Open Journal of Veterinary Medicine
Vol. 3 No. 2 (2013) , Article ID: 32867 , 5 pages DOI:10.4236/ojvm.2013.32032
ArtinM as a Neutrophil Immunostimulant in Juvenile Nile Tilapia (Oreochromis niloticus)
1Universidade Estadual de Londrina, Londrina, Brazil
2Universidade Estadual do Norte do Paraná, Jacarezinho, Brazil
3Embrapa Swine and Poultry, Concordia, Brazil
Email: *wagner.loyola@embrapa.br
Copyright © 2013 Caroline Toazza et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received November 13, 2012; revised December 18, 2012; accepted January 15, 2013
Keywords: Fish; Immune System; Protein; Lectin; Haptotaxis
ABSTRACT
Streptococcosis is one of the most important diseases in aquaculture, causing high rates of mortality in fish. ArtinM, an immunostimulant obtained from jackfruit (Artocarpus integrifolia) seed extract, enhances the innate immune response. The aim of this study was to examine the action of ArtinM on neutrophil haptotaxis to the peritoneal cavity of juvenile Nile tilapia (Oreochromis niloticus) inoculated intraperitoneally with Streptococcus agalactiae. After establishing the LD50 of S. agalactiae and the effective dose of ArtinM, 120 animals randomly distributed in 12 aquaria were divided into the following four treatment groups: G1, control; G2, via the intraperitoneal (i.p.) route inoculation with ArtinM; G3, i.p. inoculation with S. agalactiae and G4, i.p. inoculation with ArtinM and challenge with S. agalactiae. Six and 24 hours after treatment, the fish were sacrificed and peritoneal exudate and caudal vein blood samples were collected for analysis of the total number of leukocytes and neutrophils. To establish the optimal ArtinM concentration, the results were analyzed with a chi-square test at a 1% significance level. The experimental inoculation and challenge results were analyzed with the SASM-Agri software developed by Canteri et al. (2001) using the Scott-Knott’s test at a 5% significance level. The results of this study showed that i.p. inoculation with 1.0 µg ArtinM/animal has an effect on neutrophil haptotaxis to the peritoneal cavity in juvenile Nile tilapia. Therefore, ArtinM might represent a suitable prophylactic alternative in juvenile Nile tilapias inoculated with S. agalactiae.
1. Introduction
Commercial tilapia farming has increased quickly in Brazil and worldwide [1], particularly via intensive breeding systems that exploit the hardiness and high fish farming systems’ [2]. Commercial fish farming, which is often, associated with improper management practices and nutritional shortcomings, may alter water quality and favor the appearance of infectious diseases [3]. Antibiotics are the most common method used for the treatment of infections in fish. However, their indiscriminate use favors the antibiotic-resistant bacteria have a strong impact on the environment and may leave trace amounts in foodstuffs destined for human consumption [4]. An alternative to such drugs are immunostimulatory substances derived from plant, animal or bacterial extracts or other chemical compounds [5].
At the site of an injury, the accumulation of leukocytes supplied by the appropriate and the well-timed mobilization of the micro-circulation towards the inflammatory focus is essential for protection against further injury and is the main trait of inflammation [6]. Although the literature is conflicted regarding the precise cellular components that participate in infection responses in fish, studies have reported the presence of neutrophils and macrophages in different species of fish subjected to several immunostimulatory substances [7-9].
Studies have shown that lectins from plant extracts are able to stimulate the immune system of vertebrates while maintaining leukocyte integrity [10-12]. The lectin ArtinM, formerly known as KM+ or artocarpine, is extracted from jackfruit (Artocarpus integrifolia) seeds and exhibits immunostimulatory properties, including the ability to induce neutrophil migration.
Based upon the expansion of pisciculture, the commercial success of tilapia farming and the economic damage caused by streptococcosis in commercial tilapia farms, this study sought to assess the recruitment of neutrophils towards the focus of an acute infectious process 6 and 24 hours after ArtinM application in juvenile Nile tilapia challenged with Streptococcus agalactiae.
2. Materials and Methods
This study was carried out at the Fish Immunopathology Laboratory (LIPPE) at the State University of North Paraná, Bandeirantes, PR Brazil. In total, 270 juvenile Nile tilapia were used in this study. Tilapia were kept in glass aquaria with a working volume of 80 L that contained 10 fish each; aquaria were supplied with water from artesian wells at a rate of 1 L/min with continual aeration. Water temperature (28.0˚C ± 1.7˚C) was measured daily, while the pH (7.3 ± 0.3) and dissolved oxygen concentrations (5.54 ± 0.82 mg/L) were measured weekly; all parameters remained within the optimal range [13]. Aquariums were cleansed through siphoning on alternating days.
2.1. LD50 Determination
To establish the LD50, we used S. agalactiae isolated from naturally infected tilapia that was identified by [14] and [15]. Briefly, 30 tilapias were randomly distributed across 3 aquaria containing 10 fish each. Fish in each group were intraperitoneally (i.p.) injected with 104, 106 or 108 CFU/mL of S. agalactiae. The LD50 for S. agalactiae was established 15 days later [9].
2.2. Determination of Optimal ArtinM Concentration
For this purpose, 120 juvenile Nile tilapia distributed in 12 aquaria were used. Animals were randomly divided into 4 groups (n = 30 each) and subjected to treatments; treatments were performed in triplicate with 10 fish per treatment. The control group (T1) was i.p. inoculated with 1.0 mL of phosphate buffered saline solution (PBS), and groups 2 (T2), 3 (T3) and 4 (T4) were i.p. inoculated with 0.5, 1.0 or 1.5 µg ArtinM/animal, respectively. After 24 hours, fish in all groups were challenged with 1.0 mL of S. agalactiae at a concentration of 106 CFU/mL; fish were then observed for 15 days to establish the daily mortality rate. The concentration of ArtinM that was associated with the highest rates of survival was used for experimental inoculation and challenge.
2.3. Experimental Inoculation and Challenge
An additional 120 juvenile Nile tilapia were distributed in 12 aquaria. Animals were randomly divided into 4 groups (n = 30 each) and subjected to the following treatments:
Group 1 (G1), i.p. inoculated with 1.0 µL PBS and then with 1.0 mL PBS 24 hours later;
Group 2 (G2), i.p. inoculated with 1.0 µL ArtinM diluted in 0.5 mL sterile saline solution and then with 1.0 mL PBS 24 hours later; Group 3 (G3), i.p. inoculated with 1.0 µl PBS and then with 1.0 mL solution of S. agalactiae at a concentration of 106 CFU/mL 24 hours later; and Group 4 (G4), i.p. inoculated with 1.0 µl ArtinM diluted in 0.5 mL sterile saline solution and then with 1.0 mL solution of S. agalactiae at a concentration of 106 CFU/mL 24 hours later. Treatments were given in triplicate, with 10 fish receiving each treatment for each time point.
Six and 24 hours after S. agalactiae inoculation, 40 fish (10 from each group) were anesthetized with a solution of eugenol and sacrificed via spinal transection [16]. After opening the abdominal cavity, 1.5 mL of sterile RPMI 1640 (HIMEDIA® Mumbai, India) was added to the cavity and 1.0 mL of peritoneal exudate was collected using a Pasteur pipette. The samples were placed in sterile Falcon tubes and centrifuged at 4000 × g at 4˚C for 6 minutes. Cell pellets were collected and added to cover slips in contact with sterile cell culture plates.
Culture plates were incubated in a carbon dioxide (CO2) incubator at 29˚C for 30 minutes. Next, the slides were stained according to the May-Grunwald-GiemsaWright procedure [17]. To obtain total and differential leukocytes counts, 20 fields were counted using an immersion objective in Nikon microscope.
Immediately prior to sacrificing the animals, approximately 1.0 mL blood was collected from each animal through a caudal vein puncture performed with disposable syringes containing 10% EDTA. Blood smears were prepared and stained via Rosenfeld’s method (1947). Reading was performed with an immersion objective and total leukocytes and thrombocytes were indirectly quantified following the method of [18].
To establish the optimal ArtinM concentration, the results were analyzed using a chi-square test with a 1% significance level. For the experimental inoculation and challenge experiments, the results were analyzed with SASM-Agri software developed by [19]; a Scott-Knott’s test with a 5% significance level was applied.
3. Results
In this trial were used three different doses (0.5; 1.0 e 1.5 µg/animal) of ArtinM to determined the action of the lecithin on the immune system of Nile tilapias. A concentration of 1.0 μg of ArtinM/fish produced the greatest rate of survival among fish challenged i.p. with S. agalactiae (Table 1). Whereas the inferior and superior doses did not show the protector effect once the pretreated animals obtained the same survival rate than control animals. This result suggests ArtinM has an immunostimulant action that is dose-dependent.
Another factor that can indicate the immunostimulant action of a substance is it chemotactic action. The increase of leucocytes population in the peritoneal cavity results in a faster depuration contributing to resistance to infections. The largest total leukocyte averages in the peritoneal exudate samples appeared after 24 hours of inoculation in all groups but G1 (control) (Table 2). In posteriors times was observed a decrease in the number what suggests that the action of ArtinM might be timedependent.
The neutrophils are the first leucocytes to arrive at the local of infection. This phagocytes beyond its great microbicide capacity it also has a regulatory function of

Table 1. Survival rate of juvenile Nile tilapia that was i.p. inoculated with different concentrations of ArtinM and challenged with Streptococcus agalactiae (106 CFU/mL).
inflammatory response. The pre-treatment of animals with ArtinM showed that the lecithin has a chemotactic action to neutrophils. These results corroborate with studies that demonstrated the capacity of ArtinM to bind with the laminin promoting the haptotaxis of neutrophils. The greatest number of neutrophils in the peritoneal exudate samples was observed after 24 hours in the groups inoculated with ArtinM (G2) and ArtinM challenged with S. agalactiae (G4) (Table 3).
In the caudal vein blood samples, there were no significant differences in the average number of neutrophils between the four groups investigated (Table 4). These results suggest that ArtinM did not exercise a systemic action, exerting only a local action in inflammatory response stimulation.
4. Discussion
The ArtinM literature comprises studies carried out in other animal species. No published data exist regarding the use of this protein in fish. In our study, the i.p. administration of 1.0 μg of ArtinM/animal caused the highest rate of survival in juvenile Nile tilapia challenged with S. agalactiae. Lower (0.5 μg/fish) and higher (1.5 μg/fish) doses of ArtinM were not protective against S.
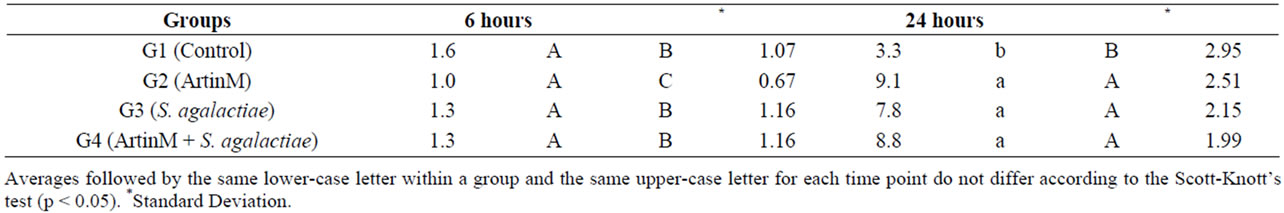
Table 2. Average number of total leukocytes in the inflammatory exudate of juvenile Nile tilapia intraperitoneally inoculated with ArtinM, Streptococcus agalactiae or ArtinM with S. agalactiae challenge.
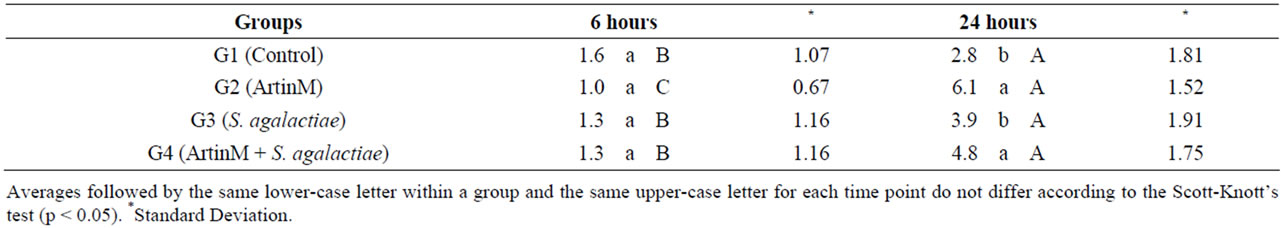
Table 3. Average number of neutrophils in the peritoneal cavity of juvenile Nile tilapia intraperitoneally inoculated with ArtinM, Streptococcus agalactiae or ArtinM with an S. agalactiae challenge.
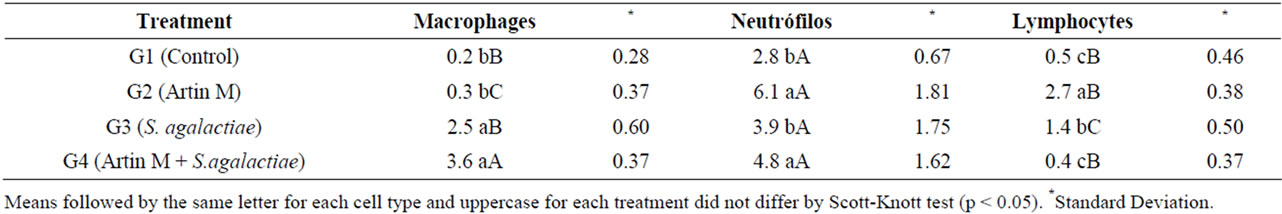
Table 4. Time effect on the population of macrophages, neutrophils and lymphocytes after induction of inflammatory exudate in juvenile Nile tilapia fish.
agalactiae challenge. Studies with ArtinM in different animal species have shown that the ideal immunomodulatory dose and its effect vary depending on species. For example, while an air pouch inoculation of 50 μg ArtinM/ animal in Leishmania amazonensis—infected mice induced the largest migration of leukocytes in 24 hours [20], mice infected with Paracoccidioides brasiliensis and treated with 0.5 μg ArtinM/animal exhibited higher resistance to infection compared to untreated mice through the increased production of IL-2 [21]. In rabbits with experimentally-induced cornea lesions, the application of a solution of 2.5 μg/mL of ArtinM accelerated the healing process [22].
In our study, the application of 1.5 μg ArtinM/fish resulted in a lower survival rate compared to a dose of 1.0 μg ArtinM/fish (Table 3). Prior studies have reported that the use of larger doses of immunostimulants can actually cause immunosuppression in fish [9]. According to Sakai (1999) the effect of immunostimulatory compounds depends directly on the ideal dose, as higher doses might not improve, and may even inhibit, the immune response. Hence, testing different doses is key in determining the most effective doses during stress-associated events of aquatic animals, such as their transportation or their reproductive cycles [23].
In general, the use of ArtinM stimulates the innate immune system of animals by causing the activation and haptotaxis of neutrophils, mast cells, dendritic cells and macrophages [21,24-29]. According to works [30], i.p. inoculation of Nile tilapia with crude Artocarpus integrifolia seed extracts increased the number of phagocytic cells in the affected area in 24 hours. In our study, despite the lack of significant differences among the inoculated groups, the largest averages of total leukocytes in the peritoneal exudate were found at 24 hours.
Neutrophils are abundant in the blood and are important in innate immunity because they are able to recognize, phagocytose and destroy pathogens without stimulation from other immune cells [31]. Reports [22] suggested that ArtinM induces neutrophil haptotaxis via the interaction of neutrophil surface glycoproteins and components of the extracellular matrix of blood vessel walls. In 2006, works [32] showed that when linked to ArtinM, the laminin present in blood vessel walls potentiates the activity of ArtinM in neutrophils, promoting their migration through haptotaxis. In our study, there was a significant increase of neutrophils in the inflammatory exudate samples collected 24 hours after inoculation in the fish that received ArtinM (G2) alone or ArtinM followed by S. agalactiae (G4).
In fish, blood composition depends on physiological and ecologic factors such as sex, stage of gonad development, stress, infection and environmental balance [31]. Moreover, it must be taken into account that in these animals, the immune response does not have the same magnitude nor does it follow the same pattern as mammals [6]. In the blood samples collected from the caudal vein 6 and 24 hours after application of ArtinM, there were no significant differences in the average number of neutrophils between the investigated groups. This result indicates that the action of ArtinM is local rather than systemic.
5. Conclusion
ArtinM administered i.p. increased the migration of neutrophils to the peritoneal cavity; further, this process depended upon the concentration of the ArtinM and duration of treatment. However, the mechanisms involved in ArtinM-induced neutrophil haptotaxis remain to be investigated. This ability of ArtinM to activate the innate immune response might be used as an immunostimulatory means to control the acute stage of infections in fish. This finding opens up possibilities for the strategic use of this protein in the periods immediately prior to the implementation of fish management techniques.
6. Acknowledgements
The authors thank Prof. Dr. Maria Cristina Roque Barreira, from USP, Ribeirão Preto; São Paulo State; Brazil; who kindly supplied the ArtinM samples that were crucial for this study.
REFERENCES
- N. M. Lopera Barrero, “Tilapicultura Semi-Intensiva em Tanques: Alternativas de Fertilização e Produção—Revisão,” Arquivos de Ciências Veterinária e Zoologia, Vol. 9, No. 1, 2006, pp. 67-76.
- F. Kubitza, “Qualidade do Alimento, Qualidade da Água e Manejo Alimentar na Produção de Peixes,” Simpósio Sobre o Manejo e Nutrição de Peixes, CBNA, Anais… Campinas, 1997, pp. 63-101.
- L. J. G. Barcellos, S. M. G. Souza and V. M. Woehl, “Estresse em Peixes: Fisiologia da Resposta ao Estresse, Causa e Consequência (Revisão),” Boletim do Instituto da Pesca, Vol. 26, No. 1, 2000, pp. 99-111.
- K. Hölmstrom, et al. “Antibiotic Use in Shrimp Farming and Implications for Environmental Impacts and Human Health,” International Journal of Food Science and Technology, Vol. 38, No. 3, 2003, pp. 255-266. doi:10.1046/j.1365-2621.2003.00671.x
- M. Sakai, “Current Reserch Status of Fish Immunostimulants,” Aquaculture, Vol. 172, No. 1-2, 1999, pp. 63-92. doi:10.1016/S0044-8486(98)00436-0
- J. Garcia Leme, M. Morato and M. Z. A. Souza, “Anti-Inflammatory Action of Glucagon in Rats,” British Journal of Pharmacology, Vol. 55, No. 1, 1975, pp. 65-68. doi:10.1111/j.1476-5381.1975.tb07611.x
- J. I. Macarthur, et al., “Peritoneal Inflammatory Cells in Plaice, Pleuronectes platessa L.: Effects of Stress and Endotoxin,” Journal of Fish Biology, Vol. 25, No. 1, 1984, pp. 69-81. doi:10.1111/j.1095-8649.1984.tb04852.x
- M. Endo, et al., “Swim Bladder as a Site for Administration of Chemical Agents: Application to Fish Immunology,” Fish e Shellfish Immunology, Vol. 7, 1997, pp. 85- 87.
- R. Salvador, “Imunização e Inflamação por Streptococcus Agalactiae em Tilápia do Nilo (Oreochromis Niloticus) Alimentadas Com Ração Suplementada Com Parede Celular de Saccharomyces cerevisiae,” Tese (Doutorado em Aquicultura)—Universidade Estadual Paulista—Centro de Aquicultura da Unesp, Jaboticabal, 2008.
- H. Lis and N. Sharon, “Lectin as Molecules and as Tools,” Annual Review of Biochemistry, Vol. 55, 1986, pp. 35-67. doi:10.1146/annurev.bi.55.070186.000343
- J. F. Kennedy, et al., “Lectins, Versatile Proteins of Recognition: A Review,” Carbohydrate Polymers, Vol. 26, No. 3, 1995, pp. 219-230. doi:10.1016/0144-8617(94)00091-7
- G. Pereira-Da-Silva, et al., “Neutropkil Activation Induced by the Lectin KM+ Involves Binding to CXCR2,” Biochimica et Biophysica Acta, Vol. 1760, No. 1, 2006, pp. 86-94. doi:10.1016/j.bbagen.2005.09.011
- C. E. Boyd, “Water Quality in Ponds for Aquaculture,” Alabama Agricultural Experiment Station, Auburn University, Auburn, 1990.
- P. Vandamme, et al., “Streptococcus difficile Is a NonHemolytic Group B, Tipe Ib Streptococcus,” International Journal of Systematic Bacteriology, Vol. 47, No. 1, 1997, pp. 81-85. doi:10.1099/00207713-47-1-81
- R. Salvador, et al., “Isolation and Characterization of Group B Streptococcus spp. from Nile Tilapia (Oreochromis niloticus) Breeding in Hapas Nets and in Earth Nurseries in the North Region of Parana State, Brazil,” Ciência Rural, Vol. 35, No. 6, 2005, pp. 1374-1378. doi:10.1590/S0103-84782005000600023
- A. S. Pedrazzani, et al., “Senciência e Bem-Estar de Peixes: Uma Visão de Futuro do Mercado Consumidor,” Panorama da Aqüicultura, Vol. 102, 2007, pp. 24-29.
- W. Loyola, “Ação Protetora de Extrato de Sementes de Artocarpus Integrifólia Contra Infecção por Candida Albicans Através do Aumento das Funções de Fagócitos,” Tese (Doutorado em Microbiologia)—Universidade Estadual de Londrina, Londrina, 2008.
- T. C. Hrubec and S. A. Smith, “Hematology of Fish,” In: B. F. Feldman, J. G. Zinkl and N. C. Jain, Eds., Schalm’s Veterinary Hematology, 5th Edition, W. W. Lippincott, Sydney, 1998, pp. 1120-1125.
- M. G. Canteri, et al., “SASM-Agri: Sistema Para Análise e Separação de Médias em Experimentos Agrícolas Pelos Métodos Scoft-Knott, Tukey e Duncan,” Revista Brasileira de Agrocomputação, Vol. 1, No. 2, 2001, pp. 18- 24.
- C. R. Teixeira, et al., “Potential of KM+ Lectin in Immunization against Leishmania amazonensis Infection,” Vaccine, Vol. 24, No. 15, 2006, pp. 3001-3008. doi:10.1016/j.vaccine.2005.11.067
- K. C. Coltri, et al., “Therapeutic Administration of KM Lectin Protects Mice against Paracoccidioides brasiliensis Infection via Interleukin-12 Production in a Toll-Like Receptor 2-Dependent Mechanism,” The American Journal of Pathology, Vol. 173, No. 2, 2008, pp. 423-432. doi:10.2353/ajpath.2008.080126
- F. Chahud, et al., “The Lectin KM+ Induces Corneal Epithelial Wound Healing in Rabbits,” International Journal of Experimental Pathology, Vol. 90, No. 2, 2009, pp. 166-173. doi:10.1111/j.1365-2613.2008.00626.x
- I. Bricknell and R. A. Dalmo, “The Use of Immunostimulants in Fish Larval Aquaculture,” Fish & Shellfish Immunology, Vol. 19, No. 5, 2005, pp. 457-472. doi:10.1016/j.fsi.2005.03.008
- L. Ganiko, et al., “Neutrophil Haptotaxis Induced by the Lectin KM+,” Glycoconjugate Journal, Vol. 15, No. 5, 1998, pp. 527-530. doi:10.1023/A:1006999323098
- A. Panunto-Castelo, et al., “KM(+), a Lectin from Artocarpus integrifolia, Induces IL-12 p40 Production by Macrophages and Switches from Type 2 to Type 1 CellMediated Immunity against Leishmania major Antigens, Resulting in BALB/c Mice Resistence to Infection,” Glycobiology, Vol. 11, No. 12, 2001, pp. 1035-1042. doi:10.1093/glycob/11.12.1035
- A. N. Moreno, et al., “Mast Cell Degranulation Induced by Lectins: Effect on Neutrophil Recruitment,” International Archives of Allergy and Applied Immunology, Vol. 132, 2003, pp. 221-230. doi:10.1159/000074303
- L. Ganiko, et al., “Lectin KM+-Induced Neutrophil Haptotaxis Involves Binding to Laminin,” Biochimica et Biophysica Acta, Vol. 1721, No. 1-3, 2005, pp. 152-163. doi:10.1016/j.bbagen.2004.10.012
- G. Pereira-Da-Silva, et al., “Neutropkil Activation Induced by the Lectin KM+ Involves Binding to CXCR2,” Biochimica et Biophysica Acta, Vol. 1760, No. 1, 2006, pp. 86-94. doi:10.1016/j.bbagen.2005.09.011
- L. Carandina, et al., “Avaliação da Ação Imunomoduladora de Extratos de Sementes de Artocarpus integrifolia em Infecções Experimentais em Tilápias do Nilo,” Unicentro, Guarapuava Anais... Guarapuava, 2009.
- W. Loyola, et al., “Concanavalin A Enhances Phagocytosis and Killing of Candida Albicans by Mice Peritoneal Neutrophils and Macrophages,” FEMS Immunology and Medical Microbiology, Vol. 33, No. 3, 2002, pp. 201-208. doi:10.1111/j.1574-695X.2002.tb00591.x
- M. Tavares-Dias, M. I. Mataqueiro and D. Perecin, “Total Leukocyte Counts in Fishes by Direct or Indirect Methods?” Boletim do Instituto de Pesca, Vol. 28, No. 2, 2002, pp. 155-161.
- K. A. Toledo, C. Schwartz, A. F. Oliveira and M. C. A. V. Conrado, “Neutrophil Activation Induced by Artinm: Release of Inflammatory Mediators and Enhancement of Effector Functions,” Immunology Letters, Vol. 123, No. 1, 2009, pp. 14-20. doi:10.1016/j.imlet.2009.01.009
NOTES
*Corresponding author.

