American Journal of Plant Sciences
Vol.3 No.10(2012), Article ID:24165,4 pages DOI:10.4236/ajps.2012.310179
Effect of Growth Regulators on Seed Germination and Its Significance in the Management of Aeginetia indica L. — A Root Holoparasite
![]()
1Maharani’s Science College for Women, Mysore, India; 2Department of Studies in Botany, University of Mysore, Manasagangotri, Mysore, India.
Email: *grsgyarahally@gmail.com
Received July 31st, 2012; revised August 26th, 2012; accepted September 3rd, 2012
Keywords: Aeginetia indica; Seed Germination; Suicidal Germination; Growth Regulators; Embryonal Tubules
ABSTRACT
Seed germination in root holoparasites depends on receipt of certain chemical signals from the host plant. It is possible to induce germination in such seeds without the association of hosts by using growth regulators under in vivo and in vitro conditions. IAA, GA3 and Kinetin have been used to induce seed germination in Aeginetia indica L. to analyse the possible ways of exploiting knowledge of germination for the management of this weed. Seeds pre treated with 50 mg·L–1 of GA3 showed the production of aseptate, uninucleate root hair-like tubules, which probably help in the anchorage with host root. Under in vitro, GA3 (5.0 and 7.5 mg·L–1) has been found to induce and enhance percentage of seed germination. Therefore, it is concluded that GA3 could be used to bring suicidal germination of seeds thereby manage this parasitic weed effectively. Further production of uninucleate tubules and organisation of conventional bi-polar seedling under the influence of GA3 is being reported for the first time in this taxon.
1. Introduction
Aeginetia indica L. is a herbaceous root-holoparasite causing considerable yield reduction in sugar cane, rice, maize, sorghum, fodder grasses and host of other Monocotyledonous taxa in India, China, Japan, Philippines, Indonesia etc. [1]. It produces abundant minute yellow white seeds (Figure 1(A)). Longisection of the seed shows an undifferentiated embryo consisting of radicular and plumular pole without any semblance of embryonal organs like cotyledons, hypocotyl, epicotyl etc. (Figure 1(B)).
It is established that seed germination in root-holoparasites depends on receipt of certain chemical signals from the host plant [2]. However, it is possible to induce germination in such seeds without the association of hosts in vitro [3]. Seed germination studies on Aeginetia indica are limited to Chennaveeraiah et al. [4] and French and Sherman [5]. Information on the details of seed germination and seedling morphogenesis is a prerequisite for developing methods for the management of parasitic taxa of Scrophulariaceae and Orobanchaceae [6], and hence the present study. Different growth regulators were used to induce seed germination to analyse the possible ways of exploiting knowledge of germination for the management of this parasitic weed.
2. Material and Methods
Seeds of A. indica were collected from Kerekatte, Chikamagalore District and Belthangady, South Canara District, Karnataka, India, during August and September. Seeds were harvested from mature capsules just before dehiscence, shade dried and stored in butter paper envelopes, under laboratory conditions. Both in vivo and in vitro culture methods were employed to know the effect of growth regulators like GA3, IAA and Kinetin. In vivo studies were conducted by pretreating seeds with growth regulators whereas in in vitro studies MS medium [7] was supplemented with different concentrations of growth regulators to establish their effects on seed germination.
In vivo culture was carried out in sterile petri plates lined with moist filter paper at room temperature (28˚C ± 2˚C) with constant relative humidity. Before incubation seeds were surface sterilized with nascent chlorine water and washed with distilled water. About 300 seeds were taken and arranged on the filter paper in rows to facilitate recording of mode and percentage of germination. The experiments were run in triplicates and average results were recorded.
3. Results
3.1. In Vivo Studies
In vivo seed cultures raised on moist filter paper maintained under either light or dark conditions for three months failed to show any signs of germination. Seeds water washed for two, four, six and eight days gradually turned yellow to pale and when placed on moist filter paper failed to germinate even after 10 weeks in both light and dark conditions. Cold treatment showed swelling of seeds only.
Seeds pretreated with GA3 (1 mg·L–1) did not show any signs of germination even after 30 days. Seeds kept in GA3 (50 mg·L–1) showed 63.2% germination within 20 days, while those maintained in 100 mg·L–1 showed very low percentage of germination. During germination the cells of the embryo at radicular end become swollen and spheroidal. When transferred on to moist filter paper one to six of these spheroidal cells elongate themselves into cylindrical root hair-like embryonal tubules each measuring 600 - 1240 m in length & 25 to 30 m in breadth (Figures 1(D) and (E)). The outgrowths remained unicellular and uninucleate (Figure 1(F)). No further growth and differentiation was seen. Seeds pretreated with Kinetin and IAA at different concentrations viz. 1 mg·L–1, 50 mg·L–1 and 100 mg·L–1 and sown on moist filter paper did not show any signs of germination.
3.2. In Vitro Studies
In in vitro culture of seeds inoculated on M S medium supplemented with IAA (0.1 mg·L–1) germination occurred in 28 days. The first observable change in the
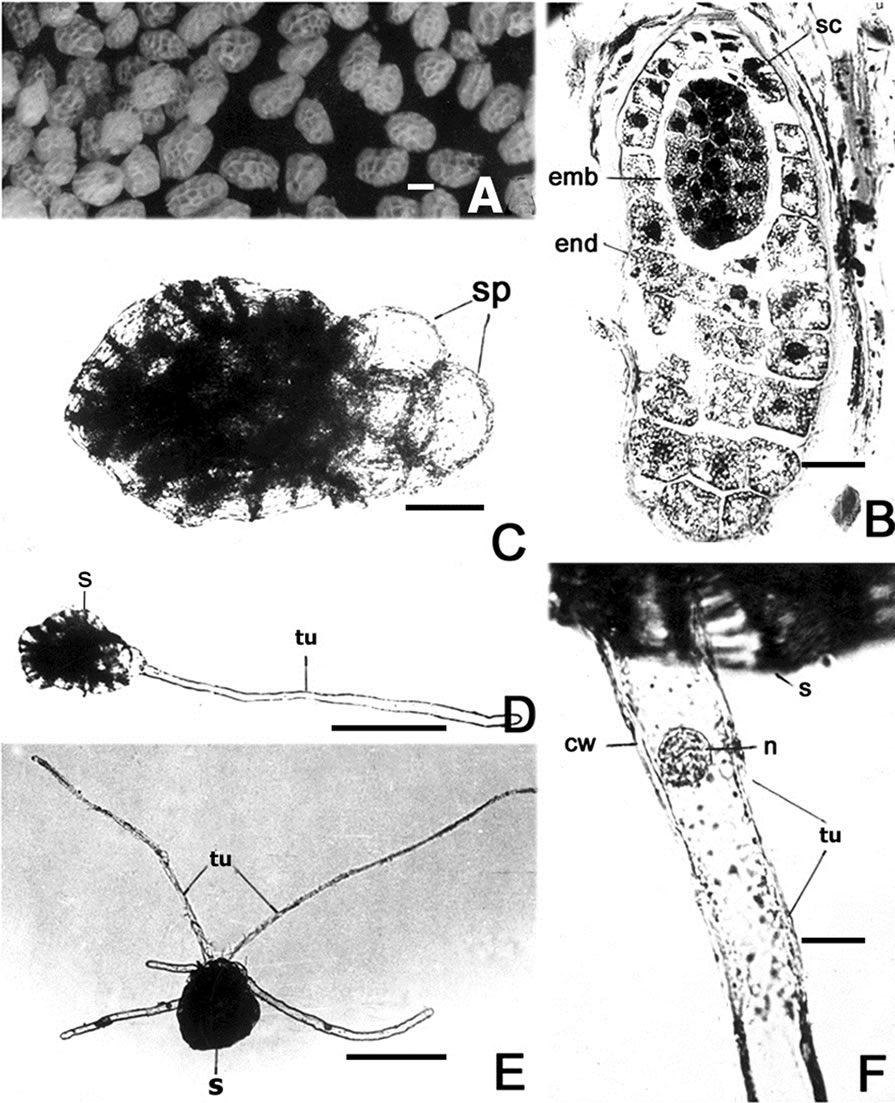
Figure 1. (A) Seeds; (B) L.S. of ripe seed showing seed coat (sc), endosperm (end) and undifferentiated embryo (emb); (C) Seed pretreated with GA3 (50 mg·L–1) in its early stage of germination, note the emerged spheroidal cells (sp); (D) and (E) Germinated seed(s) with one and five tubules (tu) respectively; (F) Enlarged basal region of the tubule. Note the presence of nucleus (n) and thickened cell wall (cw) at the tip of the tubule. Bars = 250 mm (A); 25 mm (B); 50 mm (C); 250 mm (D) and (E); 23 mm (F).
germinating seeds was an increase in volume of the embryonal cells at the radicular end, which ultimately emerge out rupturing the seed coat. The emerged part developed into a mass of rounded cells called tubercle. The percentage of germination was 1.9% at the end of 40 days. 51.2% of the germinated seeds developed tubercles. In 60 days the percentage of germination increased to 23.16%. In nearly 70.2% of the germinated seeds tubercles developed. Further development ceased and the embryo died by end of 10 weeks.
Seed germination within 40 days with IAA (1.0 mg·L–1) was 14.7%, 83.2% of which produced tubercles. In 60 days germination percentage increased to 48.16%. In 95.8% of the germinated seeds tubercles were initiated and they gradually increased in size as a mass of parenchyma cells. L.S. of the germinated seeds showed organization of the radicular and plumular meristem. In 6.2% of the seeds roots arose endogenously and are thick horn like measuring about 1 - 4 mm in length (Figure 2(A)). Proliferation of the plumular pole was observed whose cells were quite distinct with dense cytoplasm and prominent nuclei.
With IAA (2.5 mg·L–1) 6% germination occurred in 40 days. The percentage was increased to 14% by 60 days. Tubercles were initiated in 30% of the seeds. No further growth was noticed. With IAA (5 mg·L–1 and 7.5 mg·L–1) only swelling of the seeds were seen without any signs of germination even after 60 days.
GA3 (0.1 mg·L–1) has not shown any influence on seed germination even after 60 days except swelling of seeds. With GA3 (1.0 mg·L–1), 14.3% seeds germinated in 40 days out of which 48.2% produced two to five mm long tubercle. On transfer to fresh medium they produced small mounds of new tissue. In some of them roots differentiated at the free surface. The percentage of germination increased to 52.4% by the end of 60 days. After ten weeks they turned sepia brown and died.
With GA3 (2.5 mg·L–1) 29.4% seeds germinated by the end of 40 days, it was 58.3% by the 60th day. 66.4% of the seeds germinated in 40 days and 83.1% in 60 days. The radicular pole emerged through the micropyle to form a golden-yellow tubercle which was globular with smooth surface. Comparatively the peripheral cells of the tubercle were rich in starch. Ultimately, a large lobed yellowish mass of varied shape resulted. Roots developed endogenously and emerged through its exposed surface. Further growth was not seen.
The germination was 6.45% in 25 days with GA3 (5.0 mg·L–1). By 40 days it recorded 46.3% and was 68.2% in 60 days. Of the 84.2% of germinated seeds, cells of the radicular end of embryo increased in size and protruded out through micropyle, following rupture of seed coat. It proliferated further to form a golden yellowish tubercle. Simultaneously cells of plumular pole by cell division and cell enlargement extend out of seed coat. The shoot apex took its origin from the exposed plumular part. Due to unequal growth of the embryonal tissue, the seed coat was pushed to a side. The shoot apex developed on the lateral side. In a few cases the root developed adjacent to the shoot apex (Figure 2(B)).
Percentage of germination with GA3 (7.5 mg·L–1) showed an increase at all the observation periods, with 21.6% in 30 days, 56.2% in 40 days and 71.4% in 60 days. Tubercles developed from the radicular pole of the embryo in 52.1% of the germinated seeds in 30 days, 68.8% in 40 days and 89.1% in 60 days. In nearly 10.2% of the germinated seeds branched roots without root cap and root hairs developed from the free surface of the proliferated tissue, away from the medium. Simultaneous
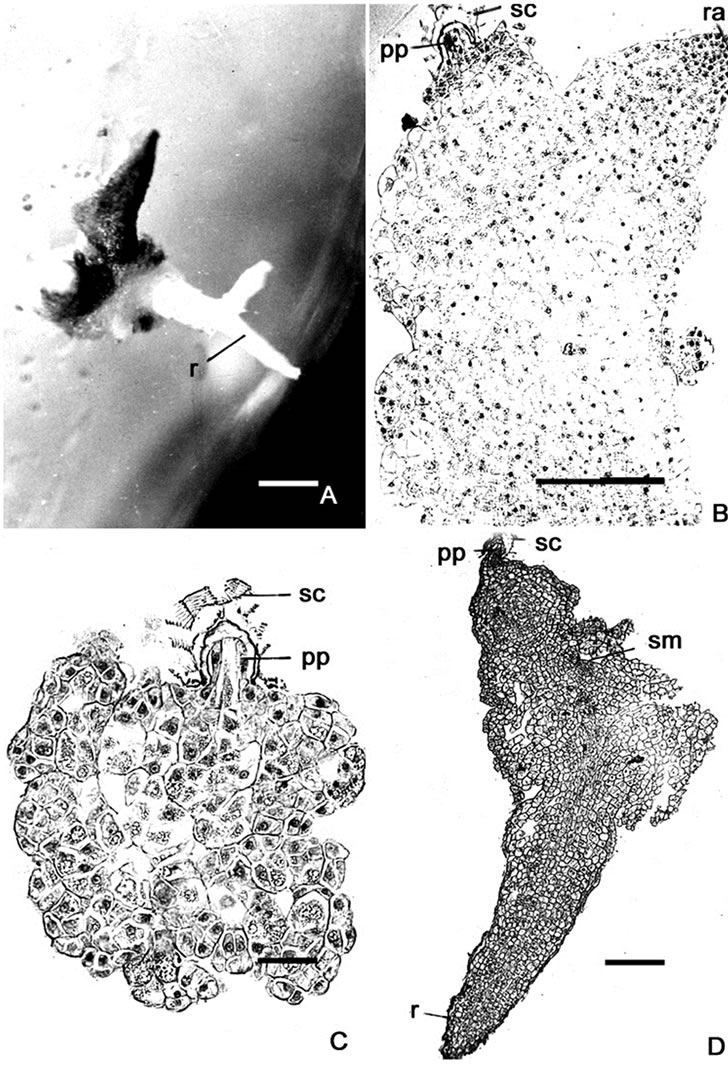
Figure 2. (A) Two months old culture on medium supplemented with IAA (1.0 mg·L–1) showing a branched root (r); (B) L.S. of callus grown on MS medium supplemented with GA3 (5.0 mg·L–1) showing plumular pole (pp) inside the seed coat (sc). Note development of radicle (ra) near the plumular pole; (C) L.S. of two and a half months old culture on medium supplemented with GA3 (7.5 mg·L–1); (D) L.S. of seedling showing root (r) and shoot meristem (sm). Bars = 50 mm (A); 250 mm (B); 40 mm (C); 240 mm (D).
with the growth of the radicular pole, the plumular pole also proliferated and extended out of the testa organizing an endogenous meristem on its lateral side.
Ontogenetic study of the embryo during seed germination on MS medium supplemented with GA3 (5.0 mg·L–1 and 7.5 mg·L–1) was made. The first noticeable change in the seeds was an increase in volume of embryonal cells at the micropylar end. As the embryo increased in size, the seed coat ruptured and exposed the epidermal cells of the radicular end. This end later developed into a mass of tissue—the tubercle by cell division and cell enlargement. On transfer to fresh medium, the tubercle produced a small mound of tissue. Sections of the germinated seeds revealed the in-situ enlargement and proliferation of the radicular pole of the embryo, while the quiescent plumular end stayed in the cup-shaped endosperm tissue enclosed by the seed coat (Figure 2(C)). As growth continued the cells of the plumular end differentiates into a shoot meristem and the seed coat is sloughed off. Initially the growth of the tubercle was uniform, later it became asymmetric. The procambium extended from the root to the shoot meristem (Figure 2(D)). The root apex consisted of densely protoplasmic cells with prominent nuclei. Further growth could not be traced as the cultures turned sepia brown and died. Anatomical observations revealed lysis of procambial elements.
On medium supplemented with Kinetin (2.5 mg·L–1), germination was initiated in 30 days in only 0.3% of seeds. In 40 days it was 2.2% and by 60 days it increased to 10% and this is the maximum percentage recorded for Kinetin treatment. In 91.2% of the germinated seeds the initial increase in size of the seed was followed by the rupture of the testa, exposing epidermal cells of radicular end of the embryo. Cells of radicular pole emerged out forming a protuberance of spheroidal cells. It grew both by cell division and cell enlargement to form a yellowish massive tissue of variable shapes. On transfer to fresh medium it proliferated further organizing mounds of tissue. The peripheral cells of the proliferated tissue had more of starch. Occasionally roots developed from the free end of the massive tissue. Sections of this region showed the presence of procambium in a few cases. Proliferation of the plumular cells occurred without further differentiation. Plumular differentiation occurs only after the establishment of haustoria with its host. Other concentrations of Kinetin have insignificant effect on germination (Table 1).
4. Discussion
The present study has revealed that seeds of A. indica germinate without any root exudate under laboratory conditions. It is apparent that host stimulus itself is not a must and could be substituted by growth regulators and
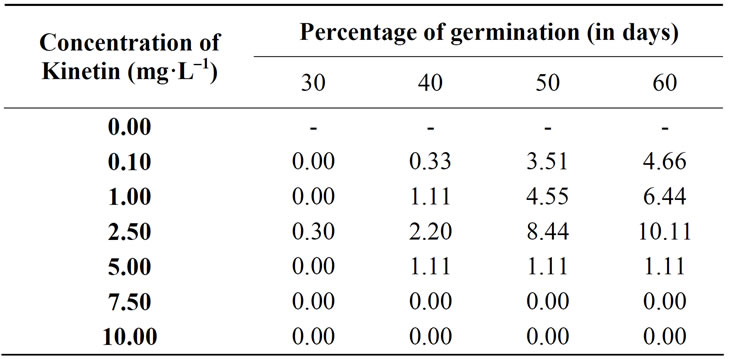
Table 1. Percentage of germination of Aeginetia indica seeds on M.S. medium supplemented with Kinetin in different concentrations.
minerals under light conditions. The study also showed that the light factor is essential for germination contrary to the observations made by French and Sherman (1976) who stated that light inhibits germination. Pretreatment with 5% sodium hypochlorite (NaOCl) was also not a prerequisite to break dormancy as the seeds readily germinated.
Chennaveeraiah et al. [4] obtained a callused embryo when pre-soaked seeds sown on modified Whites’ medium containing mineral elements supplemented with coconut milk (10%) + Kinetin (2 ppm) or both Kinetin (2 ppm) and 2,4-D (2 mg·L–1). They noticed occasional development of roots but no plumule morphogenesis.
Root hair-like tubular aseptate uninucleate outgrowths produced by seeds pretreated with GA3 (50 mg·L–1) have not been reported in any member of Orobanchaceae earlier. These tubules probably help in the anchorage of seeds with the host root and may be compared with the sticky embryonal tubules of Balanophora abbreviata Bl. of Balanophoraceae [8]. The unicellular embryonal tubules of the present study cannot be compared with the multicellular, multiseriate structures called tendrils reported by French and Sherman [5].
1.0 mg·L–1 IAA induced development of a mulberry shaped nodule/tubercle from the protruded radicular end of the embryo from which endogenous horn like roots without root caps differentiated. The plumular pole of the embryo enclosed in the seed coat becomes active and develops into shoot only after the establishment of contact with the host root in nature. While under in vitro conditions it becomes active and develops into shoot only after the formation of tubercle and endogenous roots from the radicular pole of the embryo leading to the formation of bi-polar embryo. In the present study morphogenesis of the plumular pole led to the differentiation of shoot meristem in the proliferated plumular tissue adjoining the seed coat unlike the shoot differentiation from the mass of tissue called tubercle arising from the “germtube” at the radicular pole of the embryo of Orobanche crenata [9]. Therefore, IAA appears to induce polar growth in A. indica leading to conventional bi-polar origin of root and shoot without the intervention of host plant, a feature similar to Cistanche tubulosa [10].
Of all the growth regulators maximum percentage of germination occurred at GA3 (5 mg·L–1 and 7.5 mg·L–1) and almost all the seedlings exhibited plumule morphogenesis. The organization of a conventional bi-polar seedling under the influence of GA3 (5.0 mg·L–1) has never been recorded earlier in this taxon. It is clear from the above findings that it is possible to grow this parasitic plant in culture without the influence of host root exudate. Therefore it is concluded that GA3 (5.0 and 7.5 mg·L–1) could be used to bring suicidal germination of seeds in the management of this parasitic weed.
5. Acknowledgements
Financial assistance from the UGC in the form of FIP teacher fellow to C.R. Vijay is gratefully acknowledged.
REFERENCES
- C. Parker and C. R. Riches, “Parasitic Plants as Weeds,” In: M. C. Press and J. D. Graves, Eds., Parasitic Plants, Chapman and Hall, London, 1995, pp. 27-255.
- R. G. Stewart and M. C. Press, “The Physiology and Biochemistry of Parasitic Angiosperms,” Annual Review of Plant Physiology and Plant Molecular Biology, Vol. 41, 1990, pp. 127-151. doi:10.1146/annurev.pp.41.060190.001015
- K. R. Shivanna and N. S. Rangaswamy, “Seed Germination and Seedling Morphogenesis of the Root Parasite Sopubia delphinifolia G. Don,” Zeitschrift für Pflanzenphysiologie, Vol. 80, No. 2, 1976, pp. 112-119.
- M. S. Chennaveeraiah, K. Nataraja and P. S. Chikkannaiah, “In Vitro Culture of the Seeds of Root Parasite: Aeginetia indica Linn.,” Current Science, Vol. 40, No. 24, 1971, pp. 668-669.
- R. C. French and L. J. Sherman, “Factors Affecting Dormancy, Germination and Seeding Development of Aeginetia indica L. (Orobanchaceae),” American Journal of Botany, Vol. 63, No. 5, 1976, pp. 558-570. doi:10.2307/2441819
- A. Sahai and K. R. Shivanna, “Seed Germination and Seedling Morphogenesis in Parasitic Angiosperms of the Scrophulariaceae and Orobanchaceae,” Seed Science and Technology, Vol. 10, No. 3, 1982, pp. 565-583.
- T. Murashige and F. Skoog, “A Revised Medium for Rapid Growth and Bioassay with Tobacco Tissue Culture,” Physiologia Plantarum, Vol. 15, 1962, pp. 473-479. doi:10.1111/j.1399-3054.1962.tb08052.x
- D. A. Govindappa and G. R. Shivamurthy, “Seed Germination in Balanophora abbreviata Blume,” Phytomorphology, Vol. 26, No. 2, 1976, pp. 135-138.
- R. Kadry and R. Tewfic, “Seed Germination in Orobanche crenata Forsk.,” Svensk Botanisk Tidskrift, Vol. 50, 1956, pp. 270-286.
- T. S. Rangan and N. S. Rangaswamy, “Morphogenic Investigations on Parasitic Angiosperms. l. Cistanche tubulosa (Orobanchaceae),” Canadian Journal of Botany, Vol. 46, 1968, pp. 263-266.
NOTES
*Corresponding author.

