Paper Menu >>
Journal Menu >>
 Vol.2, No.4, 379-387 (2010) Natural Science http://dx.doi.org/10.4236/ns.2010.24046 Copyright © 2010 SciRes. OPEN ACCESS Study the effect of formulation variables in the development of timed-release press-coated tablets by Taguchi design Chikkanna Narendra1*, Mayasandra SrinavasaIyengar Srinath2 1Department of Pharmaceutics, Visveswarapura Institute of Pharmaceutical Sciences, Bangalore, India; narendragcp@rediffmail.com 2Department of Pharmaceutics, SET’S College of Pharmacy, Dharwad, India Received 30 November 2009; revised 28 December 2009; accepted 3 February 2010. ABSTRACT In this investigation, the effect of formulation variables on the release properties of timed- release press-coated tablets was studied using the Taguchi method of experimental design. Formulations were prepared based on Taguchi orthogonal array design with different types of hydrophilic polymers (X1), varying hydrophilic polymer/ethyl cellulose ratio (X2), and addition of magnesium stearate (X3) as independent variables. The design was quantitatively evalu- ated by best fit mathematical model. The results from the statistical analysis revealed that factor X1, X3 and interaction factors between X1X2 and X1X3 were found to be significant on the re- sponse lag time (Y1), where as only factor X1 was found to be significant on the response percent drug release at 8 hrs (Y2). A numerical optimization technique by desirability function was used to optimize the response variables, each having a different target. Based on the re- sults of optimization study, HPC was identified as the most suitable hydrophilic polymer and incorporation of hydrophobic agent magnesium stearate, could significantly improve the lag time of the timed-release press-coated tablet. Keywords: Press-Coated Tablet; Taguchi Design; Hydrophilic Polymers; Timed-Release; Hydrophobic Agents 1. INTRODUCTION During the recent years timed-release preparations has received increasing attention, which release the drug rapidly and completely after a lag time following oral drug administration. This type of delivery system is not only rate controlled but also time and /or site controlled to deliver the drug when it is required. Such time and/or site controlled formulations has been widely investigated for a number of diseases and therapies [1,2]. Over a period, many different approached have been used for delivering the drugs as time and /or site specific which includes, Timeclock® system [3], Chronotropic® system [4], Pulsincap® sysem [5], Port® system [6], Ti m eR x® system [7] and Geomatrix® system [8]. These systems are developed with intention to meet the needs of chronopathologies with symptoms mostly recurring at night time or early morning hours. The principal advan- tage of Chronotherapeutic drug delivery system includes consideration of a person’s biological rhythms in deter- mining the timing and the amount of medication to op- timize a drug's desired effects and minimize the unde- sired ones. As a consequence there is reduction of dose requirement and this likely to improve patient compli- ance [9]. In spite of the difficulties faced by releasing actives due to the variable gastrointestinal environment, orally administered timed-release delivery systems are most preferred because they offer flexibility in dosage-form design and are relatively safe. Press-coated tablet com- posed of an inner core that contains an active pharma- ceutical ingredient and inert excipients surrounded by an outer coating layer. The outer coating material may be compressed onto the inner core as compression coated which dissolves or erodes or disintegrates slowly to produce a lag time before the release of active ingredi- ent. Several types of hydrophilic polymers have been in- vestigated as a compression coating material including hydroxypropylmethylcellulose [10], L-hydroxypropylce- llulose [11], hydroxyethylcellulose [12], polyethylene- oxide/polyethyleneglycol [13], and pectin/ hydroxypro- pylmethylcellulose [14]. Lin et al. [15] studied the effect of hydrophilic excipients (spray-dried lactose and HPMC K4M) along with hydrophobic ethylcellulose as an outer coating shell material and concluded that addi- tion of hydrophilic excipients can be very useful in con- trolling the lag time adequately. The effect of hydroxyl-  C. Narendra et al. / Natural Science 2 (2010) 379-387 Copyright © 2010 SciRes. OPEN ACCESS 380 propylmethylcellulose acetate succinate (HPMCAS) and water soluble/insoluble plasticizers-adsorbent as outer coating material was reported by Fukui et al. [16] and the results suggested that the outer shell had a plastic deformation property due to some interaction between HPMCAS and water soluble plasticizers-adsorbent and the same would be useful for colon targeting. In another study, effect of hydrophobic additives were investigated and the results indicated that mixing of HPMCAS, mag- nesium stearate and calcium stearate at appropriate ratio prolonged the lag time [17]. Design of experiment has been widely used in phar- maceutical field to study the effect of formulation vari- ables and their interaction on dependent (response) variables. [18-20] In the present study an attempt is made to study the effect of formulation variables with the aid of Taguchi design to identify the potential con- tribution of various types of hydrophilic polymers, varying the hydrophilic/ethylcellulose ratio and presence and absence of magnesium stearate. 2. MATERIALS AND METHODS 2.1. Materials Theophylline anhydrous was received as gift sample from M/s Eros Pharma Pvt. Ltd., Bangalore, India. Hy- droxypropylmethylcllulose (HPMC, Methocel K100M), sodium carboxymethylcellulose (NaCMC, HVP), Hy- droxypropylcellulose (HPC, Klucel® EXF Pharm), Hy- droxyethylcellulose (HEC, NATROSOL® 250 HX Pharm) and ethylcellulose (EC, Ethocel® 25cPs) were supplied by M/s Strides Arco, Labs Ltd., Bangalore, In- dia as gift samples. Other materials were purchased from commercial source; magnesium stearate (Loba chemi- cals, Mumbai, India), polyvinylpyrrolidine (PVP K30) (Reidel India chemicals, Mumbai, India), sodium starch glycolate, talc (Nice chemicals, Cochin, India) and di- rectly compressible lactose (S.D. fine chemicals Ltd, Mumbai, India). All other chemicals used in the study were of analytical grade. 2.2. Experimental Design A Taguchi design [L16(45)] was implanted to study the effect of formulation variables in the development of timed release press-coated tablet. The Taguchi method utilizes orthogonal arrays are essentially fractional facto- rial experimental design to study the large number of variables with a small number of experiments. Generally a full factorial design would yield large experiments with replication of centre points. The levels of the 3 independent variables are as fol- lows; X1= Type of Hydrophillic polymer (HPMC, NaCMC, HPC and HEC) X2= Hydrophilic polymer/EC (1:1 to 4:1) X3= Amount of magnesium stearate (0 to 10%) The response variables tested include: Y1 = Lag time (time required for 10% of drug release in hour) Y2 = Percent drug release at 8 hrs. 2.3. Preparation of Core Tablet A direct compression method was adapted to prepare the core tablet. As shown in Table 1, Theophylline anhy- drous, lactose, PVP K30 and sodium starch glycolate were mixed in a suitable stainless steel vessel in a tum- bler mixer (Rimek, Karnavati Engineering Ltd. Ahmed- abad, India) at 100 rpm for 30 min. thoroughly after pass- ing through 80 mesh screen. Further, magnesium stearate and talc were added to the above powder mixture and blended for 10 min. Finally the resulting powder blend was compressed by using a 10-station rotary tablet com- pression machine (Rimek, Ahmedabad, India) fitted with 8mm standard concave punches. Preparation was per- formed in 100 tablet batches and compression was con- trolled to produce 4 ± 0.5kg/cm2 tablet crushing strength. 2.4. Preparation of Press-Coated Tablet The formulations were prepared at random following Taguchi design. Prior to compression all the ingredients were passed through 80 mesh screen. The core tablets were press-coated with an appropriate blend of polymers with or with out magnesium stearate as given in Table 2. Half the quantity of outer coating material was weighed and transferred into the die; manually the core tablet was placed carefully in the centre of the die. Then, the re- maining half quantity of outer coating material was added into the die and compressed by using 10-station rotary tablet compression machine (Rimek, Ahmedabad, India) fitted with 11 mm standard concave punches and compression was controlled to produce 14 ± 0.5kg/cm2 tablet crushing strength. 2.5. In Vitro Dissolution Studies The dissolution was performed by using USP dissolution apparatus II paddle assembly (TDT-06T, Electrolab, In- dia) at 37˚C + 1˚C using 750 ml of pH 1.2 buffer for the first 2 hours and followed by 900 ml of pH 6.8 buffer till the end of dissolution studies. The paddle rotational speed was set to 100 rpm. Aliquots samples were with- drawn at specified time intervals and the samples were Table 1. Composition of core layer of press-coated tablet. Ingredients Quantity (mg/tablet) Theophylline anhydrous 100 Sodium starch glycolate 10 Polyvinylpyrrolidone 5 Magnesium stearate 1 Talc 2 Lactose 32  C. Narendra et al. / Natural Science 2 (2010) 379-387 Copyright © 2010 SciRes. OPEN ACCESS 381 Table 2. Composition of coat layer of press-coated tablets based on Taguchi design with observed responses. Formula- tion code X1 Type X2 Ratio X3 (%) Y1 (Hr) Y2 (%) F1 HPMC 1:1 0 5.3 ± 0.6 10.51 ± 2.01 F2 HPMC 2:1 10 7.5 ± 0.5 10.05 ± 3.16 F3 HPMC 3:1 0 3.4 ± 0.3 12.30 ± 2.37 F4 HPMC 4:1 10 7.1 ± 0.5 42.40 ± 1.15 F5 NaCMC 1:1 0 1.4 ± 1.1 100* F6 NaCMC 2:1 10 3.1 ± 1.6 100* F7 NaCMC 3:1 0 2.5 ± 0.9 100* F8 NaCMC 4:1 10 4.2 ± 0.7 98.14 ± 3.34 F9 HPC 1:1 10 5.5 ± 0.5 100.81 ± 4.22 F10 HPC 2:1 0 2.3 ± 1.3 103.68 ± 3.14 F11 HPC 3:1 10 7.1 ± 0.5 98.87 ± 4.06 F12 HPC 4:1 0 2.8 ± 1.0 114.87 ± 4.13 F13 HEC 1:1 10 4.6 ± 0.3 14.13 ± 4.05 F14 HEC 2:1 0 2.5 ± 0.9 14.32 ± 3.55 F15 HEC 3:1 10 5.2 ± 0.6 11.98 ± 3.22 F16 HEC 4:1 0 2.6 ± 0.5 16.05 ± 3.37 *100% drug release was observed before 8 hrs of dissolution studies. analyzed spectrophotometrically (UV-1601, Shimadzu, Japan) at 271 nm and the amount of drug released was determined from the calibration curve. The volume of the sample withdrawn each time was replaced with the same volume of the respective buffer solution. The stud- ies were carried out in triplicate and mean values plotted verses time with standard error of mean, indicating the reproducibility of the results. 2.6. Statistical Analysis The effect of formulation variables on the response variables were statically evaluated by applying one-way ANOVA at 0.05 level using a commercially available software package Design-Expert® version 6.05 (Stat- Ease, Inc.). The design was evaluated by using a suitable model. The best fit model was selected based on the several statistical parameters including multiple correla- tion coefficient (R2), adjusted multiple correlation coef- ficient (adjusted R2) and the predicted residual sum of square (PRESS). For the model to be chosen as best fit, the PRESS valve should be small relative to the other models. Linear model Y= b0 + b1X1+ b2X2 + b3X3 Two factor interaction model Y= b0 + b1X1+ b2X2 + b3X3+ b4X1X2+b5X1X3+b6X2X3 where Y is the response variable, b0 the constant and b1, b2, b3,…,b5 is the regression coefficient. X1, X2 and X3 stand for the main effect; X1X2, X1X3 and X2X3 are the interaction terms, show how response changes when two factors are simultaneously changed. 3. RESULT AND DISCUSSION 3.1. Experimental Design Taguchi method as design of experiment was chosen for the organization of the experiments and analysis of the results. Normally a full factorial design for such experi- ment would yield 4 × 4 × 2 = 32 experiments. In the present case, L16 orthogonal array, a mixed-level design (2 factors at 4 levels and one factor at 2 levels) was con- sidered and the size of experimentation was represented by symbolic arrays i.e. 16 experiments [21]. The use of more than two factors makes it possible to study some of the eventual non-linear effects with interactions between the factors. The statistical analysis to select the model that best fits the data was obtained by analyzing the re- sults of sequential model given in the Table 3. As seen from the table, though the linear model was found to be significant but the PRESS value for a two factor interac- tion model (2FI) was found to be least hence, 2FI model was considered to analyze the response lag time. For the response percent drug release at 8 hrs, linear model was found be significant with low PRESS value and the same model was further navigated for ANOVA studies. 3.2. Effect of Type of Hydrophilic Polymers Figures 1-4 show the release profile of press-coated tablets in accordance to type of hydrophilic polymer. If HPMC as type of hydrophilic polymer, increasing the amount of HPMC in the coating layer, formulations F1, F2 and F3 exhibited a minimal drug release at the end of dissolution studies. Such a type of decrease in drug re- lease may be due to increased amount of EC in the coat- ing layer retarded the rate of hydration of HPMC which Table 3. Comparison of sequential model. Y 1 (hr) Y1 (%) Statistical Parameters Linear 2FI Quadratic Linear 2FI Quadratic R2 0.8754 0.9940 0.9941 0.9773 0.9910 0.9953 Adjusted R2 0.8132 0.9704 0.9558 0.9660 0.9550 0.9648 PRESS 16.11724 9.2977 20.88 1907.033 7752.664 9088.802 p Valve 0.0003* 0.0522 0.9563 < 0.0001* 0.713 0.3079 * denotes significant p < 0.05. 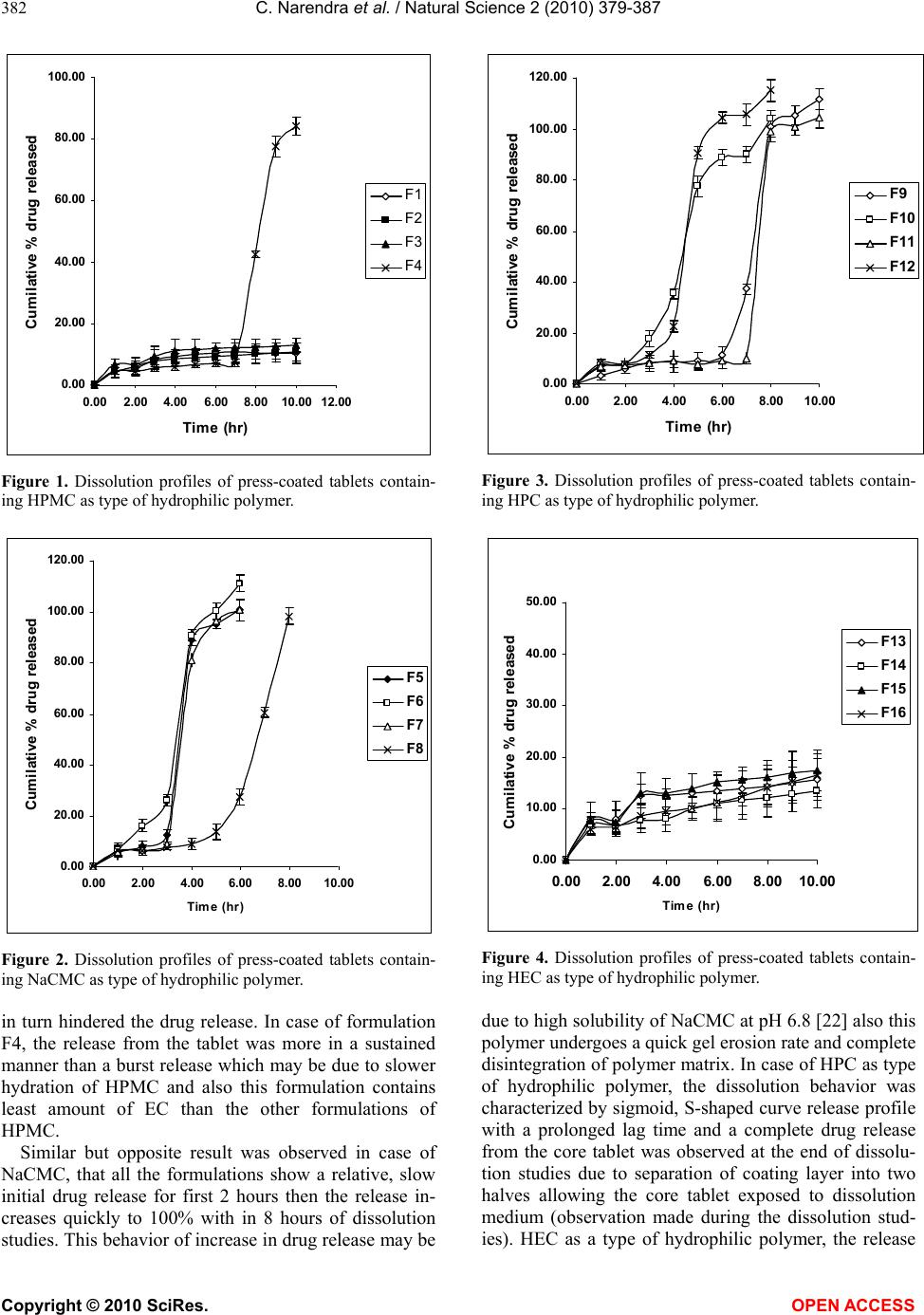 C. Narendra et al. / Natural Science 2 (2010) 379-387 Copyright © 2010 SciRes. OPEN ACCESS 382 0.00 20.00 40.00 60.00 80.00 100.00 0.00 2.004.006.008.0010.0012.00 Time (hr) Cumilative % drug released F1 F2 F3 F4 Figure 1. Dissolution profiles of press-coated tablets contain- ing HPMC as type of hydrophilic polymer. 0.00 20.00 40.00 60.00 80.00 100.00 120.00 0.00 2.00 4.006.008.0010.00 Time (hr) Cumilative % drug released F5 F6 F7 F8 Figure 2. Dissolution profiles of press-coated tablets contain- ing NaCMC as type of hydrophilic polymer. in turn hindered the drug release. In case of formulation F4, the release from the tablet was more in a sustained manner than a burst release which may be due to slower hydration of HPMC and also this formulation contains least amount of EC than the other formulations of HPMC. Similar but opposite result was observed in case of NaCMC, that all the formulations show a relative, slow initial drug release for first 2 hours then the release in- creases quickly to 100% with in 8 hours of dissolution studies. This behavior of increase in drug release may be 0.00 20.00 40.00 60.00 80.00 100.00 120.00 0.00 2.00 4.00 6.00 8.0010.00 Time (hr) Cumilative % drug released F9 F10 F11 F12 Figure 3. Dissolution profiles of press-coated tablets contain- ing HPC as type of hydrophilic polymer. 0.00 10.00 20.00 30.00 40.00 50.00 0.00 2.00 4.006.00 8.0010.00 Time (hr) Cumilative % drug released F13 F14 F15 F16 Figure 4. Dissolution profiles of press-coated tablets contain- ing HEC as type of hydrophilic polymer. due to high solubility of NaCMC at pH 6.8 [22] also this polymer undergoes a quick gel erosion rate and complete disintegration of polymer matrix. In case of HPC as type of hydrophilic polymer, the dissolution behavior was characterized by sigmoid, S-shaped curve release profile with a prolonged lag time and a complete drug release from the core tablet was observed at the end of dissolu- tion studies due to separation of coating layer into two halves allowing the core tablet exposed to dissolution medium (observation made during the dissolution stud- ies). HEC as a type of hydrophilic polymer, the release  C. Narendra et al. / Natural Science 2 (2010) 379-387 Copyright © 2010 SciRes. OPEN ACCESS 383 at the end of dissolution studies were found to be less than 18% which may be due to high viscosity of polymer, decreased water uptake to form a gel matrix [23] and presence of hydrophobic components such as EC and magnesium stearate further prevented the hydration rate. 3.3. Effect of Hydrophilic/EC Ratio EC, a cellulose ether derivative most widely used as wa- ter insoluble polymer for coating of solid dosage forms. Besides as controlled release barrier, they have also been used as moisture barrier to improve stability of hydro- lytically liable drugs [24]. The effect of hydrophilic/EC ratio in presence and absence of magnesium stearate on the release properties are summarized in Table 4. On comparison of values, increasing the hydrophilic/EC ratio, HPMC containing formulations exhibited a nega- tive effect on lag time where as a positive effect was observed in case of other hydrophilic polymers. HPMC and HEC containing formulations showed no complete drug release from the tablet even at the end of dissolu- tion studies which is probably due to slow hydration rate (because of hydrophobic components) and also the hy- drogel layer therefore formed was strong enough and could inhibit further water penetration into the inside of core tablet [25,26]. In case of NaCMC and HPC, they did not show sig- nificant difference in their release profile at the end of dissolution studies except that NaCMC containing for- mulations exhibited shorter lag time with complete drug release with in 8 hours of dissolution studies where as in case of HPC containing formulations exhibited longer lag time with complete drug release at the end of disso- lution studies. Such a type of release behavior may be due to faster hydration followed by a combination of disintegration and high erosion rate for the former where as moderate swelling with low erosion rate for the later [26,27]. 3.4. Effect of Magnesium Stearte The effect of magnesium stearate on the lag time and percent drug release at 8 hrs can be visualized from the Table 4. The formulations containing magnesium stear- ate exhibited an improved lag time but no improvement was observed in case of percent drug release at 8 hrs. The beneficial effect of magnesium stearate on the lag time is probably due to its hydrophobic nature prolongs the lag time by significantly decreasing the water uptake Figure 5. Main effect plot for type of hydrophilic polymer (X1) on lag time (Y1) by keeping factors X2 and X3 at lower level. and penetration through the coating layer [28]. 3.5. Statistical Analysis The model terms for Y1 (lag time) were found to be sig- nificant with an F value of 42.10 (0.0052), high R2 value of 0.9940 indicate the adequate fitting of two factor in- teraction model. As shown in Table 5, factors X1, X3 and interaction factors X1X2 and X1X3 were found to be sig- nificant. At lower level of factors X2 and X3, changing the type of hydrophilic polymer from HPMC to HEC the lag time decreases but at higher level of factors X2 and X3, the lag time increased to a greater value if HPMC and HPC were used as the type of hydrophilic polymer, where as in case of NaCMC and HEC the effect was found to be negative (Figures 5 & 6). Changing the factor X3 from lower to higher level, a significant positive effect on the lag time was observed with irrespective of type of hydrophilic polymer and hydrophilic /EC ratio. The interaction effect between the factors X1X2 can be studied with the help of Figures 7 & 8. In presence or absence of magnesium stearate, if X2 was increased from lower to higher level and by Table 4. Comparison of release parameters prepared from different types of hydrophilic polymers. HPMC NaCMC HPC HEC Response 1no MgSt 2MgSt 3no MgSt 4MgSt 5no MgSt 6MgSt 7no MgSt 8MgSt Y1 (Hr) 4.35 7.3 1.95 3.65 2.55 6.3 2.55 4.9 Y2 (%) 11.405 26.229 100 99.07 109.275 99.84 15.185 13.055 Mean values from the formulations 1F1-F3, 2F2-F4, 3F5-F7, 4F6-F8, 5F10-F12, 6F9-F11, 7F14-F16, 8F13-F15. Y1= Lag time HPMCNaCMC HPC HEC 0.80 2.47 4.15 5.82 7.50 X1  C. Narendra et al. / Natural Science 2 (2010) 379-387 Copyright © 2010 SciRes. OPEN ACCESS 384 Table 5. Summary of ANOVA table for dependent variables from Taguchi design. Source d.f. Sum square Mean square F value Probability Y1 (Hr) R2 = 0.9940 Model 12 55.04 4.59 42.10 0.0052* X1 3 19.29 6.43 59.01 0.0036* X2 1 0.46 0.46 4.18 0.1334 X3 1 26.34 26.34 241.70 0.0006* X1X2 3 3.30 1.10 10.10 0.0446* X1X3 3 4.38 1.46 13.41 0.0304* X2X3 1 0.60 0.60 5.51 0.1005 Y2 (%) R2 = 0.9773 Model 5 29611.65 5922.33 86.34 < 0.0001* X1 3 29388.78 9796.26 142.82 < 0.0001* X2 1 221.52 221.52 3.23 0.1025 X3 1 1.36 1.36 0.02 0.8910 d.f. denotes degree of freedom; * denotes significant p < 0.05. Figure 6. Main effect plot for type of hydrophilic polymer (X1) on lag time (Y1) by keeping factors X2 and X3 at higher level. changing the type of hydrophilic polymer, only HPMC containing formulations showed negative effect where as other hydrophilic polymers showed positive effect on the lag time. The interaction effect between the factors X1X3 can be studied with the help of Figures 9 & 10. From this figures it may be concluded that presence of magnesium stearate in the coating layer exhibited a posi- tive effect on the lag time with irrespective levels of factors X1 and X2. A linear model for Y2 (percentage drug release at 8 hrs) was found to be significant. In this case, only factor X1 was found to be significant (Table 5). As the factor X1 was increased from lower to higher level, NaCMC and Figure 7. Interaction effect plot between type of hydro- philic polymer (X1) and hydrophilic polymer/EC ratio (X2) on lag time (Y1) at lower level of factor X3. (▪ Lower level; ∆ Higher level). HPC containing formulations exhibited an increased amount of drug release where as incase of HPMC and HEC containing formulations exhibited very less amount of drug release (Figures 11 & 12). This type of behavior may be attributed due to low hydration rate of these polymers in presence to EC and magnesium stearate and if so hydrated they formed a dense layer which further decreases the water diffusion into the core layer and de- layed the release of drug from the dosage form [29]. 4. OPTIMIZATION To optimize the studied responses with different targets, Y1= Lag time X1 HPMC NaCMC HPC HEC 0.82 2.49 4.16 5.83 X2 X1 HPMC NaCMC HPC HEC 1.40 3.20 5.01 6.82 8.62 Y1= Lag time  C. Narendra et al. / Natural Science 2 (2010) 379-387 Copyright © 2010 SciRes. OPEN ACCESS 385 Figure 8. Interaction effect plot between type of hydrophilic polymer (X1) and hydrophilic polymer/EC ratio (X2) on lag time (Y1) at higher level of factor X3. (▪ Lower level; ∆ Higher level). Figure 9. Interaction effect plot between type of hydrophilic polymer (X1) and amount of magnesium stearate (X3) on lag time (Y1) at lower level of factor X2.(▪ Lower level; ∆ Higher level). Figure 10. Interaction effect plot between type of hydrophilic polymer (X1) and amount of magnesium stearate (X3) on lag time (Y1) at higher level of factor X2 (▪ Lower level; ∆ Higher level). Figure 11. Main effect plot for type of hydrophilic polymer (X1) on % drug release at 8 hrs (Y2) by keep- ing factors X2 and X3 at lower level. a multi-criteria decision approach, like numerical opti- mization technique by the desirability function was used to generate the optimum settings for the formulation. [30, 31] The variables were optimized for the response Y1 and Y2 and the optimized formulation settings were ar- rived by maximizing the percent drug release at 8 hrs and lag time was kept at range between 6 to 7 hours. According to the statistical prediction, the optimal values obtained Figure 12. Main effect plot for type of hydrophilic polymer (X1) on % drug release at 8 hrs (Y2) by keeping factors X2 and X3 at higher level. was: HPC for type of hydrophilic polymer, hydrophilic polymer/EC ratio ranged between 2.5: 1 to 4: 1 and magnesium stearate also was ranged between 26-30 mg. Since, the Taguchi design is used to screen the formula- tion variables and to study their significant effect [32], the results from optimization studies was found to be in wider range and suggesting further studies to arrive to the optimal settings. Y2 = % drug release at 8 hrs HPMC NaCMC HPC HEC 10.05 37.13 64.20 91.27 118.34 X1 X1 HPMC NaCMC HPC HEC 0.33 28.96 57.60 86.23 114.87 Y2 = % drug release at 8 hrs  C. Narendra et al. / Natural Science 2 (2010) 379-387 Copyright © 2010 SciRes. OPEN ACCESS 386 5. CONCLUSIONS A Taguchi design was performed to screen the effect of formulation variables on the response lag time and per- cent drug release at 8 hrs in the development of timed- release press-coated tablets by applying computer opti- mization technique. Type of hydrophilic polymer was found to be the major factor affecting studied responses and also the results demonstrated that the hydrophobic agent, magnesium stearate could significantly prolonged the lag time. Among the type of different hydrophilic polymers studied, HPC was found to be more suitable and other hydrophilic polymers did not demonstrate beneficial effect (with in the studied variable limits) in the development of timed-release press-coated tablets. Based on the results of optimization studies it was con- cluded that further studies are required to obtain the op- timal settings. REFERENCES [1] Ueda, S., Hata, T., Asakura, S., Yamaguchi, H., Kotani, M. and Ueda, Y. (1994) Development of a novel drug release system, time-controlled explosion system (TES). I. Con- cept and design. Journal of Drug Targeting, 2(1), 35-44. [2] Krögel, I. and Bodmeier, R. (1998) Pulsatile drug release from an insoluble capsule body controlled by an erodible plug. Pharmaceutical Research, 15(3), 474-481. [3] Pozzi, F., Furlani, P., Gazzaniga, A., Davis, S.S. and Wilding, I.R. (1994) The TIME CLOCK* system: A new oral dosage form for fast and complete release of drug after a predetermined lag time. Journal of Controlled Release, 31(1), 99-108. [4] Gazzaniga A., Sangalli, M. and Giordano, F. (1994) Oral chronotropic drug delivery systems: Achievement of time and/or site specificity. European Journal of Pharmaceu- tics and Biopharmaceutics, 40, 246-250. [5] McNeil, M.E., Rashid, A. and Stevens, H.N.E. (1994) Dispensing device. U.S. Patent 5,342,624. [6] Crison, J.R., Siersma, P.R., Taylor, M.D. and Amidon, G.L. (1995) Programmable oral release technology, Port Systems & Mac226: A novel dosage form for time and site specific oral drug delivery. Proceedings of Interna- tional Symposium on Control Release of Bioactive Mate- rials, 22, 278-279. [7] Conte, U., Maggi, L., Torre, P., Giunchedi, P. and La-Manna, A. (1993) Press-coated tablets for time pro- grammed release of drugs. Biomaterials, 14(13), 1017- 1023. [8] Conte, U. and Maggi, L. (1996) Modulation of the dis- solution profiles from Geomatrix® multi-layer matrix tablets containing drugs of different solubility. Biomate- rials, 17(9), 889-896. [9] Lemmer, B. (2007) Chronobiology, drug-delivery, and chronotherapeutics. Advanced Drug Delivery Reviews, 59(9-10), 825-827. [10] Wu, B., Shun, N., Wei, X. and Wu, W. (2007) Charac- terization of 5-fluorouracil release from hydroxypropyl- methylcellulose compression-coated tablets. Pharmaceu- tical Development and Technology, 12(2), 203-210. [11] Fukui, E., Uemura, K. and Kobayashi, M. (2000) Studies on applicability of press-coated tablets using hy- droxypropylcellulose (HPC) in the outer shell for timed- release preparations. Journal of Controlled Release, 68(2), 215-223. [12] Matsuo, M., Arimori, K., Nakamura, C. and Nakano, M. (1996) Delayed-release tablets using hydroxyethylcellu- lose as a gel-forming matrix. International Journal of Pharmaceutics, 138(2), 225-235. [13] Sawada, T., Kondo, H., Nakashima, H., Sako, K. and Hayashi, M. (2004) Time-release compression-coated core tablet containing nifedipine for chronopharmaco- therapy. International Journal of Pharmaceutics, 280(1-2), 103-111. [14] Ugurlu, T., Turkoglu, M., Gurer, U.S. and Akarsu, B.G. (2007) Colonic delivery of compression coated nisin tablets using pectin/HPMC polymer mixture. European Journal of Pharmaceutics and Biopharmaceutics, 67(1), 202-210. [15] Lin, S.Y., Li, M.J. and Lin, K.H. (2004) Hydrophilic excipients modulate the time lag of time-controlled dis- integrating press-coated tablets. AAPS PharmSciTech, 5(4), Article 54. [16] Fukui, E., Miyamura, N., Yoneyama, T. and Kobayashi, M. (2001) Drug release from and mechanical properties of press-coated tablets with hydroxypropylmethylcellu- lose acetate succinate and plasticizers in the outer shell. International Journal of Pharmaceutics, 217(1-2), 33-43. [17] Fukui, E., Miyamura, N. and Kobayashi, M. (2001) Ef- fect of magnesium stearate or calcium stearate as addi- tives on dissolution profiles of diltiazem hydrochloride from press-coated tablets with hydroxypropylmethylcel- lulose acetate succinate in the outer shell. International Journal of Pharmaceutics, 216(1-2), 137-146. [18] Narendra, C., Srinath M.S. and Rao, B.P. (2005) Devel- opment of three layered buccal compact containing metoprolol tartrate by statistical optimization technique. International Journal of Pharmaceutics, 304(1-2), 102- 114. [19] Lewis, G.A., Mathieu, D. and Phan-Tan-Luu, R. (1999) Pharmaceutical experimental design. Marcel Dekker, New York. [20] Li, S., Lin, S., Daggy, B.P., Mirchandani, H.L. and Chein, Y.W. (2003) Effect of HPMC and carbopol on the release and floating properties of gastric floating drug delivery system using factorial design. International Journal of Pharmaceutics, 253(1-2), 13-22. [21] Nouranian, S., Garambi, H. and Mohammadi, N. (2007) Taguchi-based optimization of adhesion of polyurethane to plasticized poly (vinyl chloride) in synthetic leather. Journal of Adhesion Science and Technology, 21(8), 705- 724. [22] Conti, S., Maggia, L., Segalea, L., Machiste, E.O., Conte, U., Grenier, P. and Vergnault, G. (2007) Matrices con- taining NaCMC and HPMC 1. Dissolution performance characterization. International Journal of Pharmaceutics, 333(1-2), 136-142. [23] Matsuo, M., Nakamura, C., Arimori, K. and Nakano, M. (1995) Evaluation of hydroxyethylcellulose as a hydro- philic swellable material for delayed release tablets.  C. Narendra et al. / Natural Science 2 (2010) 379-387 Copyright © 2010 SciRes. OPEN ACCESS 387 Chemical & Pharmaceutical Bulletin, 43(2), 311-314. [24] Mahato, R.I. (2005) Biomaterials for delivery and tar- geting of proteins and nucleic acids. CRC Press, Florida. [25] Enayatifard, R., Saeedi, M., Akbari, J. and Tabatabaee, Y.H. (2009) Effect of hydroxypropylmethylcellulose and ethylcellulose content on release profile and kinetics of dilti- azem hcl from matrices. Tropical Journal of Pharmaceutical Research, 8(5), 425-432. [26] Roy, D.S. and Rohera, B.D. (2002) Comparative evalua- tion of rate of hydration and matrix erosion of HEC and HPC and study of drug release from their matrices. European Journal of Pharmaceutical Sciences, 16(3), 193-199. [27] Hilton, A.K. and Deasy, P.B. (1992) In vitro and in vivo evaluation of an oral sustained-release floating dosage form of amoxycillin trihydrate. International Journal of Pharmaceutics, 86(1), 79-88. [28] Durig, T. and Fassihi, R. (1997) Mechanistic evaluation of binary effects of magnesium stearate and talc as dis- solution retardants at 85% drug loading in an experimen- tal extended-release formulation. Journal of Pharmaceu- tical Sciences, 86(10), 1092-1098. [29] Minarro, M., Garcia-Montoya, E. and Sune-Negre, J.M. (2001) Study of formulation parameters by factorial de- sign in metoprolol tartrate matrix systems. Drug Devel- opment and Industrial Pharmacy, 27(9), 965-973. [30] Narendra, C., Srinath, M.S. and Moin, A. (2008) Study the effect of formulation variables on in vitro floating time and release properties of floating drug delivery sys- tem by statistical optimization technique. Chemical In- dustry & Chemical Engineering Quarterly, 14, 17-26. [31] Sanchez-Lafuente, C., Furlanetto, S., Fernandez-Arevalo, M., Alvarez-Fuentes, J., Rabasco, A.M., Faucci, T., Pin- zauti, S. and Mura, P. (2002) Didanosine extended- re- lease matrix tablets: Optimization of formulation vari- ables using statistical experimental design. International Journal of Pharmaceutics, 237(1-2), 107-118. [32] Kuo, C.F.J. and Wu, Y.-S. (2006) Optimization of the film coating process for polymer blends by the grey- based Taguchi method. International Journal of Ad- vanced Manufacturing Technology, 27(5-6), 525-530. |

