 Open Journal of Respiratory Diseases, 2012, 2, 1-8 http://dx.doi.org/10.4236/ojrd.2012.21001 Published Online February 2012 (http://www.SciRP.org/journal/ojrd) 1 Effects of Clarithromycin at Sub-Minimum Inhibitory Concentrations on Early ermB Gene Expression, Metabolic Activity and Growth of an erm(B)-Expressing Macrolide-Resistant Strain of Streptococcus pneumoniae Riana Cockeran1*, H. C. Steel1, N. Wolter2, L. de Gouveia2, A. von Gottberg2, K. P. Klugman2,3, A. T. Leanord4, D. J. Inverarity5, T. J. Mitchell5, C. Feldman6, R. Anderson1 1Medical Research Council (MRC) Unit for Inflammation and Immunity, Department of Immunology, Faculty of Health Sciences, University of Pretoria, and Tshwane Academic Division of the National Health Laboratory Service, Pretoria, South Africa 2Respiratory and Meningeal Pathogens Research Unit, National Institute for Communicable Diseases of the National Health Laboratory Service and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa 3Hubert Department of Global Health, Rollins School of Public Health, and Division of Infectious Diseases, School of Medicine, Emory University, Atlanta, USA 4Microbiology Department, Southern General Hospital, Glasgow, UK 5Division of Infection and Immunity, Glasgow Biomedical Research Centre, University of Glasgow, Glasgow, UK 6Division of Pulmonology, Department of Internal Medicine, Charlotte Maxeke Johannesburg Academic Hospital and University of the Witwatersrand, Johannesburg, South Africa Email: *riana.cockeran@up.ac.za Received October 17, 2011; revised December 25, 2011; accepted December 10, 2011 ABSTRACT Aim: To investigate the effects of exposure of a macrolide-resistant [erm(B)-expressing] strain of Streptococcus pneumo- niae (strain 2507) to clarithromycin (0.5 and 5 mg/L) added at the outset and 6 hours after initiation of culture on early gene expression, energy metabolism, and growth. Methods: Bacterial growth was determined by turbidometric and colony counting procedures, energy metabolism by measurement of ATP, while analysis of gene expression was per- formed using rever se tran scriptio n-PCR and sequen cing. Results: Addition of clarithromycin , at either con centration , at the outset of cu lture, caused transient suppression of growth of 10 - 12 hou rs duration, while d elayed additio n of antib i- otic (during the logarithmic phase) resu lted in an abru pt halt in growth followed by recovery. These inhibitory effects of clarithromycin on bacterial growth were associated with up-regulation of expression of erm(B), decreased ATP and protein synthesis, and were unaffected by inclusion of either catalase (500 and 1000 kunits/L), or compe- tence-stimulating peptide (CSP-1, 0.5 mg/L). The inhibitory effects could, however, be overcome by pre-exposure of the bacteria to the antibiotic. Moreover, clarithromycin appeared to potentiate the antimicrobial actions of ceftriaxone, at sub-MIC concentrations, for strain 2507. Conclusions: Unlike several other common bacterial pathogens, the full expression of erm(B)-mediated macrolide resistance by the pneumococcus has a slow onset, which is associated with transient susceptibility to macr olides and inhibition of growth . Keywords: Clarithromycin; Macrolide-Resistance; Pneumococcus 1. Introduction Macrolide antibiotics are used in the management of pa- tients with community-acquired pneumonia in a number of settings, for example as monotherapy in young, other- wise healthy patients in the outpatient setting, in whom macrolide resistance among pneumococci is unlikely to exist. They are also used in combination therapy, to- gether with beta-lactam agents, in more severely ill, hos- pitalised patients being admitted for intravenous ther- apy. However, macrolide resistance is increasing world- wide and clearly limits the clinical utility of this class of antimicrobial agent, particularly when used alone. Nev- ertheless, it is noteworthy that therapeutic efficacy of these agents in the setting of macrolide resistance has been documented in a number of reports in humans and animal models of experimental infection [1-6]. Several mechanisms have been proposed to explain the suscepti- bility of ostensibly resistant pathogens to macrolides; these include possible non-ribosomal antimicrobial activities of macrolides [7-9] and/or beneficial immunomodulatory/an- ti-inflammatory effects of these agents [10-13]. In addi- tion, we have recently proposed that delayed onset of inducible resistance mechanisms may result in transient C opyright © 2012 SciRes. OJRD  R. COCKERAN ET AL. 2 susceptibility to macrolides [14-16]. The current study has been designed to probe this latter possibility, with par- ticular emphasis on the early effects of clarithromycin on early erm(B) gene expression, metabolic activity and growth of an erm(B)-expressing strain of the pneumo- coccus, as well as the effects of sub-minimum inhibitory concentrations of this agent in combination with ceftri- axone on growth. 2. Materials and Methods 2.1. Bacteria Although focused primarily on strain 2507, one clinical macrolide-susceptible strain of Streptococcus pneumoni- ae (strain 172) and seven additional macrolide-resistant strains (strains 1791, 1832, 2235, 2506, 3328, 3502 and 4916), were also included in this study. Isolates 172, 2506 and 2507 hav e been described previously [14]. Iso- lates were collected as part of an active laboratory-based surveillance system for invasive pneumococcal disease. Isolates were selected from adult cases (>15 years of age) and had been isolated from blood culture specimens. 2.2. Phenotypic Characterisation of Bacterial Strains Bacterial identification was p erformed using standardised methodologies [14]. MICs were determined by Etest (AB Biodisk, Solna, Sweden). Pneumococci were serotyped by the Quellung method using specific antisera (Statens Serum Institut, Copenhagen, Denmark). 2.3. Genotypic Characterisation of Bacterial Strains Bacterial strains were boiled at 95˚C for 10 min in order to extract chromosomal DNA, and screened for the pres- ence of erm(B) and mef(A/E) by a duplex PCR [19]. The erm(B) gene was amplified using forward primer ermBF (5’-CTTAGAAGCAAACTTAAGAG-3’) and reverse pri- mer ermBR (5’-ATCGATACAAATTCCCCGTAG-3’). For each 50 L reaction, 3 L of chromosomal DNA was added to a mix containing 2.5 U of Taq DNA polymerase, 1x reaction buffer, 1.5 mM MgCl2, 200 µM each of dA- TP, dCTP, dGTP and dTTP and 800 nM each of forward and reverse primer. Cycling parameters were: 94˚C for 2 min; 94˚C for 1 min, 52˚C for 1 min and 72˚C for 3 min for 30 cycles; and 72˚C for 5 min. Amplified products were purified with QIAquick PCR purification kit (Qia- gen, Surrey, UK) and the erm(B) gene sequenced using an Applied Biosystems 3130 Genetic Analyzer. Multilo- cus Sequence Typing (MLST) was performed as previ- ously described [17]. Sequence types were assigned by submission of the allele sequences to the MLST website (http://spneumoniae.mlst.net). 2.4. Antimicrobial Agents and Other Chemicals Clarithromycin was kindly provided by Abbott Labora- tories, North Chicago, IL, USA. Clarithromycin and cef- triaxone were made to a stock solution of 5 g/L in sterile distilled water. The pneumococcal competence phero- mone, competence-stimulating peptide-1 (CSP-1), was kindly provided by Dr. D. A. Morrison, Laboratory of Molecular Biology, University of Illinois, Chicago, IL, USA, while all other chemicals and reagents, including bovine liver catalase and ceftriaxone, were purchased from the Sigma Chemical Co, St. Louis, Missouri, USA. 2.5. Bacterial Growth Studies Strains were grown in tryptone soy broth (TSB; Biolab Diagnostics, Johannesburg, South Africa) for 18 hours at 37˚C in an atmosphere of 5% CO2, after which the bacte- ria were pelleted by centrifugation, resuspended in 0.15 M phosphate-buff ered saline (PBS, pH 7.4), and adjusted turbidometrically to a concentration of 1 × 107 colony- forming units (cfu)/mL. For all growth studies, TSB was inoculated with a standard inoculum of 2 × 105 cfu/mL of each strain and growth monitored for up to 24 hours us- ing a microplate turbidometric method (Powerwavex [Bio- Tek Instruments Inc., Winooski, Vermont, USA]), at a wa- velength of 540 nm, and colony-counting procedures. The effects of clarithromycin at fixed, final concentra- tions of 0.5 and 5 mg/L on bacterial growth were invest- tigated using two strategies. Firstly, continuous exposure of each of the eight resistant pneumococcal strains to cla- rithromycin, added from the outset, and secondly, addi- tion of the antibiotic 6 hours after the initiatio n of culture. Because of the differences in rates of growth of the dif- ferent strains, these results are expressed as the time tak- en for the clarithromycin-free control systems and the cor- responding clarithromycin-treated (5 mg/L only, added at the outset) systems, to enter the exponential phase of growth. Strain 2507 was subjected to pulsed addition of clari- thromycin at 6 hourly intervals from time 0 to 24 hours. In addition, this strain was also investigated for the ef- fects of pre-exposure to clarithromycin for 18 hours fol- lowed by dilution (1:20,000 approximately) or washing on growth during re-exposure to the antimicrobial agent. CSP-1 (0.1 - 0.5 mg/L) and catalase (500 - 1000 kunits/L) were used to investigate interference with quorum sens- ing and increased sensitivity to the auto-toxic actions of hydrogen peroxide as potential mechanisms of clarithro- mycin-mediated transient inhibition of growth of strain 2507. To investigate possible interactive antimicrobial effe- cts of clarithromycin and ceftriaxone, strain 2507 was treat- ed with a combination of the macrolide (5 mg/L) and -lactam (0.05, 0.1, 0.25 and 0.5 mg/L); clarithromycin Copyright © 2012 SciRes. OJRD  R. COCKERAN ET AL. 3 was added from the ouset, followed by ceftriaxone 45 min later, and the bacteria monitored for growth using turbidity measurement (540 nm) and colony-counting pro- cedures after an incubation period of 16 hours at 37˚C, 5% CO2. The effects of sub-MIC concentrations of clarithromy- cin (0.01 and 0.025 mg/L) on the growth of the suscepti- ble strain, 172, were also investigated. 2.6. Measurement of Bacterial ATP The effects of clarithromycin (0.5 and 5 mg/L) on the en- ergy metabolism of strain 2507 were measured by a che- miluminescence procedure using numerically standard- ised bacterial suspensions in indicator-free tissue culture medium RPMI 1640 (Highveld Biological (Pty) Ltd., Johannesburg, South Africa). Bacteria cultured for 18 hours in TSB were pelleted by centrifugation, washed once, and resuspended to a concentration of 5 × 107 cfu/mL in RPMI 1640 without or with clarithromycin and incubated for 1 hour at 37˚C, 5% CO2 followed by addition of bovine serum albumin (5 g/L). After further incubation periods of 1 and 5 hours (total incubation pe- riods of 2 and 6 hours respectively), viability was meas- ured using colony counting procedures, while bacterial ATP concentrations were determined using the Bac- Titer-GloTM Microbial Cell Viability Assay (Promega Corporation, Madison, WI, USA). Briefly, 0.2 mL of a 1:50 dilution of the bacteria were mixed with an equal volume of BacTiter-GloTM solution (a composite mix consisting of a prokaryotic cell ATP extraction agent and luciferase/luciferin for the detection of ATP) and incu- bated for 5 min at room temperature followed by meas- urement of chemiluminescence using a 20/20n chemilu- minometer (Turner Biosystems Inc., Sunnyvale, CA, USA). The results are expressed as relative light units (rlu). 2.7. Bacterial Protein Synthesis The effects of clarithromycin (0.5 and 5 mg/L) on protein synthesis by strain 2507 were measured using a radiome- tric procedur e. Bacteria grown for 18 hours in TSB were resuspended to a concentration of 5 × 107 cfu/mL in RP- MI 1640 supplemented with 0.5 mCi/L of a radiolabelled amino acid mixture (L-amino acid mixture 14C[U], 37 mBq; Du Pont-NEN Products, Boston, MA, USA) and incubated at 37˚C, 5% CO2. After 2, 4, 6 and 8 hours of incubation, the bacteria were pelleted by centrifugation and washed, followed by the addition of warm 5% tri- chloroacetic acid to release bacterial proteins and radio- activity in the lysates was measured by liquid scintilla- tion spectrometry. 2.8. Quantification of erm(B) Gene Expression The expression of the erm(B) gene was measured for strain 2507. Three 50 mL aliquots of fresh Brain Heart Infusion broth (Oxoid, UK) taken from the same batch were each inoculated with 500 L of a freshly thawed glycerol stock of the bacterium (total viable count = log10 8.392 cfu/mL). These were incubated in a water bath at 37˚C and the optical density (OD) monitored until it reach- ed midlog. At this point, 10 mL of each of the three cultures was removed and centrifuged at 5087× g at room temperature. The supernatant was decanted and the pellet immediately frozen in liquid nitrogen and stored at –80˚C prior to RNA extraction. The remaining culture was split into paired 18 ml ali- quots and clarithromycin was added to a final concentra- tion of 5 mg/L to one of each pair, and both samples re- incubated at 37˚C. After 15 minutes (when the cultures were still in the logarithmic growth phase), 10 mL of cul- ture from each pair were extracted, centrifuged at room temperature and frozen in liquid nitrogen as above. RNA extraction began by adding 200 L of fresh lysozyme TE buffer to the frozen pelleted cultures and continued using an RNeasy® Mini kit (Qiagen Ltd., Surrey, UK) accord- ing to the manufacturer’s instructions, including the use of Qiagen RNase-Free DNase, while the RNA was on the column and a further DNase treatment with Ambion TURBO DNA-free™ (Applied Biosystems, UK) at the conclusion of the extraction . The quality o f the RNA was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, UK) which identified all samples as hav- ing an RNA Integrity Number between 9.8 and 10. cDNA was synthesised during an overnight incubation with Superscript III (Invitrogen, UK). cDNA concentra- tions were measured using a NanoDrop® ND-1000 spec- trophotometer (NanoDrop® Technologies, USA) and the cDNA was then used in a one-step real time (RT)-PCR reaction using SYBR® Green and a Roche Lightcycler® 480 (Roche Applied Science, UK). The primers used to determine erm(B) expression levels are forward 5’-GAA- AAGGTACTCAACCAAATA-3’ and reverse 3’-AGT- AACGGTACTTAAATTG TTTAC-5’ as previously des- cribed [18]. Standard curves were constructed, RT-PCR reaction efficiencies were calculated using Microsoft Of- fice Excel 2003 (Microso ft®, UK) and comparison of the mean normalised gene expressions in the presence and absence of 5 mg/L clarithromycin at the same time point was calculated using QGene [19]. The following controls were used: 2 negative controls (nuclease free water and RNA used to synthesize the cDNA-no RT control), and a positive control (TIGR4 cDNA). Th e TIGR4 control was positive for pneumolysin and gyr-A as expected; gyr-A was used as a positive control and co mparison gene from strain 2507 to dete rmine the level of expression of erm(B). 2.9. Statistical Analysis The results of each series of experiments are presented as Copyright © 2012 SciRes. OJRD 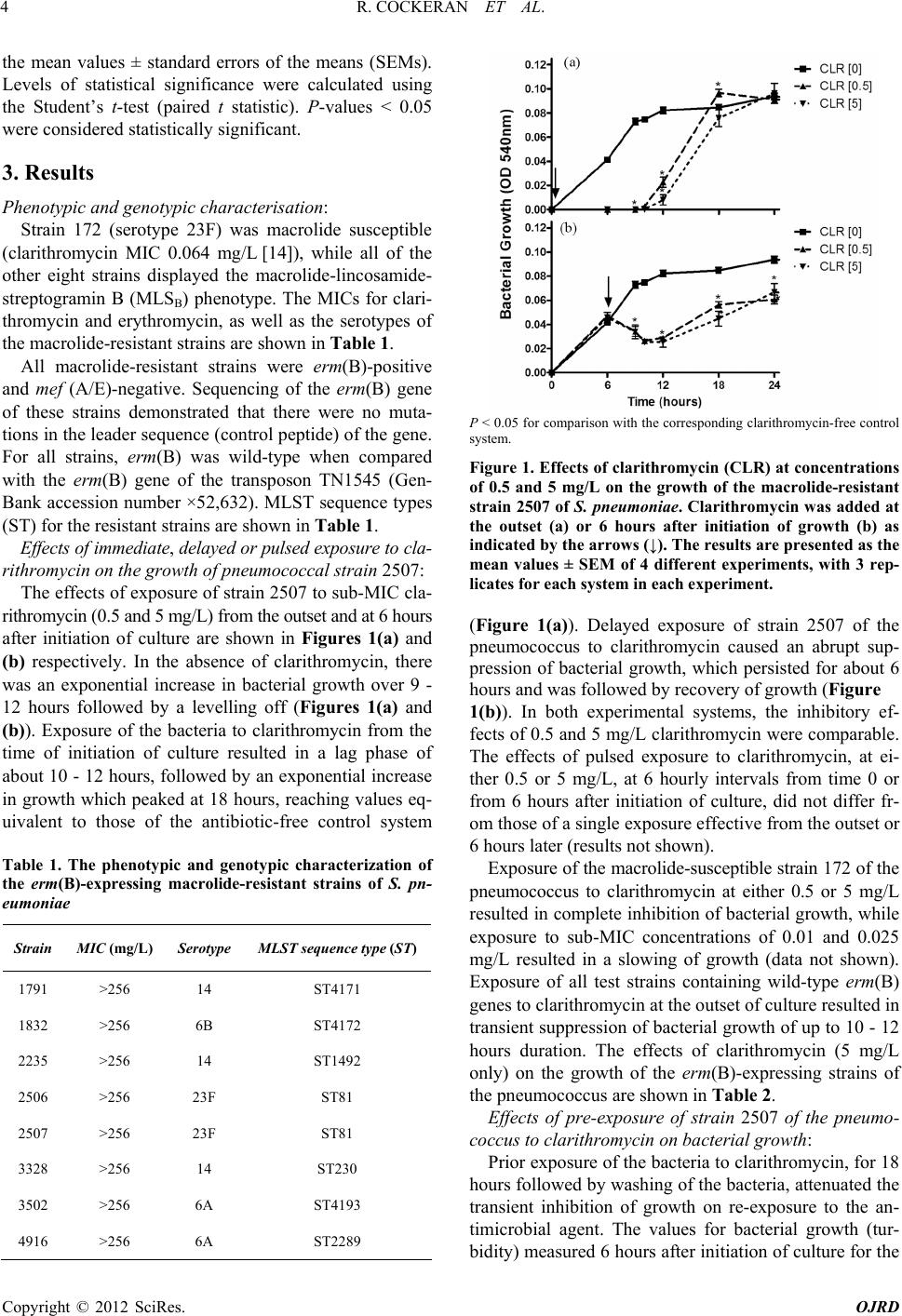 R. COCKERAN ET AL. 4 the mean values ± standard errors of the means (SEMs). Levels of statistical significance were calculated using the Student’s t-test (paired t statistic). P-values < 0.05 were considered statistically significant. 3. Results Phenotypic an d genot ypi c ch ara ct e risat i o n: Strain 172 (serotype 23F) was macrolide susceptible (clarithromycin MIC 0.064 mg/L [14]), while all of the other eight strains displayed the macrolide-lincosamide- streptogramin B (MLSB) phenotype. The MICs for clari- thromycin and erythromycin, as well as the serotypes of the macrolide-resistant strains are shown in Table 1. All macrolide-resistant strains were erm(B)-positive and mef (A/E)-negative. Sequencing of the erm(B) gene of these strains demonstrated that there were no muta- tions in the leader sequence (control peptide) of the gene. For all strains, erm(B) was wild-type when compared with the erm(B) gene of the transposon TN1545 (Gen- Bank accession number ×52,632). MLST sequence types (ST) for the resistant strains are shown in Table 1. Effects of immediate, delayed or pulsed exposure to cla- rithromycin on the gr ow t h of pneumococcal st rain 2507: The effects of exposure of strain 2507 to sub -MIC cla- rithromycin (0.5 and 5 m g/L) from the outset and at 6 hours after initiation of culture are shown in Figures 1(a) and (b) respectively. In the absence of clarithromycin, there was an exponential increase in bacterial growth over 9 - 12 hours followed by a levelling off (Figures 1(a) and (b)). Exposure of the bacteria to clarithromycin from the time of initiation of culture resulted in a lag phase of about 10 - 12 hours, followed by an exponential increase in growth which peaked at 18 hours, reaching values eq- uivalent to those of the antibiotic-free control system Table 1. The phenotypic and genotypic characterization of the erm(B)-expressing macrolide-resistant strains of S. pn- eumoniae Strain MIC (mg/L) SerotypeMLST sequence ty p e (ST) 1791 >256 14 ST4171 1832 >256 6B ST4172 2235 >256 14 ST1492 2506 >256 23F ST81 2507 >256 23F ST81 3328 >256 14 ST230 3502 >256 6A ST4193 4916 >256 6A ST2289 P < 0.0 5 for compa rison wit h the corres ponding clar ithromycin- free contr ol system. Figure 1. Effects of clarithromycin (CLR) at concentrations of 0.5 and 5 mg/L on the growth of the macrolide-resistant strain 2507 of S. pneumoniae. Clarithromycin was added at the outset (a) or 6 hours after initiation of growth (b) as indicated by the arrows (↓). The results are presented as the mean values ± SEM of 4 different experiments, with 3 rep- licates for each system in each experiment. (Figure 1(a)). Delayed exposure of strain 2507 of the pneumococcus to clarithromycin caused an abrupt sup- pression of bacterial growth, which persisted for about 6 hours and was fol l o wed by recovery of g ro wth (Figure 1(b)). In both experimental systems, the inhibitory ef- fects of 0.5 and 5 mg/L clarithromycin were comparable. The effects of pulsed exposure to clarithromycin, at ei- ther 0.5 or 5 mg/L, at 6 hourly intervals from time 0 or from 6 hours after initiation of culture, did not differ fr- om those of a single exposure effective from the outset or 6 hours later (results not shown). Exposure of the macrolide-susceptible strain 172 of the pneumococcus to clarithromycin at either 0.5 or 5 mg/L resulted in complete in hibition of bacterial g rowth, while exposure to sub-MIC concentrations of 0.01 and 0.025 mg/L resulted in a slowing of growth (data not shown). Exposure of all test strains containing wild-type erm(B) genes to clarithromycin at the outset of culture resu lted in transient suppression of bacterial growth of up to 10 - 12 hours duration. The effects of clarithromycin (5 mg/L only) on the growth of the erm(B)-expressing strains of the pneumococcus are shown in Table 2. Effects of pre-exposure of strain 2507 of the pneumo- coccus to clarithromycin on bacterial growth: Prior exposure of the bacteria to clarithro mycin, for 18 hours followed by wash ing of the b acteria, attenu ated the transient inhibition of growth on re-exposure to the an- timicrobial agent. The values for bacterial growth (tur- bidity) measured 6 hours after initiation of cu lture for the Copyright © 2012 SciRes. OJRD  R. COCKERAN ET AL. 5 Table 2. The effects of clarithromycin (CLR, 5 mg/L) on the growth of erm(B)-expressing, macrolide-resistant strains of the pneumococcus. Strain: Time to onset of the exponential phase of growth (hours) CLR [0] CLR [5] 1791 1.54 ± 0.247 10.25 ± 0.116* 1832 4.92 ± 0.651 11.54 ± 0.470* 2235 3.08 ± 1.214 5.14 ± 1.345* 2506 2.20 ± 0.839 8.23 ± 0.713* 2507 1.75 ± 0.075 12.17 ± 0.379* 3328 2.59 ± 0.748 5.15 ± 0.370* 3502 4.67 ± 1.752 6.24 ± 1.526* 4916 1.20 ± 0.330 4.85 ± 1.271* *P < 0.05 for co mpari son with the corr espon ding cl arith romycin -fr ee syst em. The resul ts ar e ex pressed as me an t i me ± SEM taken to en ter t he logarithmic phase of bacterial growth, in the antibiotic-free, and antibiotic (5 mg/L, ad- ded at out set )-t reated s ystems , (d ata f rom 3 - 4 ex p erimen ts with 3 repli cates for each system in each experiment). In the presence of clarithromycin, all strains showed a significant increase in time to onset of the exponential growth ph ase. The combined mean ± S EM maximal ODs for all the strains for the clarithromycin-free and clarithromycin-treated systems were 0.258 ± 0.030 and 0.231 ± 0.024 re spectively (no significant differences). untreated control system, and for clarithromycin-treated systems without and with prior 18 hour exposure to the antibiotic (5 mg/L) were 0.02 ± 0.00 1, 0.003 ± 0.002, and 0.03 0.002 respectively (data from 4 different experi- ments; P < 0.0 5 for comparison of the control and the pre- exposed systems with the exposed only system). Effects of CSP-1 and catalase on clarithromycin-me- diated inhibition of the growth of strain 2507 of the pn- eumococcus: Addition of CSP-1 to the culture medium, either from the outset or at 3 and 6 hours after initiation of culture fail- ed to attenuate the inhibito ry effects of clarithromycin on bacterial growth (results not shown). Although inclusion of catalase in the growth medium augmented the growth of the pneumococcus, the anti-oxidative enzyme did not affect the suppressive effects of clarithromycin on bacte- rial growth (results not shown). Interactive effects of clarithromycin and ceftriaxone on the growth of pneumococcal strain 2507: The effects of clarithromycin (0.5 mg/L) added from the outset, followed 45 min later by the addition of ceftria- xone (0.05, 0.1, 0.25 and 0.5 mg/L, with an MIC of 0.5 mg/L) are shown in Figure 2. Exposure of the pneu- mococcus to clarithromycin alone did not affect growth, while exposure to ceftriaxone (0.1 mg/L) alone resulted in a decrease in the growth of strain 2507; however, the combination of exposure to clarithromycin followed by ceftriaxone potentiated the inhibitory effect of ceftri- axone (0.1 mg/L) significantly. Effects of clarithromycin on microbial ATP levels and protein synthesis: Results are shown in Figure 3. Exposure of strain 2507 (5 × 107 cfu/mL in RPMI 1640) to clarithromycin at 0.5 *P < 0.05 for comparison with the corresponding clarithromycin (CLR) treated s ystem; ° P < 0.0 5 fo r co mpari son wi th the co rr espon d ing ceft riax one (CEF) treated system. Figure 2. Effects of clarithromycin (CLR) at a concentra- tion of 0.5 mg/L added from the outset, followed 45 min later by the addition of ceftriaxone (CEF, 0.05, 0.1, 0.25 and 0.5 mg/L) on the growth of the macrolide resistant strain 2507 of S. pneumoniae after 16 hours. The results are presented as the mean ± SEM of 5 different experiments with 3 repli- cates in each system in each experiment. and 5 mg/L for 2 and 6 hours resulted in a substantial decrease in microbial ATP levels (Figure 3(a)) which was associated with decreased viability (Figure 3(b)), and were comparable for both concentrations of antibiotic. The effects on microbial ATP levels were paralleled by significant reductions in protein synthesis (Figure 3(c)). Erm(B) gene expr ession: In the case of strain 2507 , expression analysis revealed that the baseline level of erm(B) expression was 1.97 ± 0.07, whereas after exposure to clarithromycin (5 mg/L) for 15 minutes it increased to 8.25 ± 2.09 (P = 0.02). These results represent the mean ± SEM of three bio- logical replicates. 4. Discussion In the current study, exposure of the macrolide-resistant strain, 2507, of the pneumococcus to clarithromycin (MIC > 256 mg/L), at concentrations of 0.5 and 5 mg/L, result- ed in transient inhibition of bacterial growth which per- sisted for up to 10 - 12 hours, followed by a rebound in growth which reached levels comparable with those of the correspondin g antibio tic-free con trol systems. Similar transient inhibitory effects of the antibiotic on growth were observed using 7 other resistant strain, differing ph- enotypically by serotype and genotypically by MLST se- quence type. All contained wild-type erm(B) genes and displayed extended lag phases in the presence of sub- MIC concentrations of macrolides. Addition of the anti- biotic during the logarithmic phase resulted in abrupt, tran- sient suppression of bacterial growth followed by recov- ery, albeit at a slower rate than th at of the control system, Copyright © 2012 SciRes. OJRD 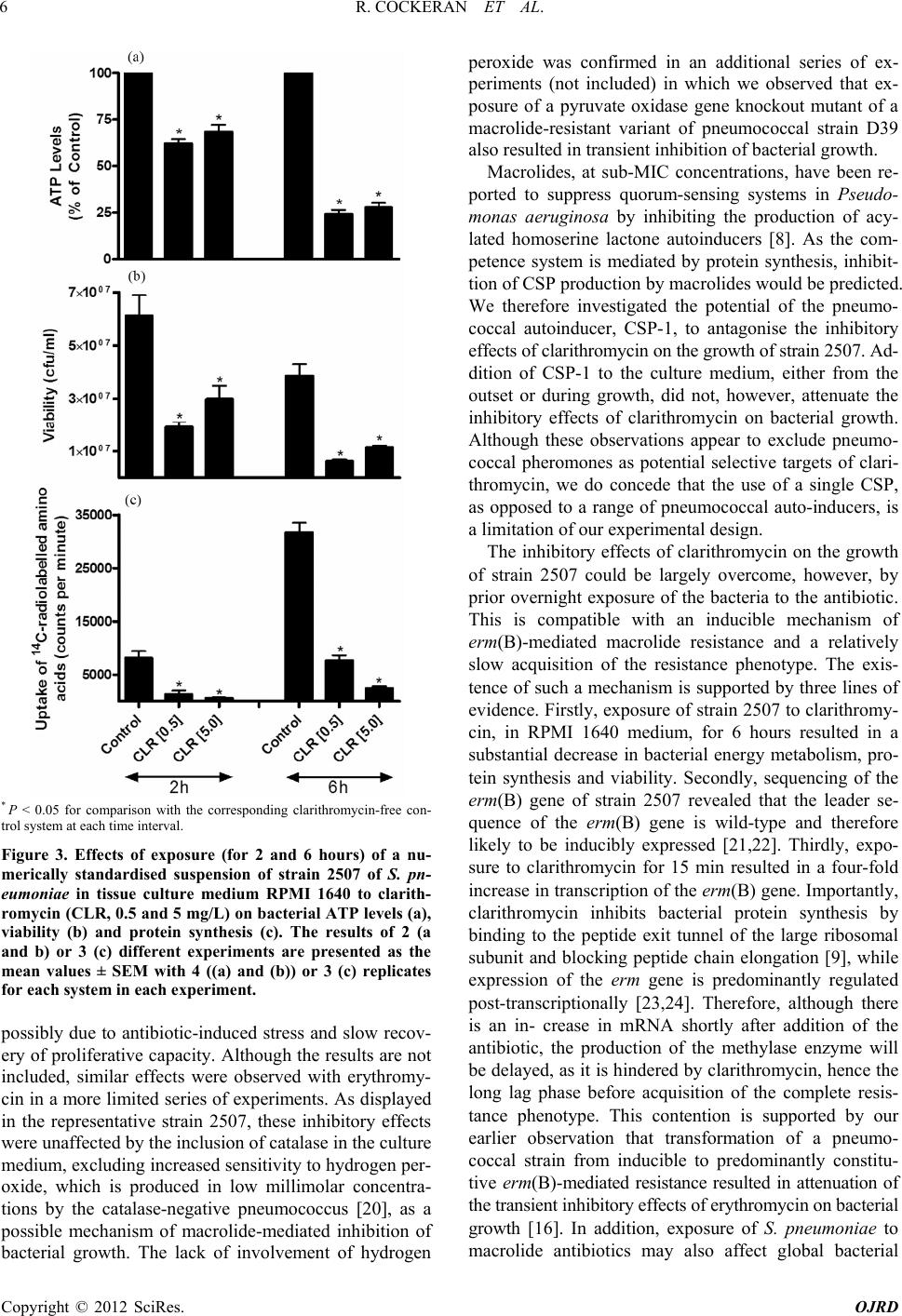 R. COCKERAN ET AL. 6 * P < 0.05 for comparison with the corresponding clarithromycin-free con- trol system at each time interval. Figure 3. Effects of exposure (for 2 and 6 hours) of a nu- merically standardised suspension of strain 2507 of S. pn- eumoniae in tissue culture medium RPMI 1640 to clarith- romycin (CLR, 0.5 and 5 mg/L) on bacterial ATP levels (a), viability (b) and protein synthesis (c). The results of 2 (a and b) or 3 (c) different experiments are presented as the mean values ± SEM with 4 ((a) and (b)) or 3 (c) replicates for each system in each experiment. possibly due to antibiotic-induced stress and slow recov- ery of proliferative capacity. Although the results are not included, similar effects were observed with erythromy- cin in a more limited series of experiments. As displayed in the representative strain 2507, these inhibitory effects were unaffected by the inclusion of catalase in the culture medium, excluding increased sensitivity to hyd rog en per- oxide, which is produced in low millimolar concentra- tions by the catalase-negative pneumococcus [20], as a possible mechanism of macrolide-mediated inhibition of bacterial growth. The lack of involvement of hydrogen peroxide was confirmed in an additional series of ex- periments (not included) in which we observed that ex- posure of a pyruvate oxidase gene knockout mutant of a macrolide-resistant variant of pneumococcal strain D39 also resulted in transient inh ibition of bacterial growth. Macrolides, at sub-MIC concentrations, have been re- ported to suppress quorum-sensing systems in Pseudo- monas aeruginosa by inhibiting the production of acy- lated homoserine lactone autoinducers [8]. As the com- petence system is mediated by protein synthesis, inhibit- tion of CSP production by macrolides would be predicted. We therefore investigated the potential of the pneumo- coccal autoinducer, CSP-1, to antagonise the inhibitory effects of clarithromycin on the growth of strain 2507. Ad- dition of CSP-1 to the culture medium, either from the outset or during growth, did not, however, attenuate the inhibitory effects of clarithromycin on bacterial growth. Although these observations appear to exclude pneumo- coccal pheromones as potential selective targets of clari- thromycin, we do concede that the use of a single CSP, as opposed to a range of pneumococcal auto-inducers, is a limitation of our experimental design. The inhibitory effects of clarithromycin on the growth of strain 2507 could be largely overcome, however, by prior overnight exposure of the bacteria to the antibiotic. This is compatible with an inducible mechanism of erm(B)-mediated macrolide resistance and a relatively slow acquisition of the resistance phenotype. The exis- tence of such a mechanism is supported by three lines of evidence. Firstly, exposure of strain 2507 to clarithromy- cin, in RPMI 1640 medium, for 6 hours resulted in a substantial decrease in bacterial energy metabolism, pro- tein synthesis and viability. Secondly, sequencing of the erm(B) gene of strain 2507 revealed that the leader se- quence of the erm(B) gene is wild-type and therefore likely to be inducibly expressed [21,22]. Thirdly, expo- sure to clarithromycin for 15 min resulted in a four-fold increase in transcription of the erm(B) gene. Importantly, clarithromycin inhibits bacterial protein synthesis by binding to the peptide exit tunnel of the large ribosomal subunit and blocking peptide chain elongation [9], while expression of the erm gene is predominantly regulated post-transcriptionally [23,24]. Therefore, although there is an in- crease in mRNA shortly after addition of the antibiotic, the production of the methylase enzyme will be delayed, as it is hindered by clarithromycin, hence the long lag phase before acquisition of the complete resis- tance phenotype. This contention is supported by our earlier observation that transformation of a pneumo- coccal strain from inducible to predominantly constitu- tive erm(B)-mediated resistance resulted in attenuation of the transient inhibitory effects of erythromycin on bacterial growth [16]. In addition, exposure of S. pneumoniae to macrolide antibiotics may also affect global bacterial Copyright © 2012 SciRes. OJRD  R. COCKERAN ET AL. 7 gene expression that could affect outcome of infection [25]. The clinical significance of clarithromycin-mediated transient inhibition of the growth of inducible erm(B)- expressing macrolide-resistant strains of the pneumo- coccus remains to be established. Together with other potentially beneficial activities such as high-level tissue penetration and accumulation, and anti-inflammatory pro- perties, these inhibitory effects of macrolides on the grow- th of macrolideresistant pneumococci may tip the host vs pathogen balance in favour of the former, as has been described in an animal model of experimental infection [4]. Furthermore, it is noteworthy from the current study that combining clarithromycin with ceftriaxone potenti- ated the activity of the -lactam for the macrolideresis- tant strain of the pneumococcus. The present treatment strategy for patients with severe pneumococcal pneumo- nia is a combination of a macrolide and a -lactam [16]. In conclusion, exposure of erm(B)-expressing macro- lide-resistant strains of the pneumococcus is associated with decreased metabolic acti vity and transient inh ibition of growth apparently as a consequence of the slow ac- quisition of the full erm(B)-mediated resistance phenol- type. We do concede, however, that this phenomenon of delayed acquisition of resistance may be limited to erm(B)-expressing pneumococci and of lesser relevance in other bacterial pathogens. 5. Acknowledgements We thank the Group for Enteric, Respiratory, and Menin- geal Disease Surveillance in South Africa (GERMS-SA) for supplying six of the isolates used in this study. CF is supported by the National Research Foundation of South Africa. REFERENCES [1] G. W. Amsden, “Pneumococcal Macrolide-Resistance— Myth or Reality?” Journal of Antimicrobial Chemo th er apy , Vol. 44, No. 1, 1999, pp. 1-6. doi:10.1093/jac/44.1.1 [2] L. E. Bermudez, K. Nash, M. Petrofsky, L. S. Young and C. B. Inderlied, “Clarithromycin-Resistant Mycobacterium Avium is still Susceptible to Treatment with Clarithro- mycin and is Virulent in Mice,” Antimicrobial Agents in Chemotherapy, Vol. 44, No. 10, 2000, pp. 2619-2622. doi:10.1128/AAC.44.10.2619-2622.2000 [3] C. Feldman, “Clinical Relevance of Antimicrobial Resis- tance in the Management of Pneumococcal Community- Acquired Pneumonia,” Journal of Laboratory and Clini- cal Medicine, Vol. 143, No. 5, 2004, pp. 269-283. doi:10.1016/j.lab.2004.02.002 [4] Y. Fukuda, K. Yanagihara, Y. Higashiyama, Y. Miyazaki, Y. Hirakata, H. Mukae, K. Tomono, Y. Mizuta, K. Tsu- kamoto and S. Kohno, “Effects of Macrolides on Pneu- molysin of Macrolide-Resistant Streptococcus Pneumo- niae,” European Respiratory Journal, Vol. 27, No. 5, 2006, pp. 1020-1025. [5] E. J. Giamarellos-Bourboulis, J.-C. Pechère, C. Routsi, D. Plachouras, S. Kollias, M. Raftogiannis, D. Zervakis, F. Baziaka, A. Koronaios, A. Antonopoulou, V. Markari, P. Koutoukas, E. Papadomichelakis, T. Tsaganos, A. Arma- ganidis, V. Koussoulas, A. Kotanidou, C. Roussos and H. Giamarellou, “Effect of Clarithromycin in Patients with Sepsis and Ventilator-Associated Pneumonia,” Clinical Infectious Diseases, Vol. 46, No. 8, 2008, pp. 1157-1164. doi:10.1086/529439 [6] M. I. Restrepo, E. M. Mortensen, G. W. Waterer, R. G. Wunderink, J. J. Coalson and A. Anzueto, “Impact of Macrolide Therapy on Mortality for Patients with Severe Sepsis Due to Pneumonia,” European Respiratory Jour- nal, Vol. 33, No. 1, 2009, pp. 153-159. doi:10.1183/09031936.00054108 [7] T. Ichimiy a, T. Yama saki, and M. Nasu, “In-Vitro Effects of Antimicrobial Agents on Pseudomonas Aeruginosa Biofilm Formation,” Journal of Antimicrobial Chemo- therapy, Vol. 34, No. 3, 1994, pp. 331-341. doi:10.1093/jac/34.3.331 [8] K. Tateda, T. J. Standiford, J. C. Pechere and K. Yamagu- chi, “Regulatory Effects of Macrolides on Bacterial Viru- lence: Potential Role as Quorum-Sensing Inhibitors,” Cur- rent Pharmacological Research, Vol. 10, No. 25, 2004, pp. 3055-3065. doi:10.2174/1381612043383377 [9] J. M. Zuckerman, “Macrolides and Ketolides: Azithro- mycin, Clarithromycin, Telithromycin,” Infectious Diseases in Clinics in North America, Vol. 18, No. 3, 2004, pp. 621-649. doi:10.1016/j.idc.2004.04.010 [10] G. W. Amsden, “Anti-Inflammatory Effects of Macroli- des—An Underappreciated Benefit in the Treatment of Community Acquired Respiratory Tract Infections and Chronic Inflammatory Pulmonary Conditions,” Journal of Antimicrobial Chemotherapy, Vol. 55, No. 1, 2005, pp. 10-21. doi:10.1093/jac/dkh519 [11] C. Feldman and R. Anderson, “The Cytoprotective Inter- actions of Antibiotics with Human Ciliated Airway Epi- thelium,” In: B. K. Rubin and J. Tamaoki, Ed., Antibiotics as Anti-Inflammatory and Immunomodulatory Agents, Birkhauser Verlag, Basel, 2005, pp. 49-63. doi:10.1007/3-7643-7310-5_3 [12] Y. Nalca, L. Jänsch, F. Bredenbruch, R. Geffers, J. Buer and S. Haussler, “Quorum-Sensing Antagonistic Activi- ties of Azithromycin in Pseudomonas Aeruginosa PA01: A Global Approach,” Antimicrobial Agents in Chemo- therapy, Vol. 50, No. 5, 2006, pp. 1680-1688. doi:10.1128/AAC.50.5.1680-1688.2006 [13] M. Shinkai, C. S. Park and B. K. Rubin, “Immunomodu- latory Effects of Macrolide Antibiotics,” Clinical and Pulmonary Medicine, Vol. 12, No. 6, 2005, pp. 341-348. doi:10.1097/01.cpm.0000187294.53696.c0 [14] R. Anderson, H. C. Steel, R. Cockeran, A. M. Smith, A. von Gottberg, L. de Gouveia, A. Brink, K. P. Klugman, T. J. Mitchell and C. Feldman, “Clarithromycin Alone and in Combination with Ceftriaxone Inhibits the Production of Pneumolysin by Both Macrolide-Susceptible and Macrolide-Resistant Strains of Streptococcus Pneumo- niae,” Journal of Antimicrobial Chemotherapy, Vol. 59, No. 2, 2007, pp. 224-229. doi:10.1093/jac/dkl479 Copyright © 2012 SciRes. OJRD  R. COCKERAN ET AL. Copyright © 2012 SciRes. OJRD 8 [15] R. Anderson, H. C. Steel, R. Cockeran, A. von Gottberg, L. de Gouveia, K. P. Klugman, T. J. Mitchell and C. Feld- man, “Comparison of the Effects of Macrolides, Amox- icillin, Ceftriaxone, Doxycycline, Tobramycin and Fluo- roquinolones, on the Production of Pneumolysin by Stre- ptococcus Pneumoniae in Vitro,” Journal of Antimicrobial Chemotherapy, Vo l. 60 , N o. 5, 20 07 , p p. 1155-1178. doi:10.1093/jac/dkm338 [16] V. Falcó, A. Sánchez, A. Pahissa and J. Rello, “Eme rging Drugs for Pneumococcal Pneumonia,” Expert Opinion in Emerging Drugs, Vol. 16, No. 3, 2011, pp. 459-477. doi:10.1517/14728214.2011.576669 [17] M. C. Enricht and B. G. Spratt, “A Multilocus Sequence Typing Scheme for Streptococcus Pneumoniae: Identifi- cation of Clones Associated with Serious Invasive Disea- se,” Microbiology, Vol. 144, No. 11, 1998, pp. 3049-3060. doi:10.1099/00221287-144-11-3049 [18] J. Sutcliffe, T. Grebe, A. Tait-Kamradt and L. Wondrack, “Detection of Erythromycin-Resistant Determinants by PCR,” Antimicrobial Agents in Chemotherapy, Vol. 40, No. 11, 1996, pp. 2562-2566. [19] P. Y. Muller, H. Janocjak, A. R. Miserez and Z. Dobbie, “Processing of Gene Expression Data Generated by Qua n- titative Real-Time PCR,” Biotechniques, Vol. 32, No. 6, 2002, pp. 1372-1379. [20] C. D. Pericone, K. Overweg, P. W. M. He rmans and J. N. Weiser, “Inhibitory and Bactericidal Effects of Hydrogen Peroxide Production by Streptococcus Pneumoniae on other Inhabitants of the Upper Respiratory Tract,” Infection and Immunity, Vol. 68, No. 7, 2000, pp. 3990-3997. doi:10.1128/IAI.68.7.3990-3997.2000 [21] A. Rosato, H. Vicarini and R. Leclercq, “Inducible and Constitutive Expression of Resistance in Clinical Isolates of Streptococci and Enterococci Cross-Resistant to Eryth- romycin and Lincomycin,” Journal of Antimicrobial Che- motherapy, Vol. 43, No. 4, 1999, pp. 559-562. doi:10.1093/jac/43.4.559 [22] N. Wolter, A. M. Smith, D. J. Farrell, J. B. Northwood, S. Douthwaite and K. P. Klugman, “Telithromycin Resis- tance in Streptococcus Pneumoniae is Conferred by a Deletion in the Leader Sequence of erm(B) that Increases rRNA Methylation,” Antimicrobial Agents in Chemothe- rapy, Vol. 52, No. 2, 2008, pp. 435-440. doi:10.1128/AAC.01074-07 [23] B. Weisblum, “Insights into Erythromycin Action from Studies of Its Activity as Inducer of Resistance,” Antim- icrobial Agents in Chemotherapy, Vol. 39, No. 4, 1995, pp. 797-805. [24] D. J. Wozniak, and R. Keyser, “Effects of Subinhibitory Concentrations of Macrolide Antibiotics on Pseudomonas Aeruginosa,” Chest, Vol. 125, No. 2, 2004, pp. 62S-69S. doi:10.1378/chest.125.2_suppl.62S [25] W.-L. Ng, K. M. Kazmierczak, G. T. Robertson, R. Gil- mour and M. E. Winkler, “Transcriptional Regulation and Signature Patterns Revealed by Microarray Analyses of Streptococcus Pneumoniae R6 Challenged with Sublethal Concentrations of Translation Inhibitors,” Journal of Bac- teriology, Vol. 185, No. 1, 2003, pp. 359-370.
|