboratory, Jimma Agricultural Research Center for their unreserved contribution of the work. Special gratitude goes to Mr. Zakir Abbanega for his technical help throughout the experiments and to Ms. Roman Getachew for her contribution during data collection. Our deep gratitude goes to the German Centre for International Migration and Development (CIM) which supported the expert in the work. We also thank European Union and MASHAV-USAID that donated Jiffy-7 peat pellets along with seedling trays and support the work respectively.
REFERENCES
- G. C. D’Eeckenbrugge and F. Leal, “Morphology, Anatomy and Taxonomy,” In: D. P. Bartholomew, R. E. Paull, and K. G. Rohrbach, Eds., The Pineapple: Botany, Production and Uses, CAB International, Wallingford, 2003, pp. 13-32. doi:10.1079/9780851995038.0013
- E. Edossa, “Spice Research Achievements and Experiences,” Research Report No. 33, IAR, Addis Ababa, 1998, pp. 16-19.
- E. Firoozabady and N. Gutterson, “Cost-Effective in Vitro Propagation Methods for Pineapple,” Plant Cell Report, Vol. 21, 2003, pp. 844-850. doi:10.1007/s00299-003-0577-x
- Z. Abebe, W, Tefera, M. Fellipe, A. Teressa and A. Mengesha, “In Vitro Multiplication of Pineapple (Ananas comosuss (L.)) and Cardamom (Elletaria cardamomum) in Ethiopia,” Proceeding of the Second Biennial Conference of Ethiopian Horticultural Science Society, Addis Ababa, 22-23 January, 2003, pp. 9-18.
- J. B. Teixeira, A. R. R. Cruz, F. R. Ferreira and J. R. Cabral, “Biotechnology Applied to Seedling Production: Production of Pineapple Plantlets,” Science and Biotechnology Development, Vol. 3, 2001, pp. 42-47.
- J. Pospisilova, I. Ticha, P. Kadleaeek, D. Haisel and S. Plazakova, “Acclimatization of Micropropagated Plants to Ex Vitro Conditions,” Biologia Plantarum, Vol. 42, No. 4, 1999, pp. 481-497. doi:10.1023/A:1002688208758
- G. Fila, J. Ghashghaie and G. Cornic, “Photosynthesis, Leaf Conductance and Water Relations of in Vitro Cultured Grapevine Rootstock in Relation to Acclimatization,” Biologia Plantarum, Vol. 102, No. 3, 1998, pp. 411-418. doi:10.1034/j.1399-3054.1998.1020309.x
- A. S. Davis, K. Eggleston, J. R. Pinto and R. K. Dumroese, “Evaluation of Three Growing Media Substrates for Western Larch Seedling Production at the USDA Forest Service Coeur d’Alene Nursery,” In: R. K. Dumroese, L. E. Riley, Eds., National Proceedings: Forest and Conservation Nursery Associations-2008, Rocky Mountain Research Station, 2009, pp. 37-41.
- S. R. Vasane and R. M. Kothari, “Optimization of Secondary Hardening Process of Banana Plantlets (Mussa paradisiacal L. var. grand Nain),” Indian Journal of Biotechnology, Vol. 5, 2006, pp. 394-399.
- L. E. B. Baldotto, M. A. Baldotto, L. P. Canellas, R. Bressan-Smith and F. L. Olivares, “Growth Promotion of Pineapple ‘Vitória’ by Humic Acids and Burkholderia spp. during Acclimatization,” Revista Brasileira de Ciência do Solo, Vol. 34, No. 5, 2010, pp.1593-1600. doi:10.1590/S0100-06832010000500012
- B. Ayenew, T. Tadesse, E. Gebremariam, A. Mengesha and W. Tefera, “Efficient Use of Temporary Immersion Bioreactor (TIB) on Pineapple (Ananas comosus L.) Multiplication and Rooting Ability,” Journal of Microbiology, Biotechnology and Food Science, Vol. 2, No. 4, 2013, pp. 2456-2465.
- B. Ayenew, A. Mengesha, T. Tadesse and E. Gebremariam, “Ensete ventricosum (Welw), Cheesman: A Cheap and Alternative Gelling Agent for pineapple (Ananas comosus var. Smooth Cayenne) in Vitro Propagation,” Journal of Microbiology, Biotechnology and Food Science, Vol. 2, No. 2, 2012, pp. 640-652.
- B. Ayenew, W. Tefera and B. Kassahun, “In Vitro Propagation of Ethiopian Ginger (Zingiber officinale Rosc.) Cultivars: Evaluation of Explant Types and Hormone Combinations,” Africa Journal of Biotechnology, Vol. 11, No. 16, 2012, pp. 3911-3918.
- D. Montgomery, “Design and Analysis of Experiments,” 6th Edition, John Wiley and Sons. Inc, New York, 2005, pp. 97-203.
- SAS Institute, “SAS/STAT User’s Guide for Personal Computers, Release 8.01,” SAS Institute, Cary, 2001.
- K. F. Salifu, M. A. Nicodemus, D. F. Jacobs and A. S. Davis, “Evaluating Chemical Indices of Media for Nursery Production of Quercus rubra Seedlings,” Horticultural Science, Vol. 41, No. 5, 2006, pp. 1342-1346.
- D. W. Reed, “Water, Media, and Nutrition for Greenhouse Crops,” Ball Publishing Inc., Illinois, 1996.
- M. Bergeron, “Peat,” Canadian Minerals Yearbook, Natural Resources, Ottawa, 37.1-37.8, 1994.
- J. E. Wyatt and J. A. Mullins, “Production of Sweat Corn from Transplants,” Horticultural Science, Vol. 24, No. 6, 1989, p. 1039.
- T. D. Landis and N. Morgan, “Growing Media Alternatives for Forest and Native Plant Nurseries,” In: R. K. Dumroese and L. E., Riley, Eds., National Proceedings: Forest and Conservation Nursery Associations-2008. Forest Service, Rocky Mountain Research Station, 2009, pp. 26-31.
- M. Raviv, “Horticultural Uses of Composted Materials,” Acta Horticulture, Vol. 469, 1998, pp. 225-234. http://www.actahort.org/books/469/469_23.htm
- S. C. Grossnickle, “Importance of Root Growth in Overcoming Planting Stress,” New Forests, Vol. 30, No. 2-3, 2005, pp. 273-294. doi:10.1007/s11056-004-8303-2
NOTES
*Corresponding author.

